Evaluation of Metal Oxide Surface Catalysts for the Electrochemical Activation of Amino Acids
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Apparatus
2.2. Attachment Method
2.3. Cyclic Voltammetry and Polarization Resistance Measurements
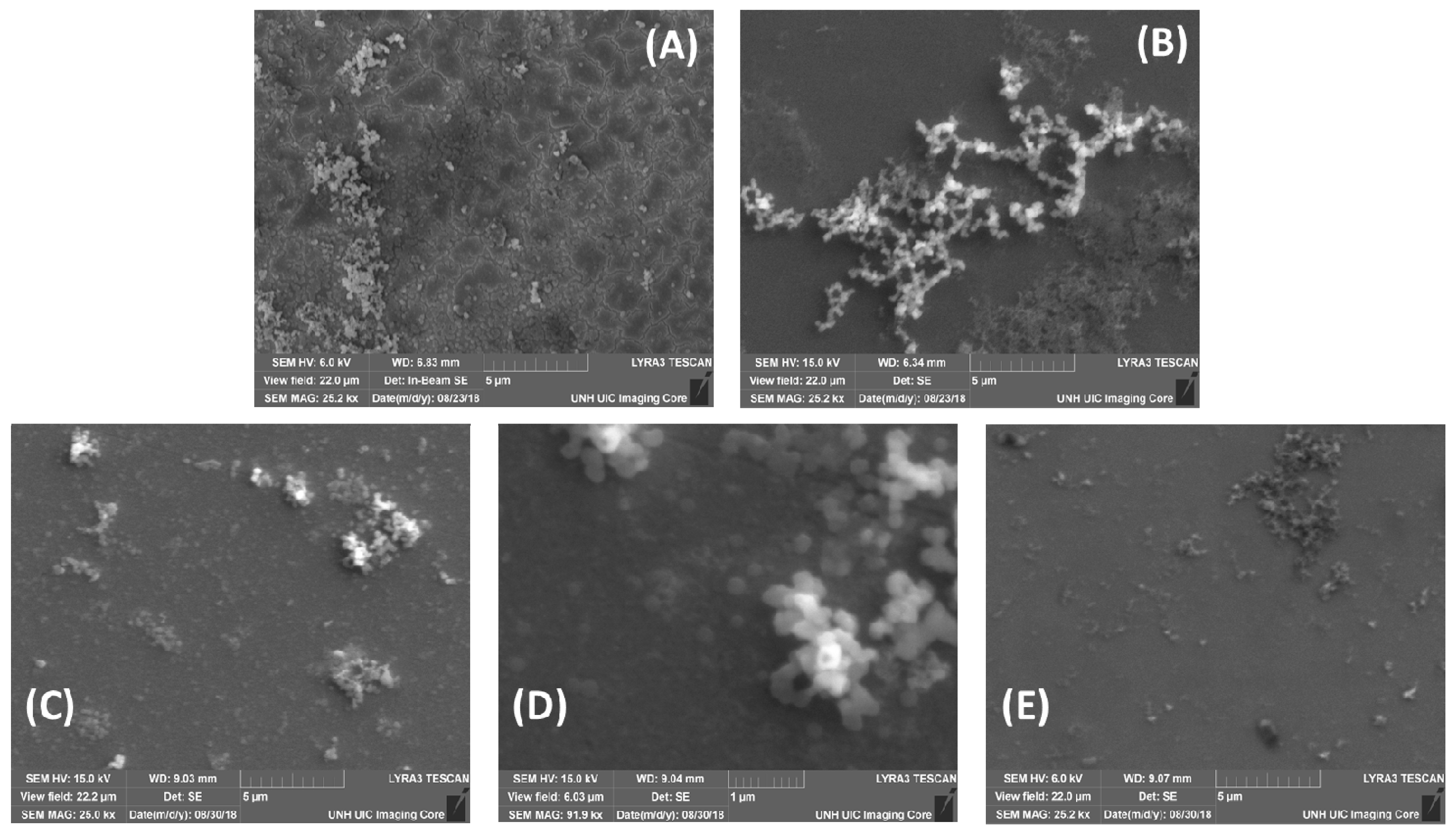
3. Results and Discussion
3.1. Amino Acid Detection at a CuO Surface
3.2. Amino Acid Detection at a Fe2O3 Surface
3.3. Amino Acid Detection at a NiO Surface
3.4. Corrosion of Nickel Oxide Surface Measured Through Polarization Resistance and Proposed Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xie, B.; Waters, M.J.; Schirra, H.J. Investigating potential mechanisms of obesity by metabolomics. J. Biomed. Biotechnol. 2012, 2012, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.-I.; Chun, M.-R.; Yang, J.-S.; Lim, S.-W.; Kim, M.-J.; Kim, S.-W.; Myung, W.-J.; Kim, D.-K.; Lee, S.-Y. Plasma amino acid profiling in major depressive disorder treated with selective serotonin reuptake inhibitors. CNS Neurosci. Ther. 2015, 21, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Gao, H.-C.; Qi, Z.-G.; Jia, J.-M.; Li, F.F.; Chen, J.J.; Wang, Y.; Guo, J.; Melgiri, N.D.; Xie, P. Peripheral metabolic abnormalities of lipids and amino acids implicated in increased risk of suicidal behavior in major depressive disorder. Metabolomics 2013, 9, 688–696. [Google Scholar] [CrossRef]
- Shao, W.; Chen, J.; Fan, S.; Lei, Y.; Xu, H.; Zhou, J.; Cheng, P.; Yang, Y.; Rao, C.; Wu, B.; et al. Combined metabolomics and proteomics analysis of major depression in an animal model: Perturbed energy metabolism in the chronic mild stressed rat cerebellum. OMICS 2015, 19, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Shushan, B. A review of clinical diagnostic applications of liquid chromatography–tandem mass spectrometry. Mass Spectrom. Rev. 2010, 29, 930–944. [Google Scholar] [CrossRef] [PubMed]
- Madsen, R.; Lundstedt, T.; Trygg, J. Chemometrics in metabolomics—A review in human disease diagnosis. Anal. Chim. Acta 2010, 659, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Gowda, G.N.; Zhang, S.; Gu, H.; Asiago, V.; Shanaiah, N.; Raftery, D. Metabolomics-based methods for early disease diagnostics. Expert Rev. Mol. Diagn. 2008, 8, 617–633. [Google Scholar] [CrossRef] [PubMed]
- Herzog, G.; Arrigan, D.W.M. Electrochemical strategies for the label-free detection of amino acids, peptides and proteins. Analyst 2007, 132, 615–632. [Google Scholar] [CrossRef] [PubMed]
- Martens-Lobenhoffer, J.; Bode-Böger, S.M. Mass spectrometric quantification of L-arginine and its pathway related substances in biofluids: The road to maturity. J. Chromatogr. B 2014, 964, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Krumpochova, P.; Bruyneel, B.; Molenaar, D.; Koukou, A.; Wuhrer, M.; Niessen, W.M.A.; Giera, M. Amino acid analysis using chromatography–mass spectrometry: An inter platform comparison study. J. Pharm. Biomed. Anal. 2015, 114, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Hernando, D.; Saurina, J.; Hernández-Cassou, S. Liquid chromatographic determination of aniline in table-top sweeteners based on pre-column derivatization with 1,2-naphthoquinone-4-sulfonate. J. Chromatogr. A 1999, 859, 227–233. [Google Scholar] [CrossRef]
- Schiesel, S.; Lämmerhofer, M.; Lindner, W. Multitarget quantitative metabolic profiling of hydrophilic metabolites in fermentation broths of β-lactam antibiotics production by HILIC–ESI–MS/MS. Anal. Bioanal. Chem. 2010, 396, 1655–1679. [Google Scholar] [CrossRef] [PubMed]
- Kaspar, H.; Dettmer, K.; Gronwald, W.; Oefner, P.J. Automated GC–MS analysis of free amino acids in biological fluids. J. Chromatogr. B 2008, 870, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Casella, I.G.; Cataldi, T.R.I.; Guerrieri, A.; Desimoni, E. Copper dispersed into polyaniline films as an amperometric sensor in alkaline solutions of amino acids and polyhydric compounds. Anal. Chim. Acta 1996, 335, 217–225. [Google Scholar] [CrossRef]
- Roushani, M.; Shamsipur, M.; Pourmortazavi, S.M. Amprometric detection of Glycine, l-Serine, and l-Alanine using glassy carbon electrode modified by NiO nanoparticles. J. Appl. Electrochem. 2012, 42, 1005–1011. [Google Scholar] [CrossRef]
- Martínez-Periñán, E.; Revenga-Parra, M.; Zamora, F.; Pariente, F.; Lorenzo, E. Nanostructured electrochemical detector for the quantification of amino acids related to metabolic diseases. Sens. Actuators B Chem. 2016, 236, 773–780. [Google Scholar] [CrossRef]
- Zen, J.-M.; Hsu, C.-T.; Senthil Kumar, A.; Lyuu, H.-J.; Lin, K.-Y. Amino acid analysis using disposable copper nanoparticle plated electrodes. Analyst 2004, 129, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; He, Z.; Hou, H.; Wang, X.; Wang, L. Architecture of Fe3O4–graphene oxide nanocomposite and its application as a platform for amino acid biosensing. Electrochim. Acta 2012, 71, 58–65. [Google Scholar] [CrossRef]
- Sigel, H.; Martin, R.B. Coordinating properties of the amide bond. Stability and structure of metal ion complexes of peptides and related ligands. Chem. Rev. 1982, 82, 385–426. [Google Scholar] [CrossRef]
- Nakamoto, K.; Morimoto, Y.; Martell, A.E. Infrared spectra of aqueous solutions. I. metal chelate compounds of amino acids 1. J. Am. Chem. Soc. 1961, 83, 4528–4532. [Google Scholar] [CrossRef]
- Legin, A.A.; Jakupec, M.A.; Bokach, N.A.; Tyan, M.R.; Kukushkin, V.Y.; Keppler, B.K. Guanidine platinum(II) complexes: Synthesis, in vitro antitumor activity, and DNA interactions. J. Inorg. Biochem. 2014, 133, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Marin-Luna, M.; Sanchez-Sanz, G.; O’Sullivan, P.; Rozas, I. Guanidine complexes of platinum: A theoretical study. J. Phys. Chem. A 2014, 118, 5540–5547. [Google Scholar] [CrossRef] [PubMed]
- Bailey, P.J.; Pace, S. The coordination chemistry of guanidines and guanidinates. Coord. Chem. Rev. 2001, 214, 91–141. [Google Scholar] [CrossRef]
- Xu, H.-B.; Fang, L.; Hu, Z.-C.; Chen, Y.-C.; Chen, J.-J.; Li, F.-F.; Lu, J.; Mu, J.; Xie, P. Potential clinical utility of plasma amino acid profiling in the detection of major depressive disorder. Psychiatry Res. 2012, 200, 1054–1057. [Google Scholar] [CrossRef] [PubMed]
- Lawton, K.A.; Berger, A.; Mitchell, M.; Milgram, K.E.; Evans, A.M.; Guo, L.; Hanson, R.W.; Kalhan, S.C.; Ryals, J.A.; Milburn, M.V. Analysis of the adult human plasma metabolome. Pharmacogenomics 2008, 9, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Zhang, F.; Baldwin, R.P. Constant-potential amperometric detection of underivatized amino acids and peptides at a copper electrode. Anal. Chem. 1991, 63, 1702–1707. [Google Scholar] [CrossRef]
- Qu, H.; Ma, H.; Zhou, W.; O’Connor, C.J. In situ surface functionalization of magnetic nanoparticles with hydrophilic natural amino acids. Inorg. Chim. Acta 2012, 389, 60–65. [Google Scholar] [CrossRef]
- Durmus, Z.; Kavas, H.; Toprak, M.S.; Baykal, A.; Altınçekiç, T.G.; Aslan, A.; Bozkurt, A.; Coşgun, S. l-lysine coated iron oxide nanoparticles: Synthesis, structural and conductivity characterization. J. Alloys Compd. 2009, 484, 371–376. [Google Scholar] [CrossRef]
- Sousa, M.H.; Rubim, J.C.; Sobrinho, P.G.; Tourinho, F.A. Biocompatible magnetic fluid precursors based on aspartic and glutamic acid modified maghemite nanostructures. J. Magn. Magn. Mater. 2001, 225, 67–72. [Google Scholar] [CrossRef]
- Pušnik, K.; Peterlin, M.; Cigić, I.K.; Marolt, G.; Kogej, K.; Mertelj, A.; Gyergyek, S.; Makovec, D. Adsorption of amino acids, aspartic acid, and lysine onto iron-oxide nanoparticles. J. Phys. Chem. C 2016, 120, 14372–14381. [Google Scholar] [CrossRef]
- Veisi, H.; Azadbakht, R.; Saeidifar, F.; Abdi, M.R. Schiff based-functionalized multi walled carbon nano tubes to immobilization of palladium nanoparticles as heterogeneous and recyclable nanocatalyst for suzuki reaction in aqueous media under mild conditions. Catal. Lett. 2017, 147, 976–986. [Google Scholar] [CrossRef]






| Rate of Corrosion (10−3 Mils per Year) | |
|---|---|
| 1 mM Arginine in 100 mM NaOH | 7.8 ± 0.6 |
| 400 μM Creatine in 100 mM NaOH | 9.5 ± 1.2 |
| 100 mM NaOH (no analyte) | 6.3 ± 0.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tooley, C.A.; Gasperoni, C.H.; Marnoto, S.; Halpern, J.M. Evaluation of Metal Oxide Surface Catalysts for the Electrochemical Activation of Amino Acids. Sensors 2018, 18, 3144. https://doi.org/10.3390/s18093144
Tooley CA, Gasperoni CH, Marnoto S, Halpern JM. Evaluation of Metal Oxide Surface Catalysts for the Electrochemical Activation of Amino Acids. Sensors. 2018; 18(9):3144. https://doi.org/10.3390/s18093144
Chicago/Turabian StyleTooley, Christian A., Charles H. Gasperoni, Sabrina Marnoto, and Jeffrey Mark Halpern. 2018. "Evaluation of Metal Oxide Surface Catalysts for the Electrochemical Activation of Amino Acids" Sensors 18, no. 9: 3144. https://doi.org/10.3390/s18093144
APA StyleTooley, C. A., Gasperoni, C. H., Marnoto, S., & Halpern, J. M. (2018). Evaluation of Metal Oxide Surface Catalysts for the Electrochemical Activation of Amino Acids. Sensors, 18(9), 3144. https://doi.org/10.3390/s18093144





