Modifications of Au Nanoparticle-Functionalized Graphene for Sensitive Detection of Sulfanilamide
Abstract
1. Introduction
2. Experimental
2.1. Materials and Reagents
2.2. Instruments
2.3. Synthesis of AuNPs/Gr Nanocomposites
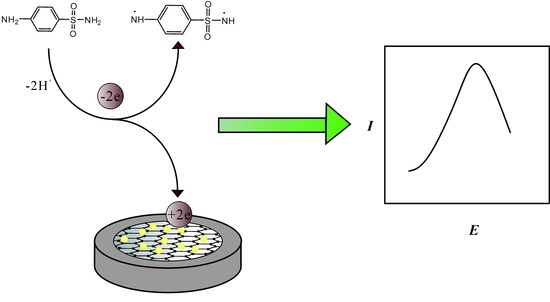
2.4. Fabrication of the AuNPs/Gr/GCE
3. Results and Discussion
3.1. Characterization of AuNPs/Gr
3.2. Surface Morphology of the Modified Electrodes
3.3. Electrochemical Response of SAM on AuNPs/Gr/GCE
3.4. Effects of Different Scanning Rates
3.5. Influence of pH
3.6. Effects of Different AuNPs/Gr Modification Volume
3.7. Determination of SAM
3.8. Interferences Study
3.9. Repeatability and Stability
3.10. Application of Sensor in a Real Sample
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Khezeli, T.; Daneshfar, A.; Sahraei, R. Emulsification liquid-liquid microextraction based on deep eutectic solvent: An extraction method for the determination of benzene, toluene, ethylbenzene and seven polycyclic aromatic hydrocarbons from water samples. J. Chromatogr. A 2015, 1425, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Liu, L.; Lv, X.; Qu, F.; You, J. Towards the determination of sulfonamides in meat samples: A magnetic and mesoporous metal-organic framework as an efficient sorbent for magnetic solid phase extraction combined with high-performance liquid chromatography. J. Chromatogr. A 2017, 1500, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Lahcen, A.A.; Amine, A. Mini-Review: Recent Advances in Electrochemical Determination of Sulfonamides. Anal. Lett. 2017, 51, 424–441. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Samanidou, V.; Stalikas, C.D. Graphene-functionalized melamine sponges for microextraction of sulfonamides from food and environmental samples. J. Chromatogr. A 2017, 1522, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Errayess, S.A.; Lahcen, A.A.; Idrissi, L.; Marcoaldi, C.; Chiavarini, S.; Amine, A. A sensitive method for the determination of Sulfonamides in seawater samples by Solid Phase Extraction and UV-Visible spectrophotometry. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 181, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhao, Q.; Jiang, L.; Li, Z.; Chen, Y.; Ding, L. Selective determination of sulfonamides from environmental water based on magnetic surface molecularly imprinting technology. Environ. Sci. Pollut. Res. 2017, 24, 9174–9186. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Li, K.; Liu, Z.; Zhang, H.; Jin, X. Novelty aqueous two-phase extraction system based on ionic liquid for determination of sulfonamides in blood coupled with high-performance liquid chromatography. Microchem. J. 2017, 136, 263–269. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Qi, M.; Zhong, S.; Wang, W.; Wang, A.J.; Chen, J. Graphene-Fe3O4 as a magnetic solid-phase extraction sorbent coupled to capillary electrophoresis for the determination of sulfonamides in milk. J. Sep. Sci. 2016, 39, 3818–3826. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Liang, H.; Xia, B.; Huang, C.; Ji, B.; Zhou, Y. Determination of Sulfonamides in Chicken Muscle by Pulsed Direct Current Electrospray Ionization Tandem Mass Spectrometry. J. Agric. Food Chem. 2017, 65, 8256–8263. [Google Scholar] [CrossRef] [PubMed]
- Hyunhee, C.; Jungbin, L.; Yunhee, C.; Kwanggeun, L. Analysis of sulfonamide and quinolone antibiotic residues in Korean milk using microbial assays and high performance liquid chromatography. Food Chem. 2009, 113, 297–301. [Google Scholar]
- Jiménez, V.; Adrian, J.; Guiteras, J.; Marco, M.P.; Companyó, R. Validation of an Enzyme-Linked Immunosorbent Assay for Detecting Sulfonamides in Feed Resources. J. Agric. Food Chem. 2010, 58, 7526–7531. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Sheng, W.; Zhang, Y.; Wang, J.; Wu, X.; Wang, S. Upconversion Nanoparticles and Monodispersed Magnetic Polystyrene Microsphere based Fluorescence Immunoassay for the Detection of Sulfaquinoxaline in Animal-derived Foods. J. Agric. Food Chem. 2016, 64, 3908–3915. [Google Scholar] [CrossRef] [PubMed]
- Dmitrienko, S.G.; Kochuk, E.V.; Tolmacheva, V.V.; Apyari, V.V.; Zolotov, Y.A. Determination of the total content of some sulfonamides in milk using solid-phase extraction coupled with off-line derivatization and spectrophotometric detection. Food Chem. 2015, 188, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Cai, Y.; Shi, Y.; Wei, F.; Liu, J.; Mou, S.; Jiang, G. Evaluation of carbon nanotubes as a solid-phase extraction adsorbent for the extraction of cephalosporins antibiotics, sulfonamides and phenolic compounds from aqueous solution. Anal. Chim. Acta 2007, 594, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Tarbin, J.A.; Clarke, P.; Shearer, G. Screening of sulphonamides in egg using gas chromatography-mass-selective detection and liquid chromatography-mass spectrometry. J. Chromatogr. B Biomed. Sci. Appl. 1999, 729, 127–138. [Google Scholar] [CrossRef]
- Berardi, G.; Bogialli, S.; Curini, R.; Di Corcia, A.; Laganá, A. Evaluation of a Method for Assaying Sulfonamide Antimicrobial Residues in Cheese: Hot-Water Extraction and Liquid Chromatography-Tandem Mass Spectrometry. J. Agric. Food Chem. 2006, 54, 4537–4543. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, R.; Mirabile, J.; Hafler, K. Determination of six illegal antibiotics in chicken jerky dog treat. J. Agric. Food Chem. 2014, 62, 3690–3696. [Google Scholar] [CrossRef] [PubMed]
- Tso, J.; Dutta, S.; Inamdar, S.; Aga, D.S. Simultaneous analysis of free and conjugated estrogens, sulfonamides, and tetracyclines in runoff water and soils using solid-phase extraction and liquid chromatography-tandem mass spectrometry. J. Agric. Food Chem. 2011, 59, 2213–2222. [Google Scholar] [CrossRef] [PubMed]
- He, B. Electrochemical Determination of Sulfonamide Based on Glassy Carbon Electrode Modified by Fe3O4/Functionalized Graphene. Int. J. Electrochem. Sci. 2017, 12, 3001–3011. [Google Scholar] [CrossRef]
- He, B.; Chen, W. Carboxyl Multiwalled Carbon Nanotubes through Ultrasonic Dispersing in Dimethylfomamide Modified Electrode as a Sensitive Amperometric Sensor for Detection of Sulfonamide. Int. J. Electrochem. Sci. 2014, 10, 4335–4345. [Google Scholar]
- Wei, X.; Xu, G.; Gong, C.; Qin, F.; Gong, X.; Li, C. Fabrication and evaluation of sulfanilamide-imprinted composite sensors by developing a custom-tailored strategy. Sens. Actuators B Chem. 2018, 255, 2697–2703. [Google Scholar] [CrossRef]
- Wang, G.; Chen, L.; Zhu, Y.; He, X.; Xu, G.; Zhang, X. Development of an electrochemical sensor based on the catalysis of ferrocene actuated hemin/G-quadruplex enzyme for the detection of potassium ions. Biosens. Bioelectron. 2014, 61, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Wei, W.; Guo, Z.X.; Shi, M.; Guo, R.P. Decreased Cezanne expression is associated with the progression and poor prognosis in hepatocellular carcinoma. J. Trans. Med. 2015, 13, 41. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, J.; Wang, X.; Wang, T.; Li, D.; Xi, F.; Wang, J.; Wang, E. Functionalization of Monolithic and Porous Three-Dimensional Graphene by One-Step Chitosan Electrodeposition for Enzymatic Biosensor. ACS Appl. Mater. Interfaces 2014, 6, 19997–20002. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Luo, L.; Zhu, L.; Ding, Y.; Deng, D.; Wang, Z. Aptamer-linked biosensor for thrombin based on AuNPs/thionine-graphene nanocomposite. Analyst 2013, 138, 5365–5370. [Google Scholar] [CrossRef] [PubMed]
- Taghdisi, S.M.; Danesh, N.M.; Lavaee, P.; Ramezani, M.; Abnous, K. An electrochemical aptasensor based on gold nanoparticles, thionine and hairpin structure of complementary strand of aptamer for ultrasensitive detection of lead. Sens. Actuators B Chem. 2016, 234, 462–469. [Google Scholar] [CrossRef]
- Kangkamano, T.; Numnuam, A.; Limbut, W.; Kanatharana, P.; Thavarungkul, P. Chitosan Cryogel with Embedded Gold Nanoparticles Decorated Multiwalled Carbon Nanotubes Modified Electrode for Highly Sensitive Flow Based Non-Enzymatic Glucose Sensor. Sens. Actuators B Chem. 2017, 246, 854–863. [Google Scholar] [CrossRef]
- He, B.S.; Du, G.A. A simple and sensitive electrochemical detection of furazolidone based on an Au nanoparticle functionalized graphene modified electrode. Anal. Methods 2017, 9, 4341–4348. [Google Scholar] [CrossRef]
- Sun, M.; Davenport, D.; Liu, H.; Qu, J.; Elimelech, M.; Li, J. Highly efficient and sustainable non-precious-metal Fe-N-C electrocatalysts for the oxygen reduction reaction. J. Mater. Chem. A 2017, 6, 2527–2539. [Google Scholar] [CrossRef]
- Shan, C.; Yang, H.; Han, D.; Zhang, Q.; Ivaska, A.; Niu, L. Electrochemical determination of NADH and ethanol based on ionic liquid-functionalized graphene. Biosens. Bioelectron. 2010, 25, 1504–1508. [Google Scholar] [CrossRef] [PubMed]
- Beiranvand, Z.S.; Abbasi, A.R.; Dehdashtian, S.; Karimi, Z.; Azadbakht, A. Aptamer-based electrochemical biosensor by using Au-Pt nanoparticles, carbon nanotubes and acriflavine platform. Anal. Biochem. 2016, 518, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Tadi, K.K.; Motghare, R.V.; Ganesh, V. Electrochemical Detection of Sulfanilamide Using Pencil Graphite Electrode Based on Molecular Imprinting Technology. Electroanalysis 2015, 26, 2328–2336. [Google Scholar] [CrossRef]












| Method | Electrode | Linear Range (μmol·L−1) | Limit of Detection | Reference |
|---|---|---|---|---|
| i-t | Fe3O4/Gr/GCE | 0.5–110 | 0.05 | [19] |
| CV | Carboxyl/DMF/MWCNTs/GCE a | 1.0–100 | 0.5 | [20] |
| FL b | MIPs/QDs@SiO2 c | 2.0–30 | 0.17 | [21] |
| DPV | Py/MIP PGE d | 0.05–1.1, 1.1–48 | 0.02 | [32] |
| DPV | Gr/AuNPs/GCE | 0.1–1000 | 0.011 | This work |
| Solution (mol·L−1) | Current (μA) | Rate of Change (%) |
|---|---|---|
| 1.0 × 10−3 SAM | 26.18 | 3.44 |
| After addition of 50-fold glucose | 25.38 | |
| 1.0 × 10−3 SAM | 22.28 | 3.00 |
| After addition of 50-fold sucrose | 22.95 | |
| 1.0 × 10−3 SAM | 27.69 | 2.60 |
| After addition of 300-fold NaCl | 28.41 | |
| 1.0 × 10−3 SAM | 29.44 | 4.55 |
| After addition of 300-fold KCl | 30.78 | |
| 1.0 × 10−3 SAM | 28.48 | 3.55 |
| After addition of 300-fold CaCl2 | 29.49 | |
| 1.0 × 10−3 SAM | 28.46 | 4.56 |
| After addition of 300-fold MgSO4 | 29.76 |
| Sample | Added (10−5 mol·L−1) | Found (10−5 mol·L−1) | Recovery (%) | Average Recovery (%) |
|---|---|---|---|---|
| SAM | 3.0 | 2.69 | 89.67 | 91.22 |
| 2.74 | 91.33 | |||
| 2.78 | 92.67 | |||
| 6.0 | 5.43 | 90.50 | 96.67 | |
| 5.85 | 97.50 | |||
| 6.12 | 102.00 | |||
| 9.0 | 7.73 | 85.89 | 94.67 | |
| 8.76 | 97.33 | |||
| 9.07 | 100.78 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, B.-S.; Yan, X.-H. Modifications of Au Nanoparticle-Functionalized Graphene for Sensitive Detection of Sulfanilamide. Sensors 2018, 18, 846. https://doi.org/10.3390/s18030846
He B-S, Yan X-H. Modifications of Au Nanoparticle-Functionalized Graphene for Sensitive Detection of Sulfanilamide. Sensors. 2018; 18(3):846. https://doi.org/10.3390/s18030846
Chicago/Turabian StyleHe, Bao-Shan, and Xiao-Hai Yan. 2018. "Modifications of Au Nanoparticle-Functionalized Graphene for Sensitive Detection of Sulfanilamide" Sensors 18, no. 3: 846. https://doi.org/10.3390/s18030846
APA StyleHe, B.-S., & Yan, X.-H. (2018). Modifications of Au Nanoparticle-Functionalized Graphene for Sensitive Detection of Sulfanilamide. Sensors, 18(3), 846. https://doi.org/10.3390/s18030846