Click Access to a Cyclodextrin-Based Spatially Confined AIE Material for Hydrogenase Recognition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
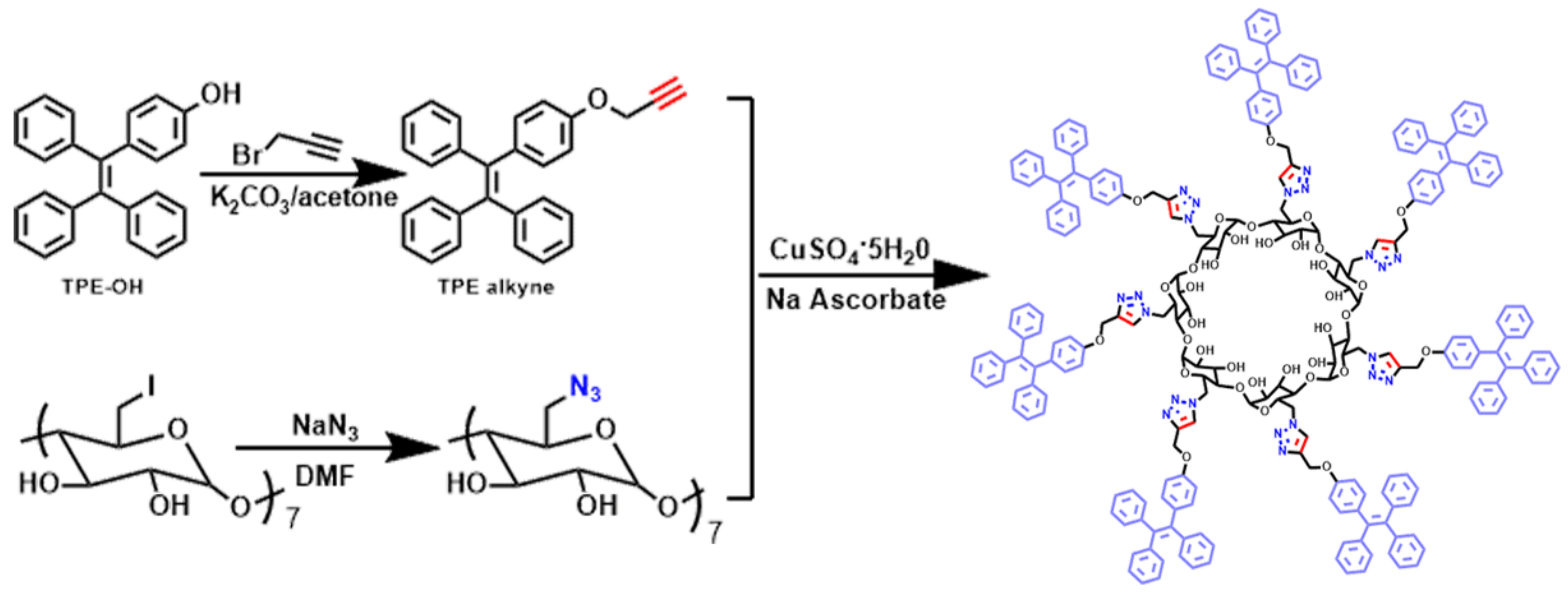
2.3. Synthesis of the Spatially Confined AIECD (SCAIECD)
3. Results and Discussion
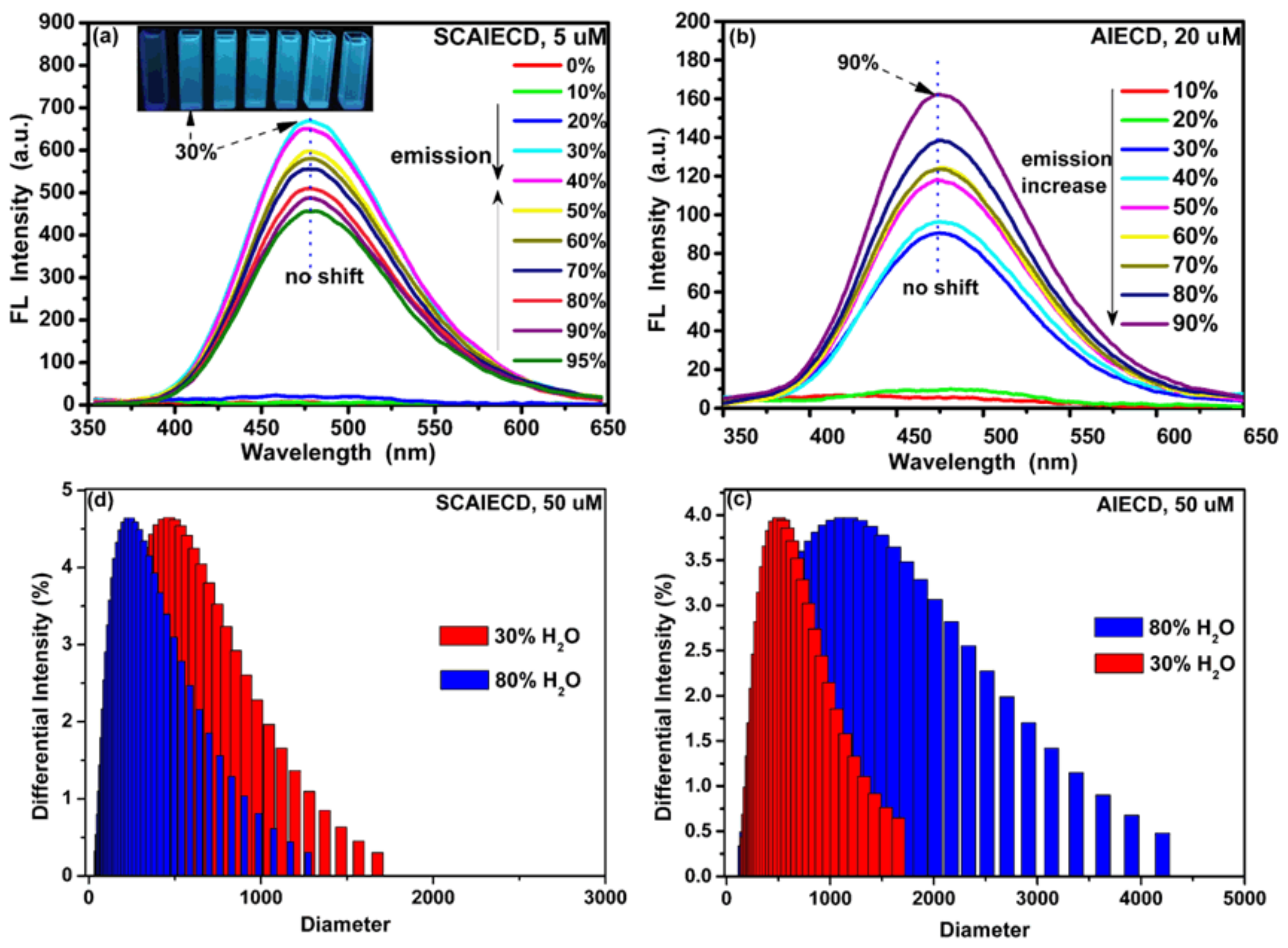
3.1. Structure Characterization and AIE Property Study of SCAIECD and AIECD
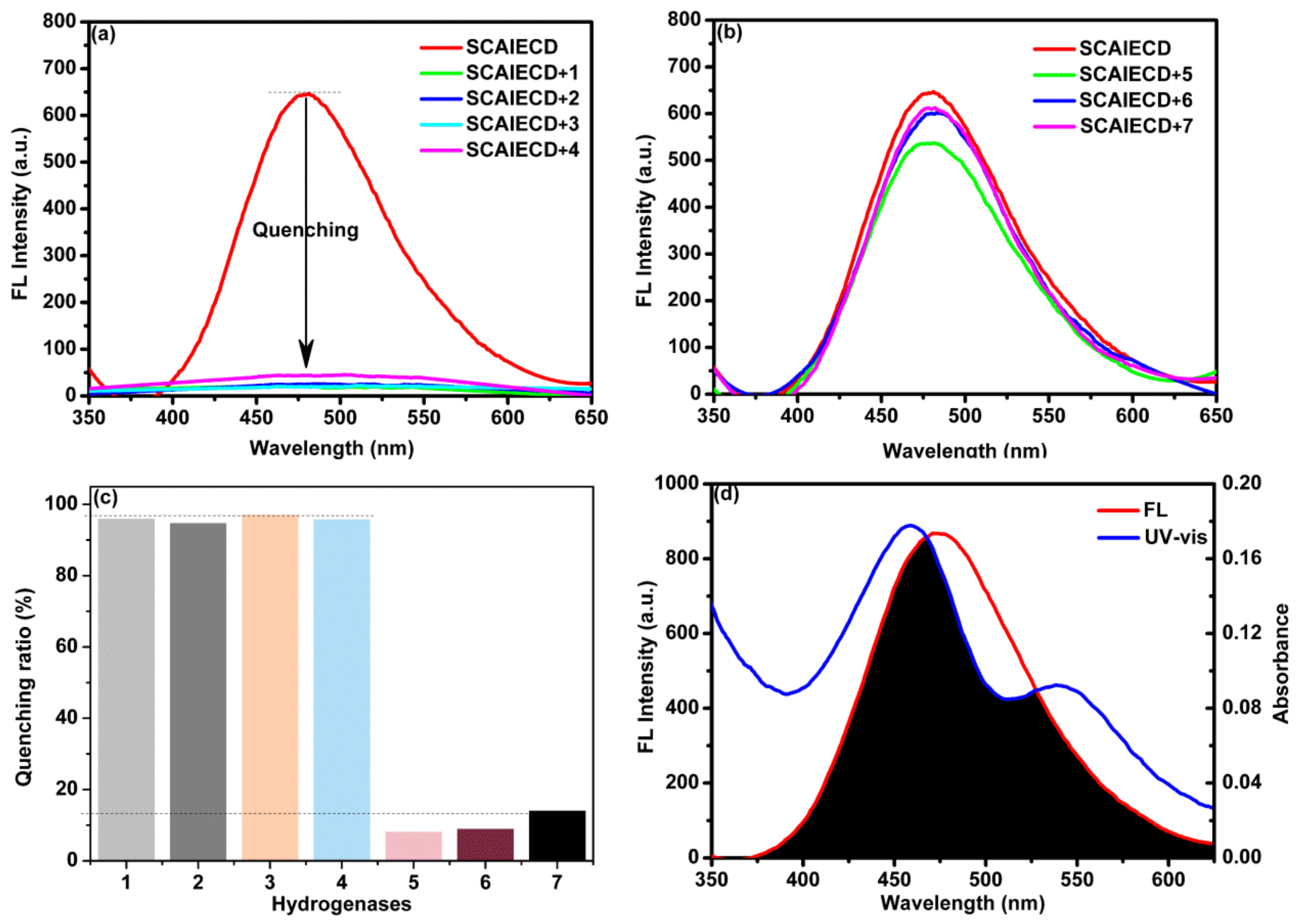
3.2. Evaluation of the Sensing Ability of SCAIECD for Hydrogenases
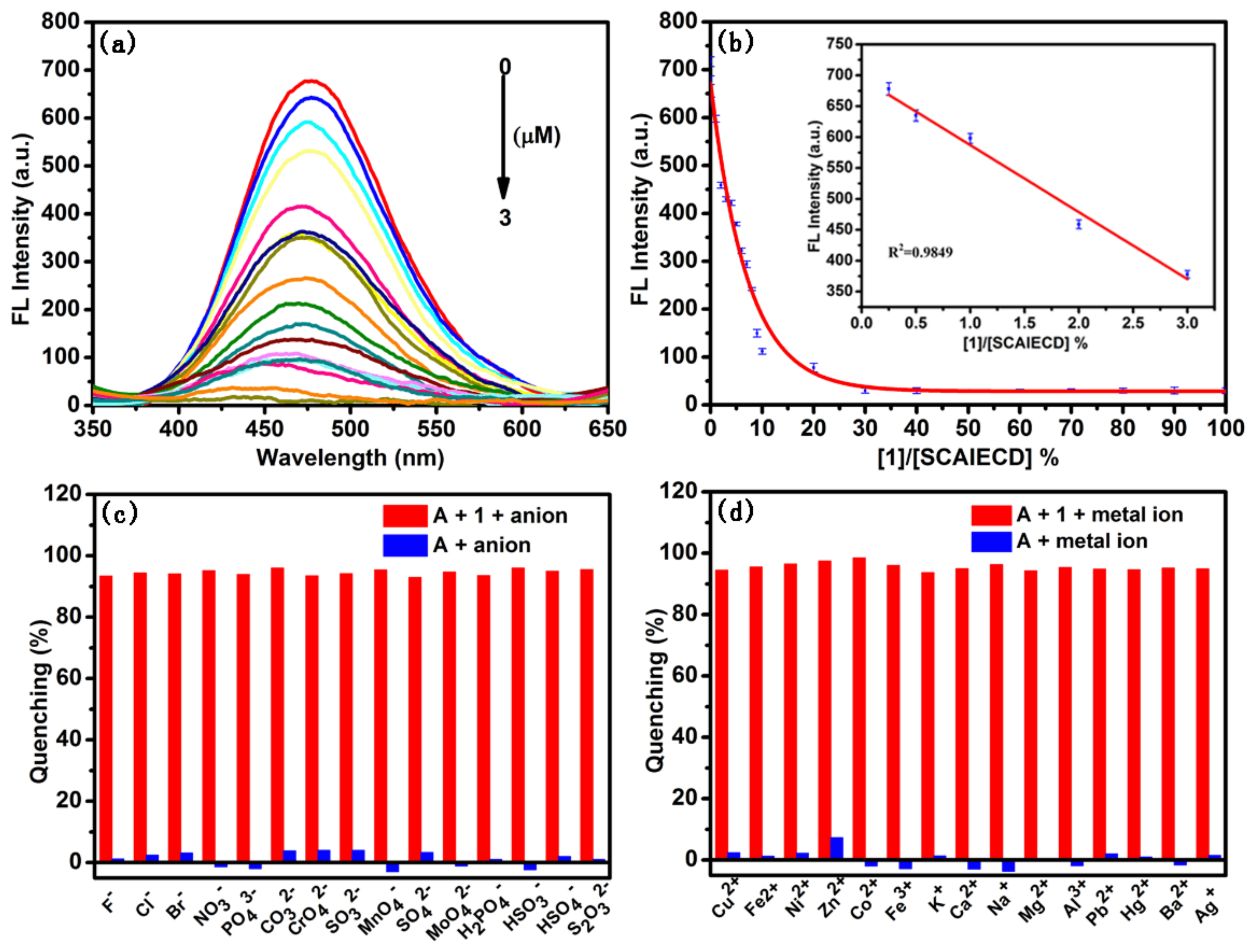
3.3. Evaluation of the Detection Limit and Reliability
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, Y.; Zhang, G.; Zhang, W.; Wang, X.; Wu, Y.; Liang, T.; Hao, X.; Fu, H.; Zhao, Y.; Zhang, D. Tuning the Solid State Emission of the Carbazole and Cyano-Substituted Tetraphenylethylene by Co-Crystallization with Solvents. Small 2016, 12, 6554–6561. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Xu, S.; Cheng, X.; Cai, X.; Liu, B. Bioorthogonal Turn-On Probe Based on Aggregation-Induced Emission Characteristics for Cancer Cell Imaging and Ablation. Angew. Chem. Int. Ed. 2016, 55, 6457–6461. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Bai, X.; Ma, J.; Xu, M.; Hu, G.; James, T.D.; Wang, L. Ultrasmall Organic Nanoparticles with Aggregation-Induced Emission and Enhanced Quantum Yield for Fluorescence Cell Imaging. Anal. Chem. 2016, 88, 7853–7857. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zeng, F.; Wu, S. A fluorescent assay for γ-glutamyltranspeptidase via aggregation induced emission and its applications in real samples. Biosens. Bioelectron. 2016, 85, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Mei, J.; Hu, R.; Sun, J.Z.; Qin, A.; Tang, B.Z. Click Synthesis, Aggregation-Induced Emission, E/Z Isomerization, Self-Organization, and Multiple Chromisms of Pure Stereoisomers of a Tetraphenylethene-Cored Luminogen. J. Am. Chem. Soc. 2012, 134, 9956–9966. [Google Scholar] [CrossRef] [PubMed]
- Kashapov, R.R.; Mamedov, V.A.; Zhukova, N.A.; Kadirov, M.K.; Nizameev, I.R.; Zakharova, L.Y.; Sinyashin, O.G. Controlling the binding of hydrophobic drugs with supramolecular assemblies of β-cyclodextrin. Colloids Surf. A 2017, 527, 55–62. [Google Scholar] [CrossRef]
- Huang, H.; Xu, D.; Liu, M.; Jiang, R.; Mao, L.; Huang, Q.; Wan, Q.; Wen, Y.; Zhang, X.; Wei, Y. Direct encapsulation of AIE-active dye with β cyclodextrin terminated polymers: Self-assembly and biological imaging. Mater. Sci. Eng. C 2017, 78, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zou, H.; Yuan, H.; Gu, S.; Yuan, W.; Li, M. Triple stimuli-responsive supramolecular assemblies based on host-guest inclusion complexation between β-cyclodextrin andazobenzene. Eur. Polym. J. 2017, 91, 396–407. [Google Scholar] [CrossRef]
- Li, X.; Jin, X.; Yao, X.; Ma, X.; Wang, Y. Thioether bridged cationic cyclodextrin stationary phases: Effect of spacer length, selector concentration and rim functionalities on theenantio separation. J. Chromatogr. A 2016, 1467, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Ren, F.; Gao, H.; Wu, Q.; Zhu, F.; Tang, B.Z. Bioinspired Fluorescent Nanosheets for Rapid and Sensitive Detection of Organic Pollutants in Water. ACS Sens. 2016, 1, 1272–1278. [Google Scholar] [CrossRef]
- Liao, R.; Lv, P.; Wang, Q.; Zheng, J.; Feng, B.; Yang, B. Cyclodextrin-based biological stimuli-responsive carriers for smart and precision medicine. Biomater. Sci. 2017, 5, 1736–1745. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.A.; Wodrich, M.D.; Hu, X.; Corminboeuf, C. Toward Functional Type III[Fe]-Hydrogenase Biomimics for H2 Activation: Insights from Computation. Chem. Eur. J. 2015, 21, 3987–3996. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Chen, D.; Hu, X. Hydrogen-activating models of hydrogenases. Coord. Chem. Rev. 2015, 303, 32–41. [Google Scholar] [CrossRef]
- Darensbourg, M.Y.; Weigand, W. Sulfoxygenation of Active Site Models of [NiFe] and [FeFe] Hydrogenases—A Commentary on Possible Chemical Models of Hydrogenase Enzyme Oxygen Sensitivity. Eur. J. Inorg. Chem. 2011, 2011, 994–1004. [Google Scholar] [CrossRef]
- Li, B.; Liu, T.; Popescu, C.V.; Bilko, A.; Darensbourg, M.Y. Synthesis and Mossbauer Characterization of Octahedral Iron (II) Carbonyl Complexes FeI2(CO)3L and FeI2(CO)2L2: Developing Models of the [Fe]-H2ase Active Site. Inorg. Chem. 2009, 48, 11283–11289. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, B.; Popescu, C.V.; Bilko, A.; Perez, L.M.; Hall, M.B.; Darensbourg, M.Y. Analysis of a Pentacoordinate Iron Dicarbonyl as Synthetic Analogue of the Hmd or Mono-Iron Hydrogenase Active Site. Chem. Eur. J. 2010, 16, 3083–3089. [Google Scholar] [CrossRef] [PubMed]
- Shu, T.; Su, L.; Wang, J.; Lu, X.; Liang, F.; Li, C.; Zhang, X. Value of the Debris of Reduction Sculpture: Thiol Etching of Au Nanoclusters for Preparing Water-Soluble and Aggregation-Induced Emission-Active Au(I) Complexes as Phosphorescent Copper Ion Sensor. Anal. Chem. 2016, 88, 6071–6077. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, M.; Zheng, D.; Han, K.; Dong, J.; Sun, L. Photocatalytic H2 production in aqueous solution with host-guest inclusions formed by insertion of an FeFe-hydrogenase mimic and an organic dye into cyclodextrins. Energy Environ. Sci. 2012, 5, 8220–8224. [Google Scholar] [CrossRef]
- Cheng, M.; Wang, M.; Zhang, S.; Liu, F.; Yang, Y.; Wan, B.; Sun, L. Photocatalytic H2 production using a hybrid assembly of an [FeFe]-hydrogenase model and CdSe quantumdot linked through a thiolato-functionalized cyclodextrin. Faraday Discuss. 2017, 198, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Singleton, M.L.; Crouthers, D.J.; Duttweiler, R.P., 3rd; Reibenspies, J.H.; Darensbourg, M.Y. Sulfonated Diiron Complexes as Water-Soluble Models of the [Fe-Fe]-Hydrogenase Enzyme Active Site. Inorg. Chem. 2011, 50, 5015–5026. [Google Scholar] [CrossRef] [PubMed]
- Singleton, M.L.; Reibenspies, J.H.; Darensbourg, M.Y. A Cyclodextrin Host/Guest Approach to a Hydrogenase Active Site Biomimetic Cavity. J. Am. Chem. Soc. 2010, 132, 8870–8871. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, T.; Li, B.; Zhang, G.; Hai, L.; Ma, X.; Wu, W. Direct synthesis of phenol by novel [FeFe]-hydrogenase model complexes as catalysts of benzene hydroxylation with H2O. RSC Adv. 2017, 7, 2934–2942. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, T.; Yang, Q.; Jiang, S.; Li, B. Synthesis and Characterization of Bio-Inspired Diiron Complexes and Their Catalytic Activity for Direct Hydroxylation of Aromatic Compounds. Eur. J. Inorg. Chem. 2015, 2015, 817–825. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, T.; Zhang, X.; Zhang, G.; Li, B. Nitrogen heterocyclic carbene containing pentacoordinate iron dicarbonyl as a [Fe]-hydrogenase active site model. Dalton Trans. 2015, 44, 16708–16712. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Zhang, T.; Zhang, X.; Zhang, G.; Hai, L.; Li, B. Synthesis, structural characterization, and chemical properties of pentacoordinate model complexes for the active site of [Fe]-hydrogenase. RSC Adv. 2016, 6, 84139–84148. [Google Scholar] [CrossRef]
- Srinivasachari, S.M.; Fichter, K.M.; Reineke, T. Polycationic β-Cyclodextrin “Click Clusters”: Monodisperse and Versatile Scaffolds for Nucleic Acid Delivery. J. Am. Chem. Soc. 2008, 130, 4618–4627. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, L.; Ching, C.; Ng, S. Synthesis and chromatographic properties of a novel chiral stationary phase derived from heptakis (6-azido-6-deoxy-2,3-di-O-phenylcarbamoylated)-β-cyclodextrin immobilized onto amino-functionalized silica gel via multiple urea linkages. J. Chromatogr. A 2002, 950, 65–74. [Google Scholar] [CrossRef]
- Pathak, R.K.; Hinge, V.K.; Mahesh, K.; Rai, A.; Panda, D.; Rao, C.P. Cd2+ complex of a triazole-based calix 4 arene conjugate as a selective fluorescent chemosensor for cys. Anal. Chem. 2012, 84, 6907–6913. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2005. [Google Scholar] [CrossRef]
- Li, H.; Yao, Y.; Han, C.; Zhan, J. Triazole-ester modified silver nanoparticles: Click synthesis and Cd2+ colorimetric sensing. Chem. Commun. 2009, 0, 4812–4814. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, R.; Tang, X.; Shi, Y.; Li, C.; Wang, Y. One-Pot Click Access to a Cyclodextrin Dimer-Based Novel Aggregation Induced Emission Sensor and Monomer-Based Chiral Stationary Phase. Sensors 2016, 16, 1985. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, W.; Yu, L.; Wang, Y. Click synthesis of a novel triazole bridged AIE active cyclodextrin probe for specific detection of Cd2+. Chem. Commun. 2015, 51, 4298–4301. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Liu, S.; Feng, A.; Yuan, J. Polymeric Nanocarriers Based on Cyclodextrins for Drug Delivery: Host−Guest Interaction as Stimuli Responsive Linker. Mol. Pharm. 2017, 14, 2475–2486. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Fu, X.; Wang, Y. Click synthesis of a triphenylamine-based fluorescent methanol probe with a unique D-π-A structure. Sens. Actuators B 2017, 245, 406–413. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, R.; Li, B.; Wang, Y.; Hu, W. Click Access to a Cyclodextrin-Based Spatially Confined AIE Material for Hydrogenase Recognition. Sensors 2018, 18, 1134. https://doi.org/10.3390/s18041134
Zhao R, Li B, Wang Y, Hu W. Click Access to a Cyclodextrin-Based Spatially Confined AIE Material for Hydrogenase Recognition. Sensors. 2018; 18(4):1134. https://doi.org/10.3390/s18041134
Chicago/Turabian StyleZhao, Rui, Bin Li, Yong Wang, and Wenping Hu. 2018. "Click Access to a Cyclodextrin-Based Spatially Confined AIE Material for Hydrogenase Recognition" Sensors 18, no. 4: 1134. https://doi.org/10.3390/s18041134
APA StyleZhao, R., Li, B., Wang, Y., & Hu, W. (2018). Click Access to a Cyclodextrin-Based Spatially Confined AIE Material for Hydrogenase Recognition. Sensors, 18(4), 1134. https://doi.org/10.3390/s18041134




