Fast Procedures for the Electrodeposition of Platinum Nanostructures on Miniaturized Electrodes for Improved Ion Sensing
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
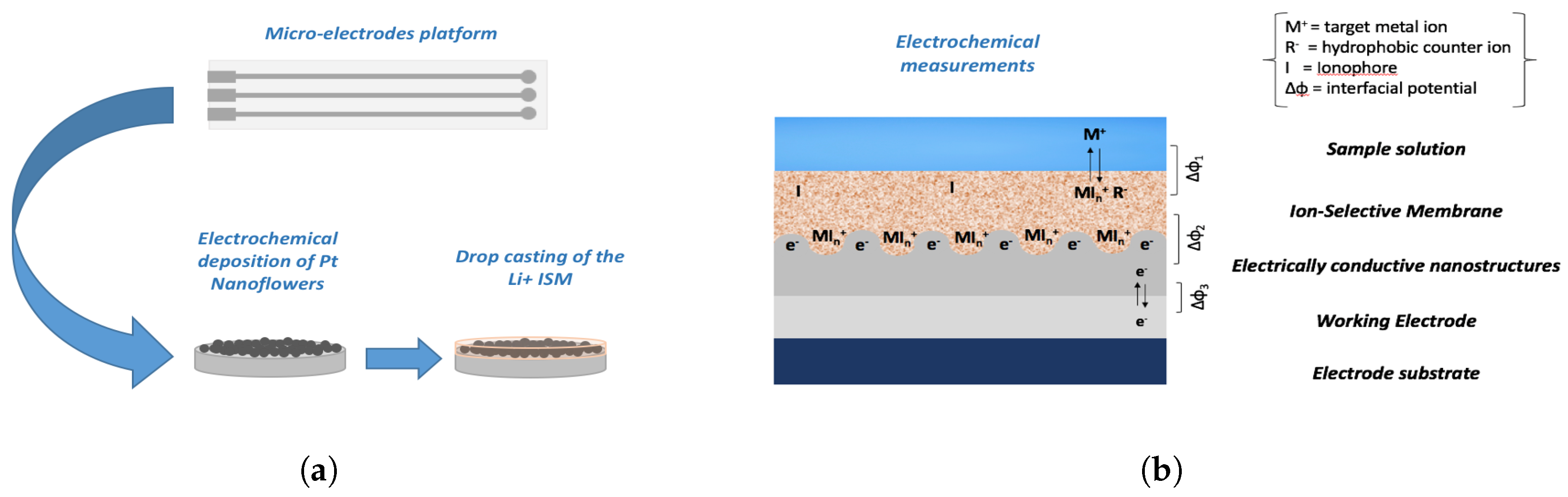
2.2. Fabrication of Micro-Electrodes
2.3. Morphological Characterization
2.4. Electrochemical Characterization
3. Results and Discussion
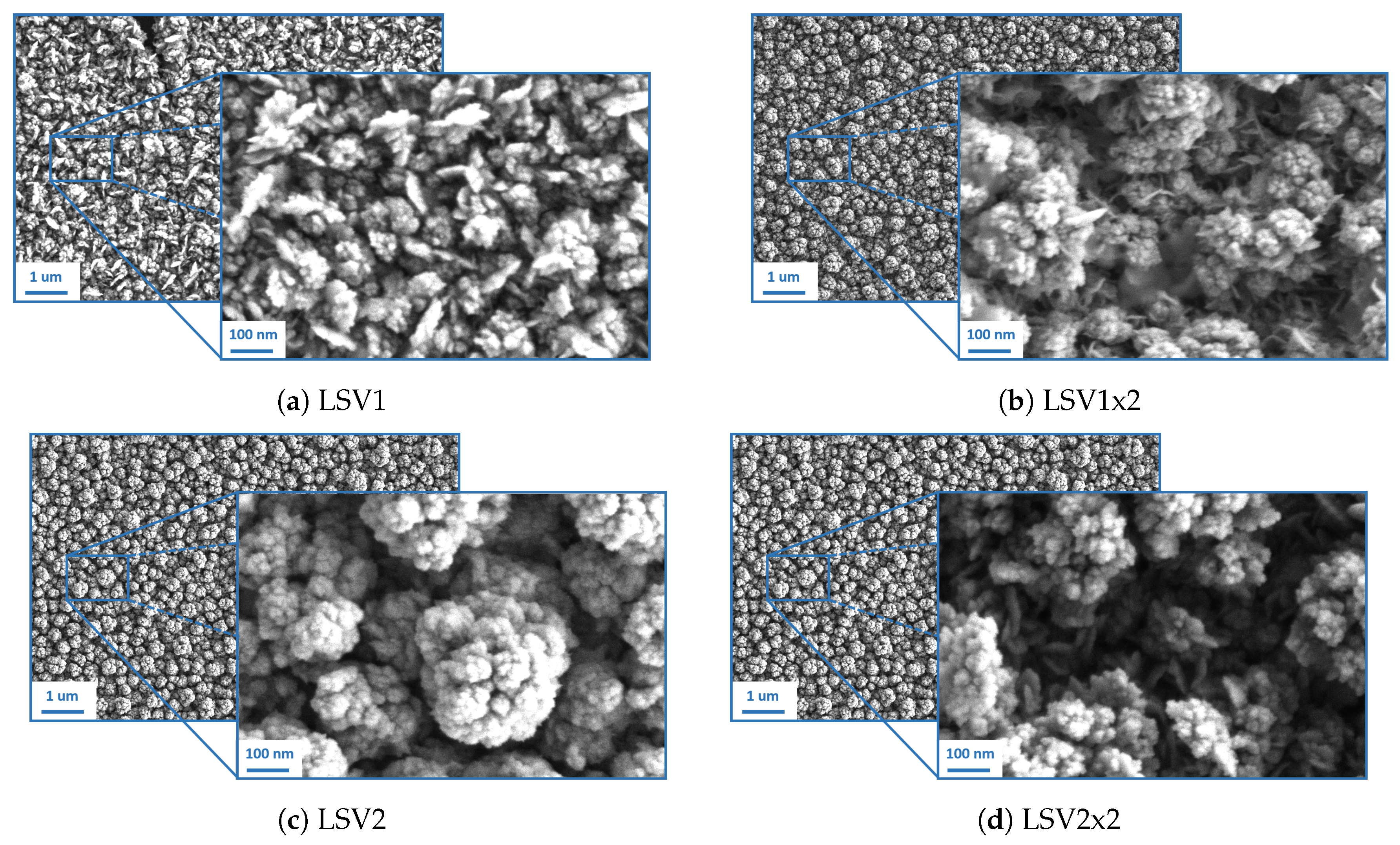
3.1. Morphological Characterization
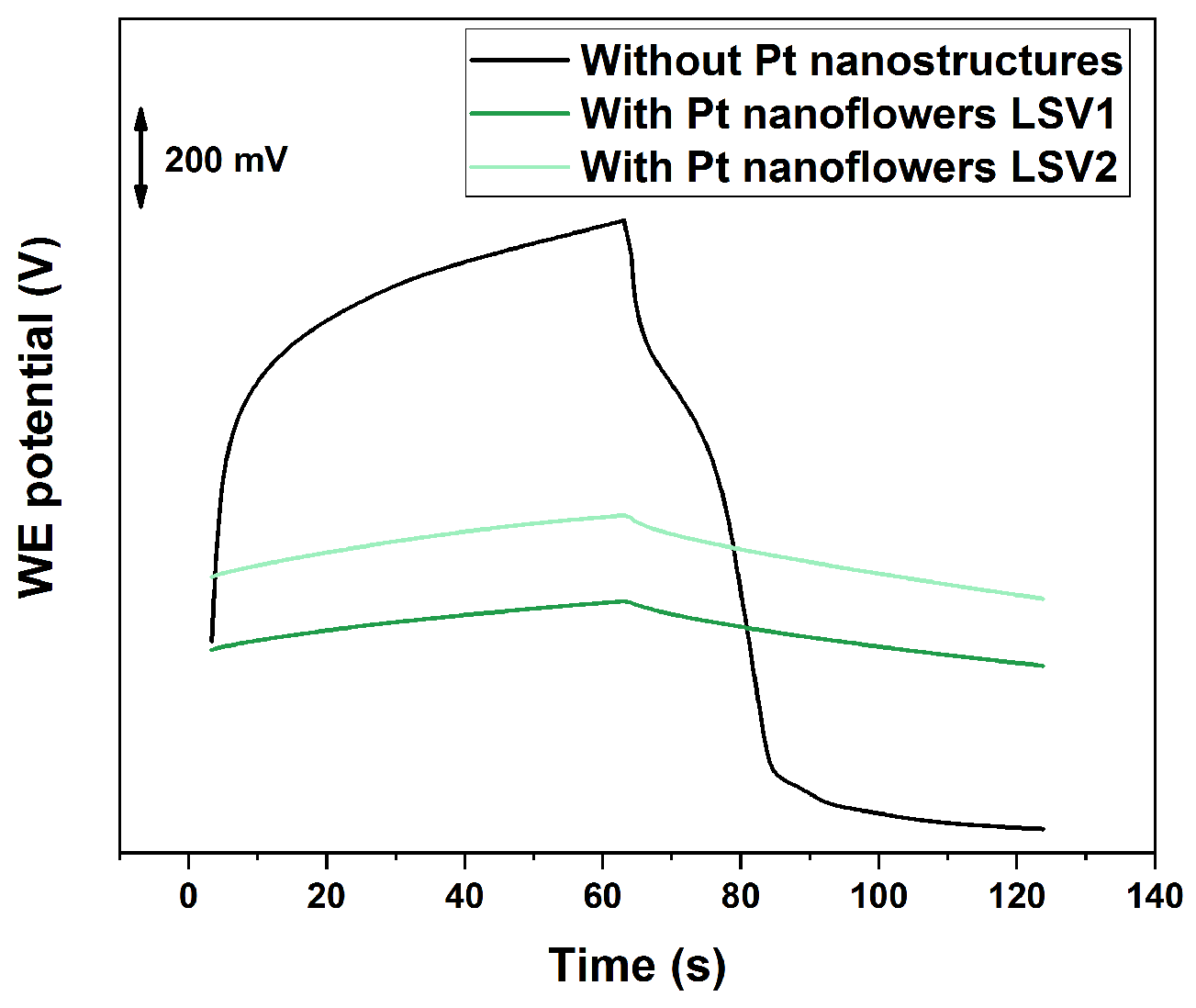
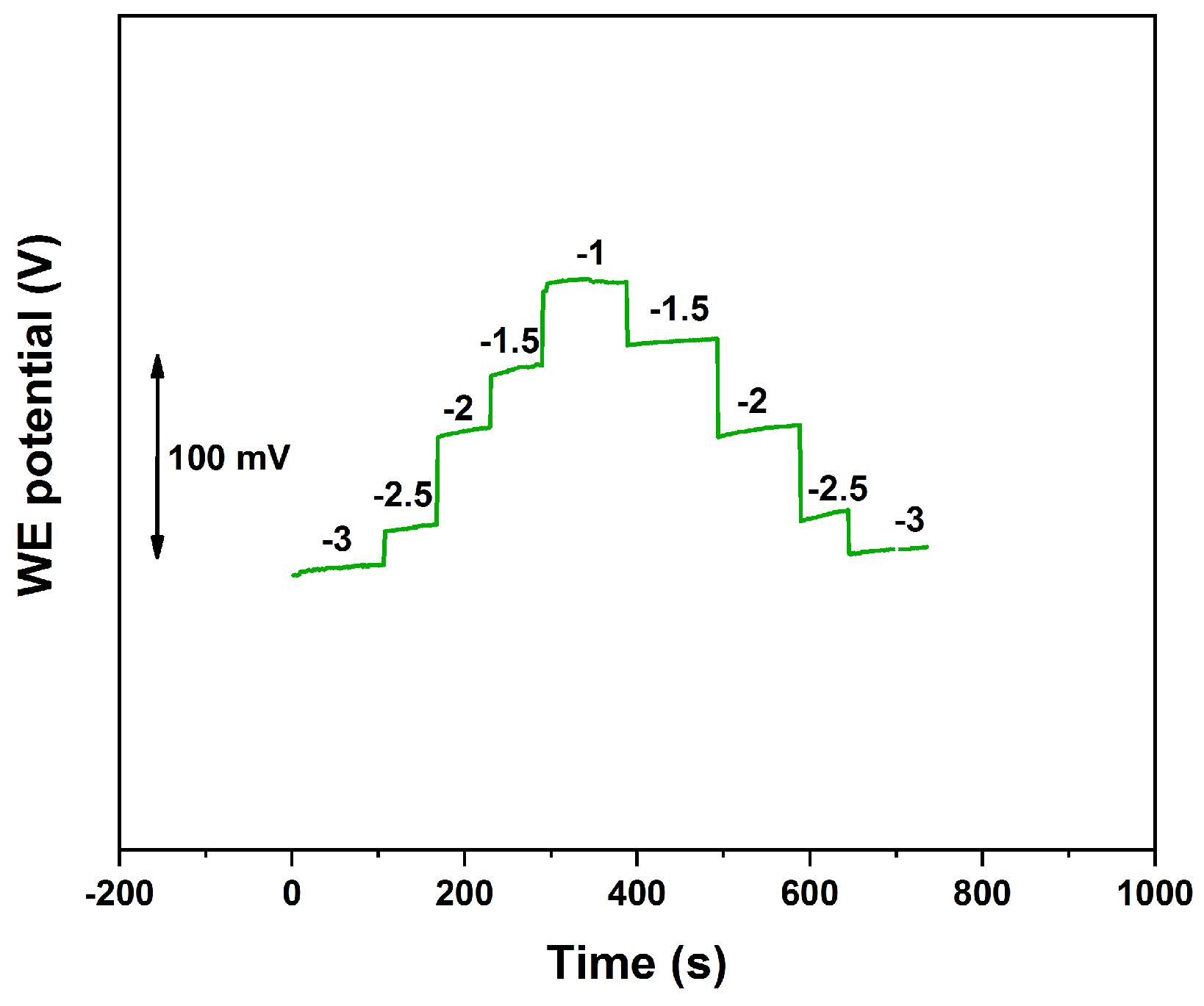
3.2. Current Reversal Chronopotentiometry (CRC) and SC Capacitance
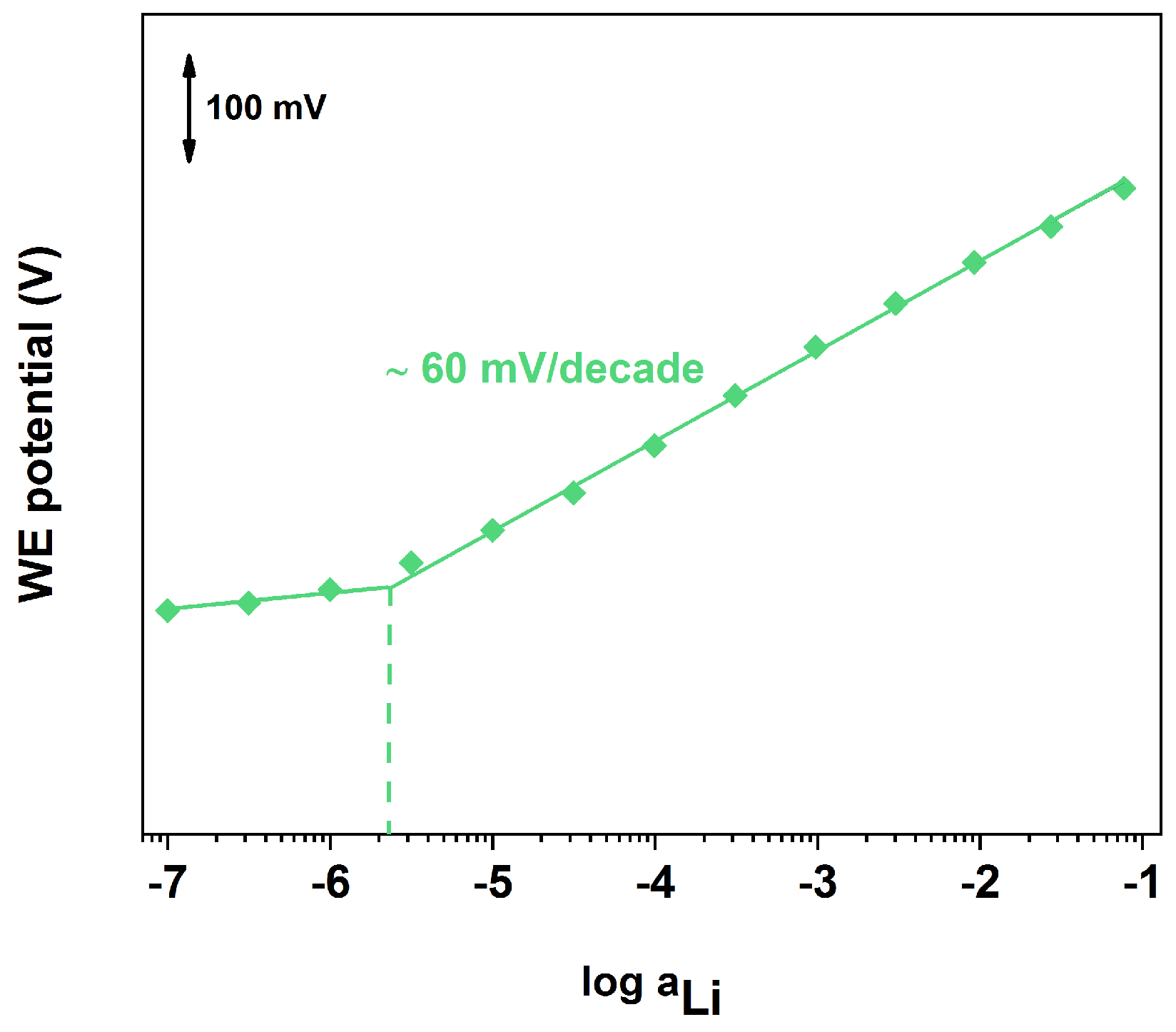
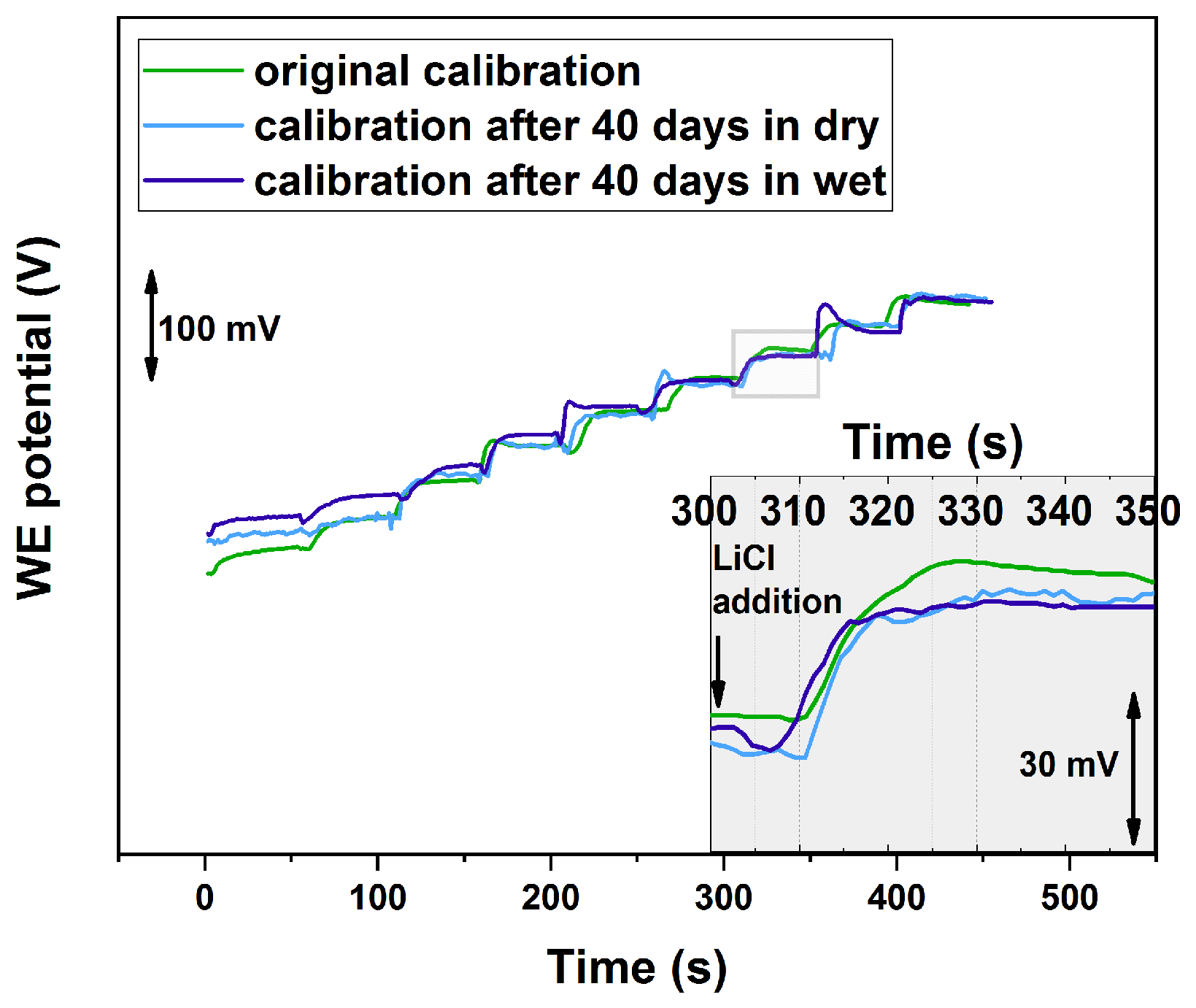
3.3. Lithium-ISE Calibration
3.4. Reversibility and Lifetime Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CRC | current reversal chronopotentiometry |
| CP | conductive polymer |
| CV | cyclic voltammetry |
| ISE | ion-selective electrode |
| ISM | ion-selective membrane |
| LOD | limit of detection |
| LSV | linear sweep voltammetry |
| POC | point-of-care |
| RE | reference electrode |
| SC | solid contact |
| SEM | scanning electron microscopy |
| WE | working electrode |
References
- Wongkaew, N.; Simsek, M.; Griesche, C.; Baeumner, A.J. Functional Nanomaterials and Nanostructures Enhancing Electrochemical Biosensors and Lab-on-a-Chip Performances: Recent Progress, Applications, and Future Perspective. Chem. Rev. 2019, 119, 120–194. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical Sensors and Biosensors Based on Nanomaterials and Nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef]
- Holzinger, M.; Goff, A.L.; Cosnier, S. Nanomaterials for biosensing applications: A review. Front. Chem. 2014, 2, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Stein, A.; Bühlmann, P. Rational design of all-solid-state ion-selective electrodes and reference electrodes. Trends Anal. Chem. 2016, 76, 102–114. [Google Scholar] [CrossRef]
- Xi, Z.; Ye, H.; Xia, X. Engineered Noble-Metal Nanostructures for in Vitro Diagnostics. Chem. Mater. 2018, 30, 8391–8414. [Google Scholar] [CrossRef]
- Schumacher, S.; Sartorius, D.; Ehrentreich-förster, E.; Bier, F.F. Miniaturization for Point-of-Care Analysis: Platform Technology for Almost Every Biomedical Assay. J. Int. Fed. Clin. Chem. Lab. Med. 2012, 23, 70–75. [Google Scholar]
- Bakker, E.; Xie, X. Shrinking Ion-Selective Sensors for Success. Anal. Sci. 2014, 20. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Wang, J. Non-invasive wearable electrochemical sensors: A review. Trends Biotechnol. 2014, 32, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Bandodkar, A.J.; Jeerapan, I.; Wang, J. Wearable Chemical Sensors: Present Challenges and Future Prospects. ACS Sens. 2016, 1, 464–482. [Google Scholar] [CrossRef]
- Windmiller, J.R.; Wang, J. Wearable Electrochemical Sensors and Biosensors: A Review. Electroanalysis 2013, 25, 29–46. [Google Scholar] [CrossRef]
- Bariya, M.; Nyein, H.Y.Y.; Javey, A. Wearable sweat sensors. Nat. Electron. 2018, 1, 160–171. [Google Scholar] [CrossRef]
- Criscuolo, F.; Taurino, I.; Carrara, S.; Micheli, G.D. A novel electrochemical sensor for non-invasive monitoring of lithium levels in mood disorders. Eng. Med. Biol. Soc. 2018, 2018, 3825–3828. [Google Scholar] [CrossRef]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 2016, 529, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Currano, L.J.; Sage, F.C.; Hagedon, M.; Hamilton, L.; Patrone, J.; Gerasopoulos, K. Wearable Sensor System for Detection of Lactate in Sweat. Sci. Rep. 2018, 8, 15890. [Google Scholar] [CrossRef] [PubMed]
- Emaminejad, S.; Gao, W.; Wu, E.; Davies, Z.A.; Nyein, H.Y.; Challa, S.; Ryan, S.P.; Fahad, H.M.; Chen, K.; Shahpar, Z.; et al. Autonomous sweat extraction and analysis applied to cystic fibrosis and glucose monitoring using a fully integrated wearable platform. Proc. Natl. Acad. Sci. USA 2017, 114, 4625–4630. [Google Scholar] [CrossRef] [PubMed]
- Schazmann, B.; Morris, D.; Slater, C.; Beirne, S.; Fay, C.; Reuveny, R.; Moyna, N.; Diamond, D. A wearable electrochemical sensor for the real-time measurement of sweat sodium concentration. Anal. Methods 2010, 2, 342–348. [Google Scholar] [CrossRef]
- Anastasova-Ivanova, S.; Crewther, B.; Bembnowicz, P.; Curto, V.; Ip, H.M.; Rosa, B.; Yang, G.Z. A wearable multisensing patch for continuous sweat monitoring. Biosens. Bioelectron. 2017, 93, 730. [Google Scholar] [CrossRef]
- Nyein, H.Y.Y.; Gao, W.; Shahpar, Z.; Emaminejad, S.; Challa, S.; Chen, K.; Fahad, H.M.; Tai, L.C.; Ota, H.; Davis, R.W.; et al. A Wearable Electrochemical Platform for Noninvasive Simultaneous Monitoring of Ca2+ and pH. ACS Nano 2016, 10, 7216–7224. [Google Scholar] [CrossRef]
- Kim, J.; Jeerapan, I.; Imani, S.; Cho, T.N.; Bandodkar, A.; Cinti, S.; Mercier, P.P.; Wang, J. Noninvasive Alcohol Monitoring Using a Wearable Tattoo-Based Iontophoretic-Biosensing System. ACS Sens. 2016, 1, 1011–1019. [Google Scholar] [CrossRef]
- Banna, M.H.; Najjaran, H.; Sadiq, R.; Imran, S.A.; Rodriguez, M.J.; Hoorfar, M. Miniaturized water quality monitoring pH and conductivity sensors. Sens. Actuators B Chem. 2014, 193, 434–441. [Google Scholar] [CrossRef]
- Veder, J.P.; Marco, R.D.; Clarke, G.; Chester, R.; Nelson, A.; Prince, K.; Pretsch, E.; Bakker, E. Elimination of undesirable water layers in solid contact polymeric ion-selective electrodes. Anal. Chem. 2008, 80, 6731–6740. [Google Scholar] [CrossRef] [PubMed]
- Lindner, E.; Gyurcsányi, R.E. Quality control criteria for solid-contact, solvent polymeric membrane ion-selective electrodes. J. Solid State Electrochem. 2009, 13, 51–68. [Google Scholar] [CrossRef]
- Matzeu, G.; Zuliani, C.; Diamond, D. Recent Progress in Disposable Ion-Selective Sensors for Environmental Applications. Adv. Sci. Technol. 2012, 77, 65–70. [Google Scholar] [CrossRef]
- Wang, S.; Wu, Y.; Gu, Y.; Li, T.; Luo, H.; Li, L.H.; Bai, Y.; Li, L.; Liu, L.; Cao, Y.; et al. Wearable Sweatband Sensor Platform Based on Gold Nanodendrite Array as Efficient Solid Contact of Ion-Selective Electrode. Anal. Chem. 2017, 89, 10224–10231. [Google Scholar] [CrossRef]
- Yin, T.; Qin, W. Trends in Analytical Chemistry Applications of nanomaterials in potentiometric sensors. Trends Anal. Chem. 2013, 51, 79–86. [Google Scholar] [CrossRef]
- Crespo, G.A.; Macho, S.; Rius, F.X. Ion-selective electrodes using carbon nanotubes as ion-to-electron transducers. Anal. Chem. 2008, 80, 1316–1322. [Google Scholar] [CrossRef]
- Parra, E.J.; Rius, F.X.; Blondeau, P. A potassium sensor based on non-covalent functionalization of multi-walled carbon nanotubes. Analyst 2013, 138, 2698. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; David-Pur, M.; Hanein, Y. Carbon Nanotube-Based Ion Selective Sensors for Wearable Applications. ACS Appl. Mater. Interfaces 2017, 9, 35169–35177. [Google Scholar] [CrossRef]
- Liang, R.; Yin, T.; Qin, W. A simple approach for fabricating solid-contact ion-selective electrodes using nanomaterials as transducers. Anal. Chim. Acta 2015, 853, 291–296. [Google Scholar] [CrossRef]
- Li, J.; Yin, T.; Qin, W. An all-solid-state polymeric membrane Pb2+-selective electrode with bimodal pore C60 as solid contact. Anal. Chim. Acta 2015, 876, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Fouskaki, M.; Chaniotakis, N. Fullerene-based electrochemical buffer layer for ion-selective electrodes. Analyst 2008, 133, 1072–1075. [Google Scholar] [CrossRef]
- Li, F.; Ye, J.; Zhou, M.; Gan, S.; Zhang, Q.; Han, D.; Niu, L. All-solid-state potassium-selective electrode using graphene as the solid contact. Analyst 2012, 137, 618–623. [Google Scholar] [CrossRef]
- Hernández, R.; Riu, J.; Bobacka, J.; Vallés, C.; Jiménez, P.; Benito, A.M.; Maser, W.K.; Rius, F.X. Reduced graphene oxide films as solid transducers in potentiometric all-solid-state ion-selective electrodes. J. Phys. Chem. C 2012, 116, 22570–22578. [Google Scholar] [CrossRef]
- Ping, J.; Wang, Y.; Wu, J.; Ying, Y. Electrochemistry Communications Development of an all-solid-state potassium ion-selective electrode using graphene as the solid-contact transducer. Electrochem. Commun. 2011, 13, 1529–1532. [Google Scholar] [CrossRef]
- Ping, J.; Wang, Y.; Ying, Y.; Wu, J. Application of electrochemically reduced graphene oxide on screen-printed ion-selective electrode. Anal. Chem. 2012, 84, 3473–3479. [Google Scholar] [CrossRef]
- Boeva, Z.A.; Lindfors, T. Few-layer graphene and polyaniline composite as ion-to-electron transducer in silicone rubber solid-contact ion-selective electrodes. Sens. Actuators B 2015, 224, 624–631. [Google Scholar] [CrossRef]
- Paczosa-Bator, B.; Cabaj, L.; Piȩk, M.; Piech, R.; Kubiak, W.W. Carbon-Supported Platinum Nanoparticle Solid-State Ion Selective Electrodes for the Determination of Potassium. Anal. Lett. 2015, 48, 2773–2785. [Google Scholar] [CrossRef]
- Zhang, T.; Lai, C.Z.; Fierke, M.A.; Stein, A.; Bühlmann, P. Advantages and limitations of reference electrodes with an ionic liquid junction and three-dimensionally ordered macroporous carbon as solid contact. Anal. Chem. 2012, 84, 7771–7778. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.Z.; Fierke, M.A.; Stein, A.; Bühlmann, P. Ion-selective electrodes with three-dimensionally ordered macroporous carbon as the solid contact. Anal. Chem. 2007, 79, 4621–4626. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Li, F.; Gan, S.; Jiang, Y.; An, Q.; Zhang, Q.; Niu, L. Using sp2-C dominant porous carbon sub-micrometer spheres as solid transducers in ion-selective electrodes. Electrochem. Commun. 2015, 50, 60–63. [Google Scholar] [CrossRef]
- Xu, J.; Jia, F.; Li, F.; An, Q.; Gan, S.; Zhang, Q.; Ivaska, A.; Niu, L. Simple and Efficient Synthesis of Gold Nanoclusters and Their Performance as Solid Contact of Ion Selective Electrode. Electrochim. Acta 2016, 222, 1007–1012. [Google Scholar] [CrossRef]
- Yin, T.; Pan, D.; Qin, W. All-solid-state polymeric membrane ion-selective miniaturized electrodes based on a nanoporous gold film as solid contact. Anal. Chem. 2014, 86, 11038–11044. [Google Scholar] [CrossRef]
- Jaworska, E.; Wójcik, M.; Kisiel, A.; Mieczkowski, J.; Michalska, A. Gold nanoparticles solid contact for ion-selective electrodes of highly stable potential readings. Talanta 2011, 85, 1986–1989. [Google Scholar] [CrossRef]
- Matzeu, G.; Zuliani, C.; Diamond, D. Solid-Contact Ion-Selective Electrodes (ISEs) based on Ligand Functionalised Gold Nanoparticles. Electrochim. Acta 2015, 159, 158–165. [Google Scholar] [CrossRef]
- Paczosa-Bator, B.; Cabaj, L.; Piech, R.; Skupień, K. Potentiometric sensors with carbon black supporting platinum nanoparticles. Anal. Chem. 2013, 85, 10255–10261. [Google Scholar] [CrossRef]
- Taurino, I.; Sanzó, G.; Mazzei, F.; Favero, G.; De Micheli, G.; Carrara, S. Fast synthesis of platinum nanopetals and nanospheres for highly-sensitive non-enzymatic detection of glucose and selective sensing of ions. Sci. Rep. 2015, 5, 15277. [Google Scholar] [CrossRef]
- Criscuolo, F.; Lobello, L.; Taurino, I.; Demarchi, D.; Carrara, S.; Micheli, G.D. Mixed gold and platinum nanostructured layers for all-solid-state ion sensors. In Proceedings of the IEEE Sensors Conference, New Delhi, India, 28–31 October 2018. [Google Scholar]
- Criscuolo, F.; Taurino, I.; Stradolini, F.; Carrara, S.; De Micheli, G. Highly-stable Li + ion-selective electrode based on noble metal nanostructures as solid-contacts. Anal. Chim. Acta 2018, 1027, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Bobacka, J. Potential Stability of All-Solid-State Ion-Selective Electrodes Using Conducting Polymers as Ion-to-Electron Transducers. Anal. Chem. 1999, 71, 4932–4937. [Google Scholar] [CrossRef] [PubMed]

| dE/dt | C | Normalized C | |
|---|---|---|---|
| [mV/s] | [F] | [F/mm] | |
| Without Pt Nanostructures (micro-electrodes) | 1.02 ± 0.22 | 0.57 ± 0.17 | 4.35 ± 1.29 |
| LSV1 (micro-electrodes) | 0.23 ± 0.08 | 2.55 ± 0.41 | 19.39 ± 1.29 |
| LSV2 (micro-electrodes) | 0.18 ± 0.01 | 2.39 ± 0.19 | 18.15 ± 3.09 |
| Literature (macro-electrodes) [48] | – | – | 15.55 ± 7.71 |
| Slope [mV/decade] | LOD | Response Time [s] | |
|---|---|---|---|
| LSV1 (micro-electrodes) | 61.7 ± 3.7 | 15–30 | |
| LSV2 (micro-electrodes) | 59.0 ± 1.0 | 15–30 | |
| Literature (macro-electrodes) [48] | 58.7 ± 0.8 | 15–30 |
| Slope [mV/decade] | LOD | Response Time [s] | |
|---|---|---|---|
| As prepared | 59.1 | ~15 s | |
| After 40 days in dry | 59.0 | 15–20 | |
| After 40 days in wet | 56.5 | 15–40 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Criscuolo, F.; Taurino, I.; Dam, V.A.; Catthoor, F.; Zevenbergen, M.; Carrara, S.; De Micheli, G. Fast Procedures for the Electrodeposition of Platinum Nanostructures on Miniaturized Electrodes for Improved Ion Sensing. Sensors 2019, 19, 2260. https://doi.org/10.3390/s19102260
Criscuolo F, Taurino I, Dam VA, Catthoor F, Zevenbergen M, Carrara S, De Micheli G. Fast Procedures for the Electrodeposition of Platinum Nanostructures on Miniaturized Electrodes for Improved Ion Sensing. Sensors. 2019; 19(10):2260. https://doi.org/10.3390/s19102260
Chicago/Turabian StyleCriscuolo, Francesca, Irene Taurino, Van Anh Dam, Francky Catthoor, Marcel Zevenbergen, Sandro Carrara, and Giovanni De Micheli. 2019. "Fast Procedures for the Electrodeposition of Platinum Nanostructures on Miniaturized Electrodes for Improved Ion Sensing" Sensors 19, no. 10: 2260. https://doi.org/10.3390/s19102260
APA StyleCriscuolo, F., Taurino, I., Dam, V. A., Catthoor, F., Zevenbergen, M., Carrara, S., & De Micheli, G. (2019). Fast Procedures for the Electrodeposition of Platinum Nanostructures on Miniaturized Electrodes for Improved Ion Sensing. Sensors, 19(10), 2260. https://doi.org/10.3390/s19102260






