Motor Planning Error: Toward Measuring Cognitive Frailty in Older Adults Using Wearables
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Demographic Information
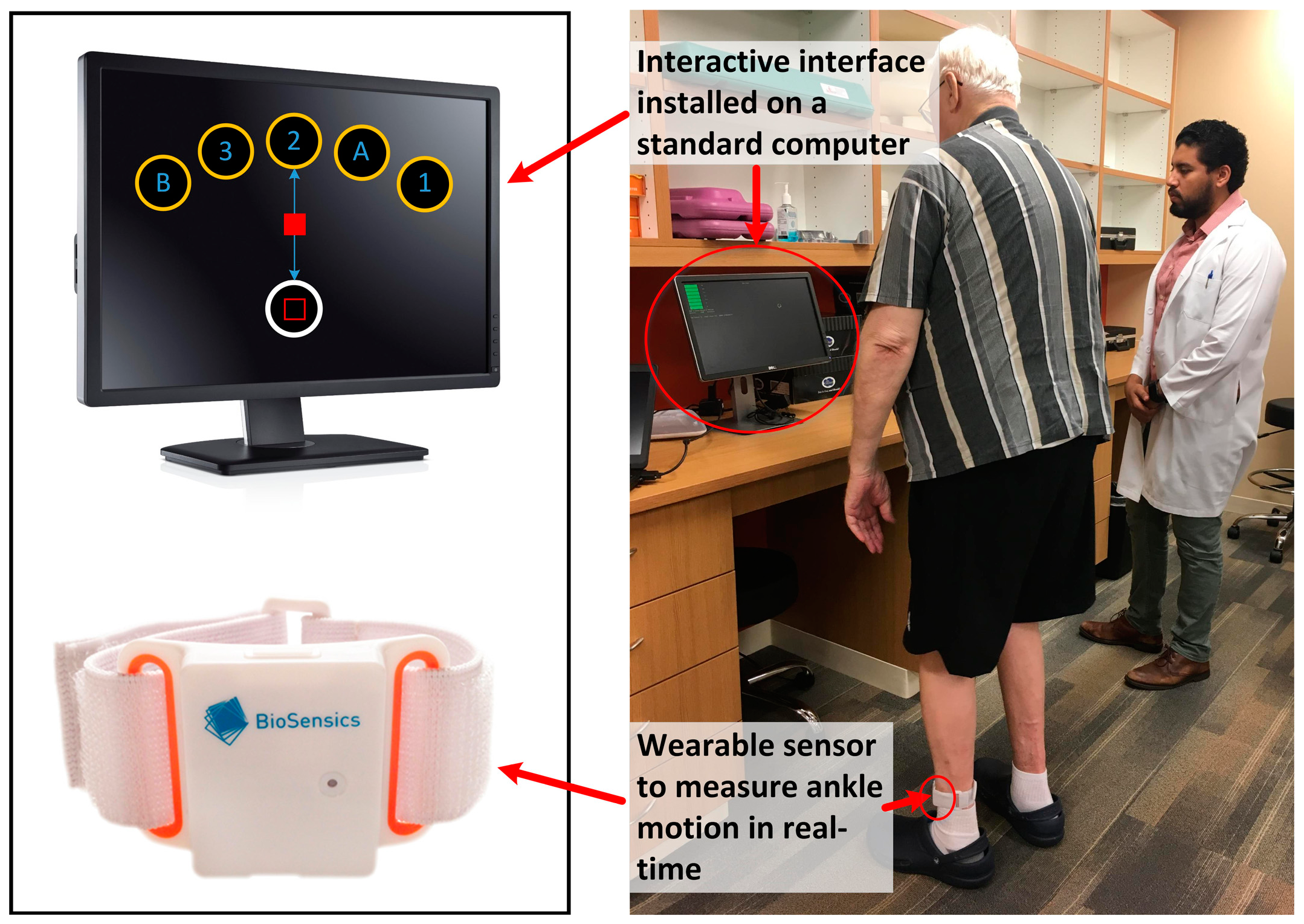
2.3. Instrumented Trail-Making Task (iTMT)
2.4. iTMT Motor Planning Error
2.5. iTMT Time
2.6. Walking Test
2.7. Statistical Analysis
Sample Size Calculation
3. Results
3.1. Study Population
3.2. iTMT Motor Planning Error and iTMT Time among Groups
3.3. Association between iTMT Motor Planning Errors and Conventional Cognitive Assessment
4. Discussion
Limitations and Future Directions
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kelaiditi, E.; Cesari, M.; Canevelli, M.; van Kan, G.A.; Ousset, P.J.; Gillette-Guyonnet, S.; Ritz, P.; Duveau, F.; Soto, M.E.; Provencher, V.; et al. Cognitive frailty: Rational and definition from an (I.A.N.A./I.A.G.G.) international consensus group. J. Nutr. Health Aging 2013, 17, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Bahureksa, L.; Najafi, B.; Saleh, A.; Sabbagh, M.; Coon, D.; Mohler, M.J.; Schwenk, M. The Impact of Mild Cognitive Impairment on Gait and Balance: A Systematic Review and Meta-Analysis of Studies Using Instrumented Assessment. Gerontology 2017, 63, 67–83. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Mental Health and Older Adults; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Cornelis, E.; Gorus, E.; Beyer, I.; Bautmans, I.; De Vriendt, P. Early diagnosis of mild cognitive impairment and mild dementia through basic and instrumental activities of daily living: Development of a new evaluation tool. PLoS Med. 2017, 14, e1002250. [Google Scholar] [CrossRef] [PubMed]
- Brooks, L.G.; Loewenstein, D.A. Assessing the progression of mild cognitive impairment to Alzheimer’s disease: Current trends and future directions. Alzheimers Res. Ther. 2010, 2, 28. [Google Scholar] [CrossRef] [PubMed]
- Dierckx, E.; Engelborghs, S.; De Raedt, R.; De Deyn, P.P.; Ponjaert-Kristoffersen, I. Mild cognitive impairment: What’s in a name? Gerontology 2007, 53, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H.; Makizako, H.; Tsutsumimoto, K.; Doi, T.; Lee, S.; Suzuki, T. Cognitive Frailty and Incidence of Dementia in Older Persons. J. Prev. Alzheimers Dis. 2018, 5, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Q.; Yu, Z.; Chen, M.; Bao, Z.; Li, J.; He, W. Cognitive frailty, a novel target for the prevention of elderly dependency. Ageing Res. Rev. 2015, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kelaiditi, E.; Canevelli, M.; Andrieu, S.; Del Campo, N.; Soto, M.E.; Vellas, B.; Cesari, M. Impact of Cholinergic Treatment Use Study/DSA Group. Frailty Index and Cognitive Decline in Alzheimer’s Disease: Data from the Impact of Cholinergic Treatment USe Study. J. Am. Geriatr. Soc. 2016, 64, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Rockwood, K.; Andrew, M.; Mitnitski, A. A comparison of two approaches to measuring frailty in elderly people. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2007, 62, 738–743. [Google Scholar] [CrossRef]
- Buta, B.J.; Walston, J.D.; Godino, J.G.; Park, M.; Kalyani, R.R.; Xue, Q.L.; Bandeen-Roche, K.; Varadhan, R. Frailty assessment instruments: Systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res. Rev. 2016, 26, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef]
- Walston, J. Frailty in older adults. In Oxford Textbook of Geriatric Medicine; Oxford University Press: Oxford, UK, 2017; p. 429. [Google Scholar]
- Montero, M.; Najafi, B.; Hinko, V.; Hoegliner, S.; Rahemi, H.; Enriquez, A.; Chung, J.; Barshes, N.; Gilani, R.; Mills, J. Using Frailty and Cognitive Assessment to Predict Adverse Events After Major Vascular Intervention: Application of Wearable Technologies. J. Vasc. Surg. 2017, 66, e56. [Google Scholar] [CrossRef]
- Millar, K.; Asbury, A.; Murray, G. Pre-existing cognitive impairment as a factor influencing outcome after cardiac surgery. Br. J. Anaesth. 2001, 86, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Fougère, B.; Daumas, M.; Lilamand, M.; Sourdet, S.; Delrieu, J.; Vellas, B.; van Kan, G.A. Association between frailty and cognitive impairment: Cross-sectional data from Toulouse frailty day hospital. J. Am. Med. Dir. Assoc. 2017, 18, 990.e1–990.e5. [Google Scholar] [CrossRef] [PubMed]
- Verghese, J.; Robbins, M.; Holtzer, R.; Zimmerman, M.; Wang, C.; Xue, X.; Lipton, R.B. Gait dysfunction in mild cognitive impairment syndromes. J. Am. Geriatr. Soc. 2008, 56, 1244–1251. [Google Scholar] [CrossRef] [PubMed]
- Boyle, P.A.; Capurso, C.; D’Introno, A.; Colacicco, A.M.; Capurso, A.; Solfrizzi, V. Mild cognitive impairment: risk of Alzheimer disease and rate of cognitive decline. Neurology 2006, 67, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Patterns of loss of basic activities of daily living in Alzheimer patients: A cross-sectional study of the French REAL cohort. Dement. Geriatr. Cogn. Disord. 2010, 29, 46–54. [CrossRef]
- Liang, F.W.; Chan, W.; Chen, P.J.; Zimmerman, C.; Waring, S.; Doody, R. Cognitively-Related Basic Activities of Daily Living Impairment Greatly Increases the Risk of Death in Alzheimers Disease. PLoS ONE 2016, 11, e0160671. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.; Lebel, K.; Bogard, S.; Goubault, E.; Boissy, P.; Clinicians, Q.P.N.; Duval, C. Using Inertial Sensors to Automatically Detect and Segment Activities of Daily Living in People with Parkinson’s Disease. IEEE Trans. Neural Syst. Rehabil. Eng. 2017. Available online: http://ieeexplore.ieee.org/stamp/stamp.jsp?arnumber=8016621 (accessed on 20 March 2018).
- Seidler, R.D.; Kwak, Y.; Fling, B.W.; Bernard, J.A. Neurocognitive mechanisms of error-based motor learning. Adv. Exp. Med. Biol. 2013, 782, 39–60. [Google Scholar] [CrossRef] [PubMed]
- Cisek, P. Integrated neural processes for defining potential actions and deciding between them: A computational model. J. Neurosci. 2006, 26, 9761–9770. [Google Scholar] [CrossRef] [PubMed]
- Tanji, J.; Shima, K. Role for supplementary motor area cells in planning several movements ahead. Nature 1994, 371, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Halsband, U.; Ito, N.; Tanji, J.; Freund, H.-J. The role of premotor cortex and the supplementary motor area in the temporal control of movement in man. Brain 1993, 116, 243–266. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, H.; Sadato, N.; Lyshkow, H.; Yonekura, Y.; Honda, M.; Nagamine, T.; Suwazono, S.; Magata, Y.; Ikeda, A.; Miyazaki, M. Both primary motor cortex and supplementary motor area play an important role in complex finger movement. Brain 1993, 116, 1387–1398. [Google Scholar] [CrossRef] [PubMed]
- Stockel, T.; Wunsch, K.; Hughes, C.M.L. Age-Related Decline in Anticipatory Motor Planning and Its Relation to Cognitive and Motor Skill Proficiency. Front. Aging Neurosci. 2017, 9, 283. [Google Scholar] [CrossRef] [PubMed]
- Raz, N.; Gunning-Dixon, F.; Head, D.; Rodrigue, K.M.; Williamson, A.; Acker, J.D. Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: Replicability of regional differences in volume. Neurobiol. Aging 2004, 25, 377–396. [Google Scholar] [CrossRef]
- Fazekas, F.; Ropele, S.; Enzinger, C.; Gorani, F.; Seewann, A.; Petrovic, K.; Schmidt, R. MTI of white matter hyperintensities. Brain 2005, 128, 2926–2932. [Google Scholar] [CrossRef] [PubMed]
- Tombaugh, T.N. Trail Making Test A and B: Normative data stratified by age and education. Arch. Clin. Neuropsychol. 2004, 19, 203–214. [Google Scholar] [CrossRef]
- Zhou, H.; Sabbagh, M.; Wyman, R.; Liebsack, C.; Kunik, M.E.; Najafi, B. Instrumented Trail-Making Task to Differentiate Persons with No Cognitive Impairment, Amnestic Mild Cognitive Impairment, and Alzheimer Disease: A Proof of Concept Study. Gerontology 2017, 63, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Cockrell, J.R.; Folstein, M.F. Mini-mental state examination. In Principles and Practice of Geriatric Psychiatry; Wiley: Chichester, UK, 2002; pp. 140–141. [Google Scholar]
- Nasreddine, Z.S.; Phillips, N.A.; Bedirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Najafi, B.; Horn, D.; Marclay, S.; Crews, R.T.; Wu, S.; Wrobel, J.S. Assessing postural control and postural control strategy in diabetes patients using innovative and wearable technology. J. Diabetes Sci. Technol. 2010, 4, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Schwenk, M.; Howe, C.; Saleh, A.; Mohler, J.; Grewal, G.; Armstrong, D.; Najafi, B. Frailty and technology: A systematic review of gait analysis in those with frailty. Gerontology 2014, 60, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Grewal, G.; Sayeed, R.; Yeschek, S.; Menzies, R.A.; Talal, T.K.; Lavery, L.A.; Armstrong, D.G.; Najafi, B. Virtualizing the assessment: A novel pragmatic paradigm to evaluate lower extremity joint perception in diabetes. Gerontology 2012, 58, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Najafi, B.; Helbostad, J.L.; Moe-Nilssen, R.; Zijlstra, W.; Aminian, K. Does walking strategy in older people change as a function of walking distance? Gait Posture 2009, 29, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Aminian, K.; Najafi, B.; Bula, C.; Leyvraz, P.F.; Robert, P. Spatio-temporal parameters of gait measured by an ambulatory system using miniature gyroscopes. J. Biomech. 2002, 35, 689–699. [Google Scholar] [CrossRef]
- Gaveau, V.; Pisella, L.; Priot, A.E.; Fukui, T.; Rossetti, Y.; Pélisson, D.; Prablanc, C. Automatic online control of motor adjustments in reaching and grasping. Neuropsychologia 2014, 55, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Vance, D.E.; Gleason, C.E.; Heidrich, S.M. Caring for older adults with mild cognitive impairment: an update for nurses. J. Gerontol. Nurs. 2012, 38, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Razjouyan, J.; Grewal, G.S.; Rishel, C.; Parthasarathy, S.; Mohler, J.; Najafi, B. Activity monitoring and heart rate variability as indicators of fall risk: Proof-of-concept for application of wearable sensors in the acute care setting. J. Gerontol. Nurs. 2017, 43, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, U.; Najafi, B.; Zijlstra, W.; Hauer, K.; Muche, R.; Becker, C.; Aminian, K. Distance to achieve steady state walking speed in frail elderly persons. Gait Posture 2008, 27, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Gerling, K.M.; Schild, J.; Masuch, M. Exergaming for elderly: Analyzing player experience and performance. In Proceedings of the Mensch & Computer, Chemnitz, Germany, 11–14 September 2011; p. 401. [Google Scholar]



| Elderly Cognitive-Intact (N) | Elderly Cognitive-Impaired (P) | p-Value (P vs. N) | Young-Healthy (H) | p-Value (N vs. H) | |
|---|---|---|---|---|---|
| Number of subject, n | 16 | 16 | - | 12 | - |
| Female, n (%) | 7.0 (44) | 5.0 (31) | 0.481 | 4.0 (33) | 0.593 |
| Age, years | 75.6 ± 9.5 | 79.0 ± 8.6 | 0.292 | 26.0 ± 5.2 | <0.001 |
| Height, cm | 170.0 ± 9.8 | 168.5 ± 12.8 | 0.723 | 170.9 ± 9.4 | 0.798 |
| Body mass, kg | 69.9 ± 14.6 | 77.7 ± 20.9 | 0.240 | 74.3 ± 15.3 | 0.450 |
| BMI, kg/m2 | 24.0 ± 3.4 | 26.7 ± 5.5 | 0.113 | 25.3 ± 3.9 | 0.387 |
| History of fall, n (%) | 4.0 (25) | 7.0 (44) | 0.279 | - | - |
| Depression, n (%) | 4.0 (25) | 2.0 (13) | 0.381 | - | - |
| STW SV, m/s | 1.00 ± 0.18 | 0.87 ± 0.21 | 0.075 | 1.27 ± 0.15 | <0.001 |
| DTW SV, m/s | 0.85 ± 0.21 | 0.68 ± 0.22 | 0.031 | 1.14 ± 0.21 | 0.001 |
| Young-Healthy | Elderly Cognitive-Intact | Elderly Cognitive-Impaired | p-Value | |
|---|---|---|---|---|
| iTMT Motor Planning Error, % | 11.1 ± 5.7 | 20.3 ± 9.6 | 34.1 ± 4.2 | <0.001 |
| iTMT Time, s | 18.5 ± 2.1 | 25.2 ± 7.9 | 50.4 ± 28.3 | <0.001 |
| iTMT Motor Planning Error | iTMT Time | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Without Adjustment | With Adjustment for Age and BMI | Without Adjustment | With Adjustment for Age and BMI | |||||||||
| Difference Mean (%) | p-Value | d | Difference Mean (%) | p-Value | d | Difference Mean (%) | p-Value | d | Difference Mean (%) | p-Value | d | |
| Elderly cognitive-intact vs. young-healthy | 9.2 (82) | 0.001 | 1.17 | −4.3 (20) | 0.547 | 0.29 | 6.7 (36) | 0.331 | 1.16 | −2.8 (11) | 0.886 | 0.07 |
| Elderly cognitive-impaired vs. young-healthy | 22.9 (207) | <0.001 | 4.56 | 8.6 (41) | 0.260 | 0.57 | 31.9 (172) | <0.001 | 1.59 | 24.2 (98) | 0.238 | 0.61 |
| Elderly cognitive-impaired vs. elderly cognitive-intact | 13.8 (68) | <0.001 | 1.86 | 12.9 (77) | <0.001 | 1.26 | 25.2 (100) | <0.001 | 1.21 | 27.0 (122) | <0.001 | 0.98 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Lee, H.; Lee, J.; Schwenk, M.; Najafi, B. Motor Planning Error: Toward Measuring Cognitive Frailty in Older Adults Using Wearables. Sensors 2018, 18, 926. https://doi.org/10.3390/s18030926
Zhou H, Lee H, Lee J, Schwenk M, Najafi B. Motor Planning Error: Toward Measuring Cognitive Frailty in Older Adults Using Wearables. Sensors. 2018; 18(3):926. https://doi.org/10.3390/s18030926
Chicago/Turabian StyleZhou, He, Hyoki Lee, Jessica Lee, Michael Schwenk, and Bijan Najafi. 2018. "Motor Planning Error: Toward Measuring Cognitive Frailty in Older Adults Using Wearables" Sensors 18, no. 3: 926. https://doi.org/10.3390/s18030926
APA StyleZhou, H., Lee, H., Lee, J., Schwenk, M., & Najafi, B. (2018). Motor Planning Error: Toward Measuring Cognitive Frailty in Older Adults Using Wearables. Sensors, 18(3), 926. https://doi.org/10.3390/s18030926






