Sensitive and Selective NH3 Monitoring at Room Temperature Using ZnO Ceramic Nanofibers Decorated with Poly(styrene sulfonate)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
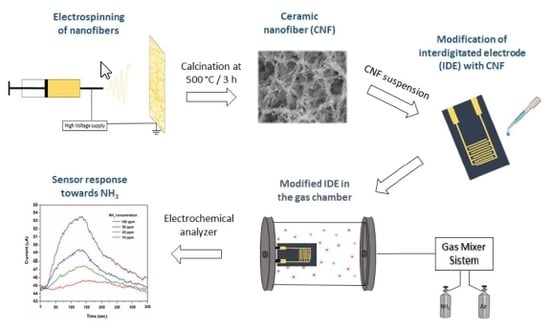
2.2. ZnO Nanofibers Fabrication
2.3. Characterization
2.4. Sensor Fabrication and Gas Testing System
2.5. NH3 Sensing at Room Temperature
3. Results and Discussion
3.1. Structural and Morphological Characterization of ZnO NFs
3.2. Gas Sensing Studies
3.3. NH3 Sensing Mechanism
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Timmer, B.; Olthuis, W.; Berg, A. Van Den Ammonia sensors and their applications—A review. Sens. Actuators B Chem. 2005, 107, 666–677. [Google Scholar] [CrossRef]
- Wang, Q.; Dong, X.; Pang, Z.; Du, Y.; Xia, X.; Wei, Q.; Huang, F. Ammonia Sensing Behaviors of TiO2-PANI/PA6 Composite Nanofibers. Sensors 2012, 12, 17046–17057. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, R.F.; Megson, L.C. The effect of uncertainties in human toxic response on hazard range estimation for ammonia and chlorine. Atmos. Environ. 1984, 18, 1195–1206. [Google Scholar] [CrossRef]
- Corkery, G.; Ward, S.; CKenny, C.; Hemmingway, P. Title Monitoring environmental parameters in poultry production facilities Author(s). In Computer Aided Process Engineering, CAPE Forum 2013; Graz University of Technology: Graz, Austria, 2013. [Google Scholar]
- Mani, G.K.; Rayappan, J.B.B. A highly selective and wide range ammonia sensor—Nanostructured ZnO:Co thin film. Mater. Sci. Eng. B 2015, 191, 41–50. [Google Scholar] [CrossRef]
- Ghosh, R.; Nayak, A.K.; Santra, S.; Pradhan, D.; Guha, P.K. Enhanced ammonia sensing at room temperature with reduced graphene oxide/tin oxide hybrid films. RSC Adv. 2015, 5, 50165–50173. [Google Scholar] [CrossRef]
- Bittman, S.; Jones, K.; Vingarzan, R.; Hunt, D.E.; Sheppard, S.C.; Tait, J.; So, R.; Zhao, J. Weekly agricultural emissions and ambient concentrations of ammonia: Validation of an emission inventory. Atmos. Environ. 2015, 113, 108–117. [Google Scholar] [CrossRef]
- Ji, B.; Gates, R.S.; Zheng, W.; Grift, T.E.; Green, A.R.; Koelkebeck, K.W. Design and Performance Evaluation of Upgraded Portable Monitoring Units for Barn Air Quality. ASABE Annu. Int. Meet. 2015, 1, 774–787. [Google Scholar]
- Zhao, Y.; Shepherd, T.A.; Li, H.; Xin, H. Environmental assessment of three egg production systems—Part I: Monitoring system and indoor air quality. Poult. Sci. 2015, 94, 518–533. [Google Scholar] [CrossRef] [PubMed]
- Ta, S.; Zhao, Y.; Li, H.; Stinn, S.; Xin, H.; Shepherd, T.A.; Zhao, Y.; Li, H.; Stinn, J.P.; Hayes, M.D.; Xin, H. Environmental assessment of three egg production systems—Part II. Ammonia, greenhouse gas, and particulate matter emissions. Poult. Sci. 2015, 94, 534–543. [Google Scholar] [CrossRef]
- Thungon, P.D.; Kakoti, A.; Ngashangva, L.; Goswami, P. Advances in developing rapid, reliable and portable detection systems for alcohol. Biosens. Bioelectron. 2017, 97, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Timsorn, K.; Lorjaroenphon, Y.; Wongchoosuk, C. Identification of adulteration in uncooked Jasmine rice by a portable low-cost artificial olfactory system. Measurement 2017, 108, 67–76. [Google Scholar] [CrossRef]
- Järvinen, T.; Lorite, G.S.; Rautio, A.-R.; Juhász, K.L.; Kukovecz, Á.; Kónya, Z.; Kordas, K.; Toth, G. Portable cyber-physical system for indoor and outdoor gas sensing. Sens. Actuators B Chem. 2017, 252, 983–990. [Google Scholar] [CrossRef]
- Hyodo, T.; Urata, K.; Kamada, K.; Ueda, T.; Shimizu, Y. Semiconductor-type SnO2-based NO2 sensors operated at room temperature under UV-light irradiation. Sens. Actuators B Chem. 2017, 253, 630–640. [Google Scholar] [CrossRef]
- Wang, Z.; Peng, X.; Huang, C.; Chen, X.; Dai, W.; Fu, X. CO gas sensitivity and its oxidation over TiO2 modified by PANI under UV irradiation at room temperature. Appl. Catal. B Environ. 2017, 219, 379–390. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, L.; Guo, F.; Liu, S.; Qi, L.; Shan, M.; Fan, X. Facial development of high performance room temperature NO2 gas sensors based on ZnO nanowalls decorated rGO nanosheets. Appl. Surf. Sci. 2017, 423, 721–727. [Google Scholar] [CrossRef]
- Abu-Hani, A.F.S.; Greish, Y.E.; Mahmoud, S.T.; Awwad, F.; Ayesh, A.I. Low-temperature and fast response H2S gas sensor using semiconducting chitosan film. Sens. Actuators B Chem. 2017, 253, 677–684. [Google Scholar] [CrossRef]
- Zhuang, Z.; Qi, D.; Ru, C.; Pan, J.; Zhao, C.; Na, H. Fast response and highly sensitive humidity sensors based on CaCl2-doped sulfonated poly (ether ether ketone)s. Sens. Actuators B Chem. 2017, 253, 666–676. [Google Scholar] [CrossRef]
- Piloto, C.; Mirri, F.; Bengio, E.A.; Notarianni, M.; Gupta, B.; Shafiei, M.; Pasquali, M.; Motta, N. Room temperature gas sensing properties of ultrathin carbon nanotube films by surfactant-free dip coating. Sens. Actuators B Chem. 2016, 227, 128–134. [Google Scholar] [CrossRef]
- Andre, R.S.; Chen, J.; Kwak, D.; Correa, D.S.; Mattoso, L.H.C.; Lei, Y. A flexible and disposable poly(sodium 4-styrenesulfonate)/polyaniline coated glass microfiber paper for sensitive and selective detection of ammonia at room temperature. Synth. Met. 2017, 233, 22–27. [Google Scholar] [CrossRef]
- Su, P.-G.; Yang, L.-Y. NH3 gas sensor based on Pd/SnO2/RGO ternary composite operated at room-temperature. Sens. Actuators B Chem. 2016, 223, 202–208. [Google Scholar] [CrossRef]
- Wang, J.; Yang, P.; Wei, X. High-Performance, Room-Temperature, and No-Humidity-Impact Ammonia Sensor Based on Heterogeneous Nickel Oxide and Zinc Oxide Nanocrystals. ACS Appl. Mater. Interfaces 2015, 7, 3816–3824. [Google Scholar] [CrossRef] [PubMed]
- Andre, R.S.; Shimizu, F.M.; Miyazaki, C.M.; Riul, A.; Manzani, D.; Ribeiro, S.J.L.; Oliveira, O.N.; Mattoso, L.H.C.; Correa, D.S. Hybrid layer-by-layer (LbL) films of polyaniline, graphene oxide and zinc oxide to detect ammonia. Sens. Actuators B Chem. 2017, 238, 795–801. [Google Scholar] [CrossRef]
- Shafiei, M.; Hoshyargar, F.; Lipton-Duffin, J.; Piloto, C.; Motta, N.; OMullane, A.P. Conversion of n-Type CuTCNQ into p-Type Nitrogen-Doped CuO and the Implication for Room-Temperature Gas Sensing. J. Phys. Chem. C 2015, 119, 22208–22216. [Google Scholar] [CrossRef] [Green Version]
- Sun, P.; Cai, Y.; Du, S.; Xu, X.; You, L.; Ma, J.; Liu, F.; Liang, X.; Sun, Y.; Lu, G. Hierarchical α-Fe2O3/SnO2 semiconductor composites: Hydrothermal synthesis and gas sensing properties. Sens. Actuators B Chem. 2013, 182, 336–343. [Google Scholar] [CrossRef]
- Masson, N.; Piedrahita, R.; Hannigan, M. Approach for quantification of metal oxide type semiconductor gas sensors used for ambient air quality monitoring. Sens. Actuators B Chem. 2015, 208, 339–345. [Google Scholar] [CrossRef]
- Feng, Q.; Li, X.; Wang, J.; Gaskov, A.M. Reduced graphene oxide (rGO) encapsulated Co3O4 composite nanofibers for highly selective ammonia sensors. Sens. Actuators B Chem. 2016, 222, 864–870. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, S.; Liu, H.; Hu, S.; Zhang, D.; Ning, H. A Survey on Gas Sensing Technology. Sensors 2012, 12, 9635–9665. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; An, S.; Ko, H.; Jin, C.; Lee, C. Synthesis of nanograined ZnO nanowires and their enhanced gas sensing properties. ACS Appl. Mater. Interfaces 2012, 4, 3650–3656. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Fu, Q.; Luo, W.; Tong, X.; Xiong, J.; Hu, Y.; Zheng, Z. Enhanced H2S gas sensing properties of undoped ZnO nanocrystalline films from QDs by low-temperature processing. Sens. Actuators B Chem. 2016, 224, 153–158. [Google Scholar] [CrossRef]
- Qiu, Y.; Yang, M.; Fan, H.; Xu, Y.; Shao, Y.; Yang, X.; Yang, S. Synthesis of ZnO nanorod arrays on Zn substrates by a gas–solution–solid method and their application as an ammonia sensor. J. Mater. Sci. 2014, 49, 347–352. [Google Scholar] [CrossRef]
- Ponnusamy, D.; Madanagurusamy, S. Nanostructured ZnO Films for Room Temperature Ammonia Sensing. J. Electron. Mater. 2014, 43, 3211–3216. [Google Scholar] [CrossRef]
- Li, C.-F.; Hsu, C.-Y.; Li, Y.-Y. NH3 sensing properties of ZnO thin films prepared via sol-gel method. J. Alloys Compd. 2014, 606, 27–31. [Google Scholar] [CrossRef]
- Guo, W.; Liu, T.; Zeng, W.; Liu, D.; Chen, Y.; Wang, Z. Gas-sensing property improvement of ZnO by hierarchical flower-like architectures. Mater. Lett. 2011, 65, 3384–3387. [Google Scholar] [CrossRef]
- Wei, A.; Pan, L.; Huang, W. Recent progress in the ZnO nanostructure-based sensors. Mater. Sci. Eng. B 2011, 176, 1409–1421. [Google Scholar] [CrossRef]
- Arshak, K.; Moore, E.; Lyons, G.M.; Harris, J.; Clifford, S. A review of gas sensors employed in electronic nose applications. Sens. Rev. 2004, 24, 181–198. [Google Scholar] [CrossRef]
- Mercante, L.A.; Scagion, V.P.; Migliorini, F.L.; Mattoso, L.H.C.; Correa, D.S. Electrospinning-based (bio)sensors for food and agricultural applications: A review. TrAC Trends Anal. Chem. 2017, 91, 91–103. [Google Scholar] [CrossRef]
- Pascariu, P.; Airinei, A.; Olaru, N.; Petrila, I.; Nica, V.; Sacarescu, L.; Tudorache, F. Microstructure, electrical and humidity sensor properties of electrospun NiO-SnO2 nanofibers. Sens. Actuators B Chem. 2016, 222, 1024–1031. [Google Scholar] [CrossRef]
- Yang, X.; Salles, V.; Kaneti, Y.V.; Liu, M.; Maillard, M.; Journet, C.; Jiang, X.; Brioude, A. Fabrication of highly sensitive gas sensor based on Au functionalized WO3 composite nanofibers by electrospinning. Sens. Actuators B Chem. 2015, 220, 1112–1119. [Google Scholar] [CrossRef]
- Wu, H.; Pan, W.; Lin, D.; Li, H. Electrospinning of ceramic nanofibers: Fabrication, assembly and applications. J. Adv. Ceram. 2012, 1, 2–23. [Google Scholar] [CrossRef]
- Piloto, C.; Shafiei, M.; Khan, H.; Gupta, B.; Tesfamichael, T.; Motta, N. Sensing performance of reduced graphene oxide-Fe doped WO3 hybrids to NO2 and humidity at room temperature. Appl. Surf. Sci. 2018, 434, 126–133. [Google Scholar] [CrossRef]
- Li, W.; Wu, X.; Chen, J.; Gong, Y.; Han, N.; Chen, Y. Abnormal n-p-n type conductivity transition of hollow ZnO/ZnFe2O4 nanostructures during gas sensing process: The role of ZnO-ZnFe2O4 hetero-interface. Sens. Actuators B Chem. 2017, 253, 144–155. [Google Scholar] [CrossRef]
- Song, X.-Z.; Qiao, L.; Sun, K.-M.; Tan, Z.; Ma, W.; Kang, X.-L.; Sun, F.-F.; Huang, T.; Wang, X.-F. Triple-shelled ZnO/ZnFe2O4 heterojunctional hollow microspheres derived from Prussian Blue analogue as high-performance acetone sensors. Sens. Actuators B Chem. 2018, 256, 374–382. [Google Scholar] [CrossRef]
- Li, Y.; Luo, N.; Sun, G.; Zhang, B.; Ma, G.; Jin, H.; Wang, Y.; Cao, J.; Zhang, Z. Facile synthesis of ZnO/ZnFe2O4/α-Fe2O3 porous microrods with enhanced TEA-sensing performance. J. Alloys Compd. 2018, 737, 255–262. [Google Scholar] [CrossRef]
- Poloju, M.; Jayababu, N.; Ramana Reddy, M.V. Improved gas sensing performance of Al doped ZnO/CuO nanocomposite based ammonia gas sensor. Mater. Sci. Eng. B 2018, 227, 61–67. [Google Scholar] [CrossRef]
- Pang, Z.; Yang, Z.; Chen, Y.; Zhang, J.; Wang, Q.; Huang, F.; Wei, Q. A room temperature ammonia gas sensor based on cellulose/TiO2/PANI composite nanofibers. Colloids Surf. A Physicochem. Eng. Asp. 2016, 494, 248–255. [Google Scholar] [CrossRef]
- Lin, Y.; Huang, L.; Chen, L.; Zhang, J.; Shen, L.; Chen, Q.; Shi, W. Fully gravure-printed NO2 gas sensor on a polyimide foil using WO3-PEDOT:PSS nanocomposites and Ag electrodes. Sens. Actuators B Chem. 2015, 216, 176–183. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, Y.; Zhang, L.; Gao, P.-X.; Lei, Y. CeO2 nanofibers for in situ O2 and CO sensing in harsh environments. RSC Adv. 2012, 2, 5193–5198. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, X.; Zhou, Z.; Lei, Y. Electrospun Ce-Ni-O composite nanofibers for highly selective propane detection at high temperature based on its rapid reaction kinetics. J. Mater. Chem. A 2014, 2, 14038–14047. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, Y.; La, A.; Lei, Y. Preparation, characterization and gas sensing application of novel conducting polypyrrole composite nanofibers. In Polypyrrole: Properties, Performance and Applications; Mason, E.C., Weber, A.P., Eds.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2011; p. 17. ISBN 978-1-61209-143-3. [Google Scholar]
- Rahman, M.M.; Khan, S.B.; Marwani, H.M.; Asiri, A.M.; Alamry, K.A.; Rub, M.A.; Khan, A.; Khan, A.A.P.; Azum, N. Facile synthesis of doped ZnO-CdO nanoblocks as solid-phase adsorbent and efficient solar photo-catalyst applications. J. Ind. Eng. Chem. 2014, 20, 2278–2286. [Google Scholar] [CrossRef]
- Cai, X.; Hu, D.; Deng, S.; Han, B.; Wang, Y.; Wang, Y.; Wu, J. Isopropanol sensing properties of coral-like ZnO-CdO composites by flash preparation via self-sustained decomposition of metal-organic complexes. Sens. Actuators B Chem. 2014, 198, 402–410. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, S.; Yoo, J.; Lim, K.-H.; Lee, E.; Kim, K.; Kim, J.; Kim, Y.S. Aqueous zinc ammine complex for solution-processed ZnO semiconductors in thin film transistors. RSC Adv. 2014, 4, 11295–11299. [Google Scholar] [CrossRef]
- Hsieh, P.-T.; Chen, Y.-C.; Kao, K.-S.; Wang, C.-M. Luminescence mechanism of ZnO thin film investigated by XPS measurement. Appl. Phys. A 2007, 90, 317–321. [Google Scholar] [CrossRef]
- Seekaew, Y.; Lokavee, S.; Phokharatkul, D.; Wisitsoraat, A.; Kerdcharoen, T.; Wongchoosuk, C. Low-cost and flexible printed graphene-PEDOT:PSS gas sensor for ammonia detection. Org. Electron. 2014, 15, 2971–2981. [Google Scholar] [CrossRef]
- Xu, S.; Kan, K.; Yang, Y.; Jiang, C.; Gao, J.; Jing, L.; Shen, P.; Li, L.; Shi, K. Enhanced NH3 gas sensing performance based on electrospun alkaline-earth metals composited SnO2 nanofibers. J. Alloys Compd. 2015, 618, 240–247. [Google Scholar] [CrossRef]
- Bai, S.; Tian, Y.; Cui, M.; Sun, J.; Tian, Y.; Luo, R.; Chen, A.; Li, D. Polyaniline@SnO2 heterojunction loading on flexible PET thin film for detection of NH3 at room temperature. Sens. Actuators B Chem. 2016, 226, 540–547. [Google Scholar] [CrossRef]
- Sundara Venkatesh, P.; Dharmaraj, P.; Purushothaman, V.; Ramakrishnan, V.; Jeganathan, K. Point defects assisted NH3 gas sensing properties in ZnO nanostructures. Sens. Actuators B Chem. 2015, 212, 10–17. [Google Scholar] [CrossRef]
- Lin, Q.; Li, Y.; Yang, M. Tin oxide/graphene composite fabricated via a hydrothermal method for gas sensors working at room temperature. Sens. Actuators B Chem. 2012, 173, 139–147. [Google Scholar] [CrossRef]
- Loyon, L.; Burton, C.H.; Misselbrook, T.; Webb, J.; Philippe, F.X.; Aguilar, M.; Doreau, M.; Hassouna, M.; Veldkamp, T.; Dourmad, J.Y.; et al. Best available technology for European livestock farms: Availability, effectiveness and uptake. J. Environ. Manag. 2016, 166, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Deng, J.; Wang, L.; Wang, R.; Fei, T.; Zhang, T. A class of hierarchical nanostructures: ZnO surface-functionalized TiO2 with enhanced sensing properties. RSC Adv. 2013, 3, 3131–3136. [Google Scholar] [CrossRef]
- Wang, T.; Kou, X.; Zhao, L.; Sun, P.; Liu, C.; Wang, Y.; Shimanoe, K.; Yamazoe, N.; Lu, G. Flower-like ZnO hollow microspheres loaded with CdO nanoparticles as high performance sensing material for gas sensors. Sens. Actuators B Chem. 2017, 250, 692–702. [Google Scholar] [CrossRef]
- Lim, Y.T.; Son, J.Y.; Rhee, J.-S. Vertical ZnO nanorod array as an effective hydrogen gas sensor. Ceram. Int. 2013, 39, 887–890. [Google Scholar] [CrossRef]
- Rai, P.; Khan, R.; Ahmad, R.; Hahn, Y.-B.; Lee, I.-H.; Yu, Y.-T. Gas sensing properties of single crystalline ZnO nanowires grown by thermal evaporation technique. Curr. Appl. Phys. 2013, 13, 1769–1773. [Google Scholar] [CrossRef]
- Gurav, K.V.; Patil, U.M.; Shin, S.W.; Pawar, S.M.; Kim, J.H.; Lokhande, C.D. Morphology evolution of ZnO thin films from aqueous solutions and their application to liquefied petroleum gas (LPG) sensor. J. Alloys Compd. 2012, 525, 1–7. [Google Scholar] [CrossRef]
- Navaneethan, M.; Patil, V.L.; Ponnusamy, S.; Muthamizhchelvan, C.; Kawasaki, S.; Patil, P.S.; Hayakawa, Y. Sensitivity enhancement of ammonia gas sensor based on Ag/ZnO flower and nanoellipsoids at low temperature. Sens. Actuators B Chem. 2018, 255, 672–683. [Google Scholar] [CrossRef]
- Patil, N.B.; Nimbalkar, A.R.; Patil, M.G. ZnO thin film prepared by a sol-gel spin coating technique for NO2 detection. Mater. Sci. Eng. B 2018, 227, 53–60. [Google Scholar] [CrossRef]
- Wang, Y.; Akhigbe, J.; Ding, Y.; Brückner, C.; Lei, Y. meso-Tritolylcorrole-Functionalized Single-walled Carbon Nanotube Donor Acceptor Nanocomposites for NO2 Detection. Electroanalysis 2012, 24, 1348–1355. [Google Scholar] [CrossRef]
- Ganbavle, V.V.; Inamdar, S.I.; Agawane, G.L.; Kim, J.H.; Rajpure, K.Y. Synthesis of fast response, highly sensitive and selective Ni:ZnO based NO2 sensor. Chem. Eng. J. 2016, 286, 36–47. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, W.; Strout, T.; Ding, Y.; Lei, Y. Preparation, Characterization and Sensitive Gas Sensing of Conductive Core-sheath TiO2-PEDOT Nanocables. Sensors 2009, 9, 6752–6763. [Google Scholar] [CrossRef] [PubMed]







| Material | Response (ΔR/Rg) | Response Time (s) | Recovery Time (s) | NH3 Concentration | Reference |
|---|---|---|---|---|---|
| ZnO/PSS nanofibers | 17% | 51 | 160 | 100 ppm | This work |
| Polyaniline (PANI)/SnO2 | - | 33 | - | 100 ppm | [57] |
| ZnO | 10% | 49 | 19 | 100 ppm | [58] |
| Cellulose/TiO2/PANI nanofibers | 3.5 | 83 | 130 | 100 ppm | [46] |
| rGO/AgNWs * | 15% | 60 | 150 | 100 ppm | [59] |
| rGO/Co3O4 nanofibers | 53.6% | 4 | 300 | 50 ppm | [27] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andre, R.S.; Kwak, D.; Dong, Q.; Zhong, W.; Correa, D.S.; Mattoso, L.H.C.; Lei, Y. Sensitive and Selective NH3 Monitoring at Room Temperature Using ZnO Ceramic Nanofibers Decorated with Poly(styrene sulfonate). Sensors 2018, 18, 1058. https://doi.org/10.3390/s18041058
Andre RS, Kwak D, Dong Q, Zhong W, Correa DS, Mattoso LHC, Lei Y. Sensitive and Selective NH3 Monitoring at Room Temperature Using ZnO Ceramic Nanofibers Decorated with Poly(styrene sulfonate). Sensors. 2018; 18(4):1058. https://doi.org/10.3390/s18041058
Chicago/Turabian StyleAndre, Rafaela S., Dongwook Kwak, Qiuchen Dong, Wei Zhong, Daniel S. Correa, Luiz H. C. Mattoso, and Yu Lei. 2018. "Sensitive and Selective NH3 Monitoring at Room Temperature Using ZnO Ceramic Nanofibers Decorated with Poly(styrene sulfonate)" Sensors 18, no. 4: 1058. https://doi.org/10.3390/s18041058
APA StyleAndre, R. S., Kwak, D., Dong, Q., Zhong, W., Correa, D. S., Mattoso, L. H. C., & Lei, Y. (2018). Sensitive and Selective NH3 Monitoring at Room Temperature Using ZnO Ceramic Nanofibers Decorated with Poly(styrene sulfonate). Sensors, 18(4), 1058. https://doi.org/10.3390/s18041058