Insect-Mimetic Imaging System Based on a Microlens Array Fabricated by a Patterned-Layer Integrating Soft Lithography Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of a Microlens Array with a Gold Screening Layer
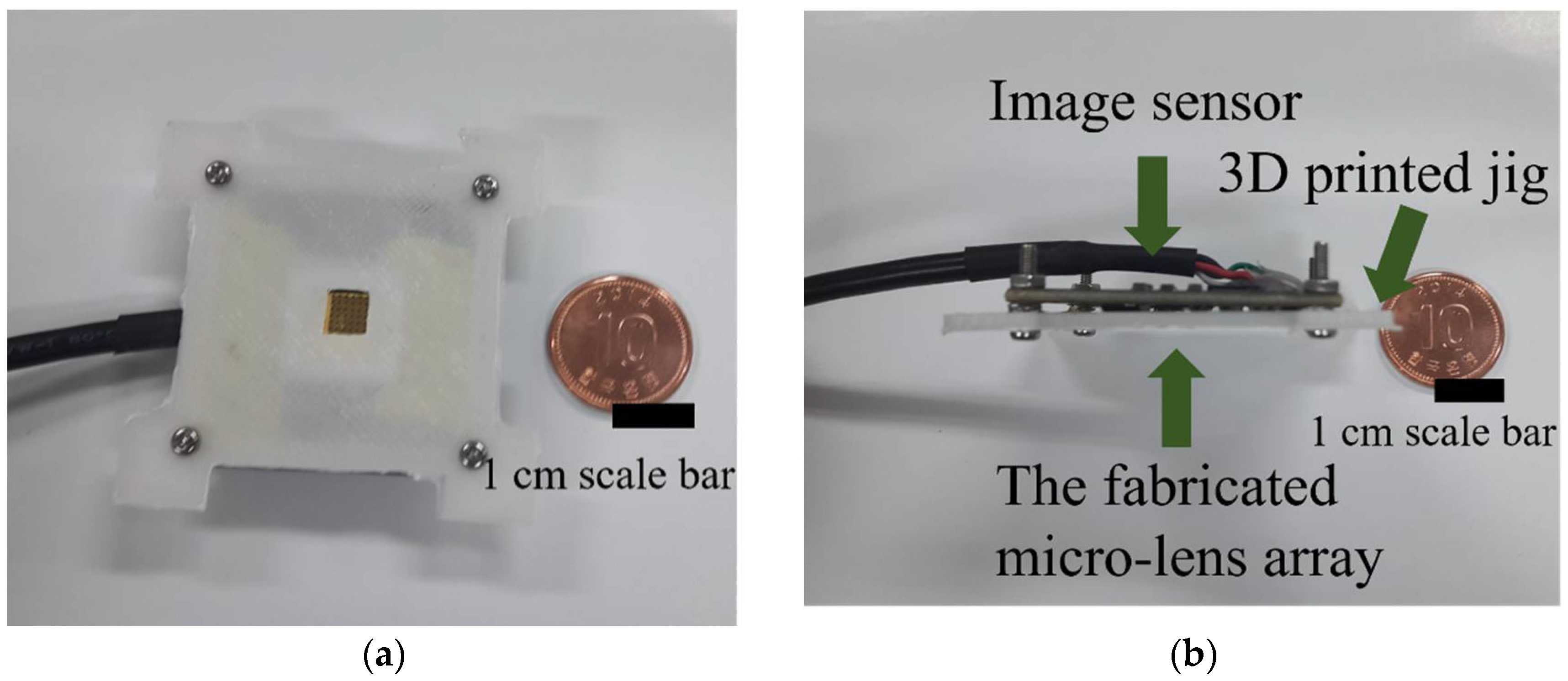
2.2. Imaging System Setup Using a 3D Printed Jig
3. Results
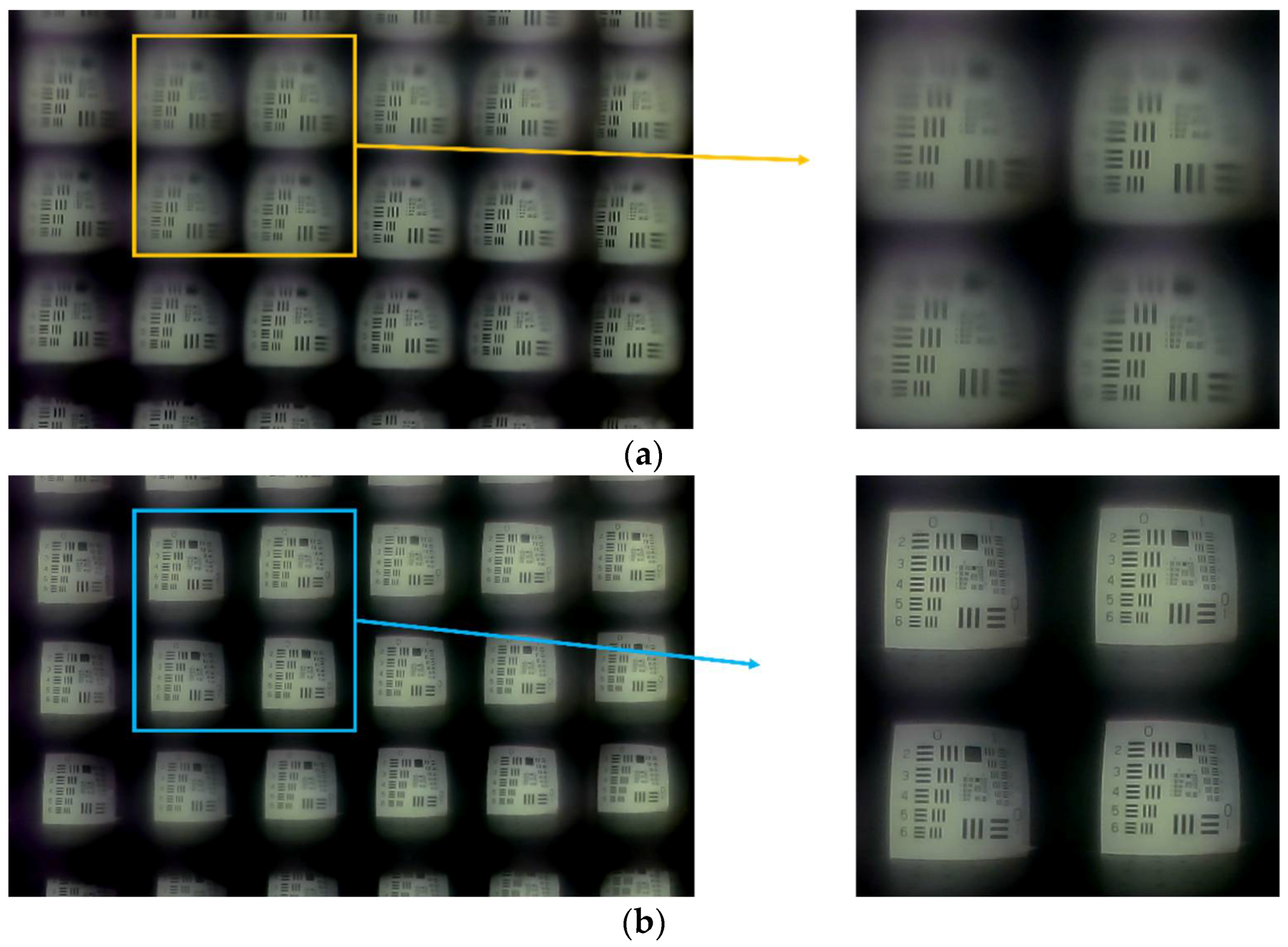
3.1. Characteristics of the Microlens Array
3.2. Integrated Imaging System with a Conventional CCD Image Sensor
4. Discussion & Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahuja, N.A.; Bose, N.K. Design of large field-of-view high-resolution miniaturized imaging system. EURASIP J. Adv. Signal Process. 2007, 2007, 1–10. [Google Scholar] [CrossRef]
- Land, M.F. Compound eyes: Old and new optical mechanisms. Nature 1980, 287, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Horridge, G.A. The separation of visual axes in apposition compound eyes. Philos. Trans. R. Soc. B Biol. Sci. 1978, 285, 1–59. [Google Scholar] [CrossRef]
- Snyder, A.W. Physics of vision in compound eyes. In Comparative Physiology and Evolution of Vision in Invertebrates: A: Invertebrate Photoreceptors; Autrum, H., Bennett, M.F., Diehn, B., Hamdorf, K., Heisenberg, M., Järvilehto, M., Kunze, P., Menzel, R., Miller, W.H., Snyder, A.W., et al., Eds.; Springer: Berlin/Heidelberg, Germnay, 1979; pp. 225–313. [Google Scholar]
- McIntyre, P.; Caveney, S. Graded-index optics are matched to optical geometry in the superposition eyes of scarab beetles. Philos. Trans. R. Soc. B Biol. Sci. 1985, 311, 237–269. [Google Scholar] [CrossRef]
- Rafael, N.; Nicolas, F. On image quality of microlens arrays in diurnal superposition eyes. Pure Appl. Opt. J. Eur. Opt. Soc. Part A 1998, 7, L69. [Google Scholar]
- Park, H.S.; Hoskinson, R.; Abdollahi, H.; Stoeber, B. Compact near-eye display system using a superlens-based microlens array magnifier. In Proceedings of the 2015 28th IEEE International Conference on Micro Electro Mechanical Systems (MEMS), Estoril, Portugal, 18–22 January 2015; pp. 952–955. [Google Scholar]
- Brückner, A.; Duparré, J.; Dannberg, P.; Bräuer, A.; Tünnermann, A. Artificial neural superposition eye. Opt. Express 2007, 15, 11922–11933. [Google Scholar] [CrossRef] [PubMed]
- Stollberg, K.; Brückner, A.; Duparré, J.; Dannberg, P.; Bräuer, A.; Tünnermann, A. The gabor superlens as an alternative wafer-level camera approach inspired by superposition compound eyes of nocturnal insects. Opt. Express 2009, 17, 15747–15759. [Google Scholar] [CrossRef] [PubMed]
- Floreano, D.; Pericet-Camara, R.; Viollet, S.; Ruffier, F.; Bruckner, A.; Leitel, R.; Buss, W.; Menouni, M.; Expert, F.; Juston, R.; et al. Miniature curved artificial compound eyes. Proc. Natl. Acad. Sci. USA 2013, 110, 9267–9272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buschbeck, E.K. The compound lens eye of strepsiptera: Morphological development of larvae and pupae. Arthropod Struct. Dev. 2005, 34, 315–326. [Google Scholar] [CrossRef]
- James, M.; Nandamuri, S.P.; Stahl, A.; Buschbeck, E.K. The unusual eyes of Xenos peckii (strepsiptera: Xenidae) have green- and uv-sensitive photoreceptors. J. Exp. Biol. 2016, 219, 3866–3874. [Google Scholar] [CrossRef] [PubMed]
- Keum, D.; Jeon, D.S.; Kim, M.H.; Jeong, K.H. Artificial compound eye inspired by imaging principle of xenos peckii. In Proceedings of the 2015 Transducers—2015 18th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS), Anchorage, AK, USA, 21–25 June 2015; pp. 403–406. [Google Scholar]
- Borrelli, N.F.; Morse, D.L.; Bellman, R.H.; Morgan, W.L. Photolytic technique for producing microlenses in photosensitive glass. Appl. Opt. 1985, 24, 2520–2525. [Google Scholar] [CrossRef] [PubMed]
- Tanida, J.; Kumagai, T.; Yamada, K.; Miyatake, S.; Ishida, K.; Morimoto, T.; Kondou, N.; Miyazaki, D.; Ichioka, Y. Thin observation module by bound optics (tombo): Concept and experimentalverification. Appl. Opt. 2001, 40, 1806–1813. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.M.; Xie, Y.; Malyarchuk, V.; Xiao, J.; Jung, I.; Choi, K.-J.; Liu, Z.; Park, H.; Lu, C.; Kim, R.-H.; et al. Digital cameras with designs inspired by the arthropod eye. Nature 2013, 497, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Daly, D.; Stevens, R.F.; Hutley, M.C.; Davies, N. The manufacture of microlenses by melting photoresist. Meas. Sci. Technol. 1990, 1, 759. [Google Scholar] [CrossRef]
- Norland Optical Adhesive 72. Available online: https://www.norlandprod.com/adhesives/noa%2072.html (accessed on 23 March 2018).
- Ikjoo, B.; Anthony, W.C.; Beomjoon, K. Transfer of thin au films to polydimethylsiloxane (pdms) with reliable bonding using (3-mercaptopropyl)trimethoxysilane (MPTMS) as a molecular adhesive. J. Micromech. Microeng. 2013, 23, 085016. [Google Scholar]
- Axelevitch, A.; Apter, B.; Golan, G. Simulation and experimental investigation of optical transparency in gold island films. Opt. Express 2013, 21, 4126–4138. [Google Scholar] [CrossRef] [PubMed]
- Sung Cheol, P.; Min Kyu, P.; Moon Gi, K. Super-resolution image reconstruction: A technical overview. IEEE Signal Process. Mag. 2003, 20, 21–36. [Google Scholar] [CrossRef]
- Kim, J.; Jung, J.-H.; Jeong, Y.; Hong, K.; Lee, B. Real-time integral imaging system for light field microscopy. Opt. Express 2014, 22, 10210–10220. [Google Scholar] [CrossRef] [PubMed]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, M.; Seo, J.-M.; Cho, D.-i.“.; Koo, K.-i. Insect-Mimetic Imaging System Based on a Microlens Array Fabricated by a Patterned-Layer Integrating Soft Lithography Process. Sensors 2018, 18, 2011. https://doi.org/10.3390/s18072011
Seo M, Seo J-M, Cho D-i“, Koo K-i. Insect-Mimetic Imaging System Based on a Microlens Array Fabricated by a Patterned-Layer Integrating Soft Lithography Process. Sensors. 2018; 18(7):2011. https://doi.org/10.3390/s18072011
Chicago/Turabian StyleSeo, Minwon, Jong-Mo Seo, Dong-il “Dan” Cho, and Kyo-in Koo. 2018. "Insect-Mimetic Imaging System Based on a Microlens Array Fabricated by a Patterned-Layer Integrating Soft Lithography Process" Sensors 18, no. 7: 2011. https://doi.org/10.3390/s18072011
APA StyleSeo, M., Seo, J.-M., Cho, D.-i. “., & Koo, K.-i. (2018). Insect-Mimetic Imaging System Based on a Microlens Array Fabricated by a Patterned-Layer Integrating Soft Lithography Process. Sensors, 18(7), 2011. https://doi.org/10.3390/s18072011






