Clinical Applicability of a Textile 1-Lead ECG Device for Overnight Monitoring
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Overview
2.2. Study Participants
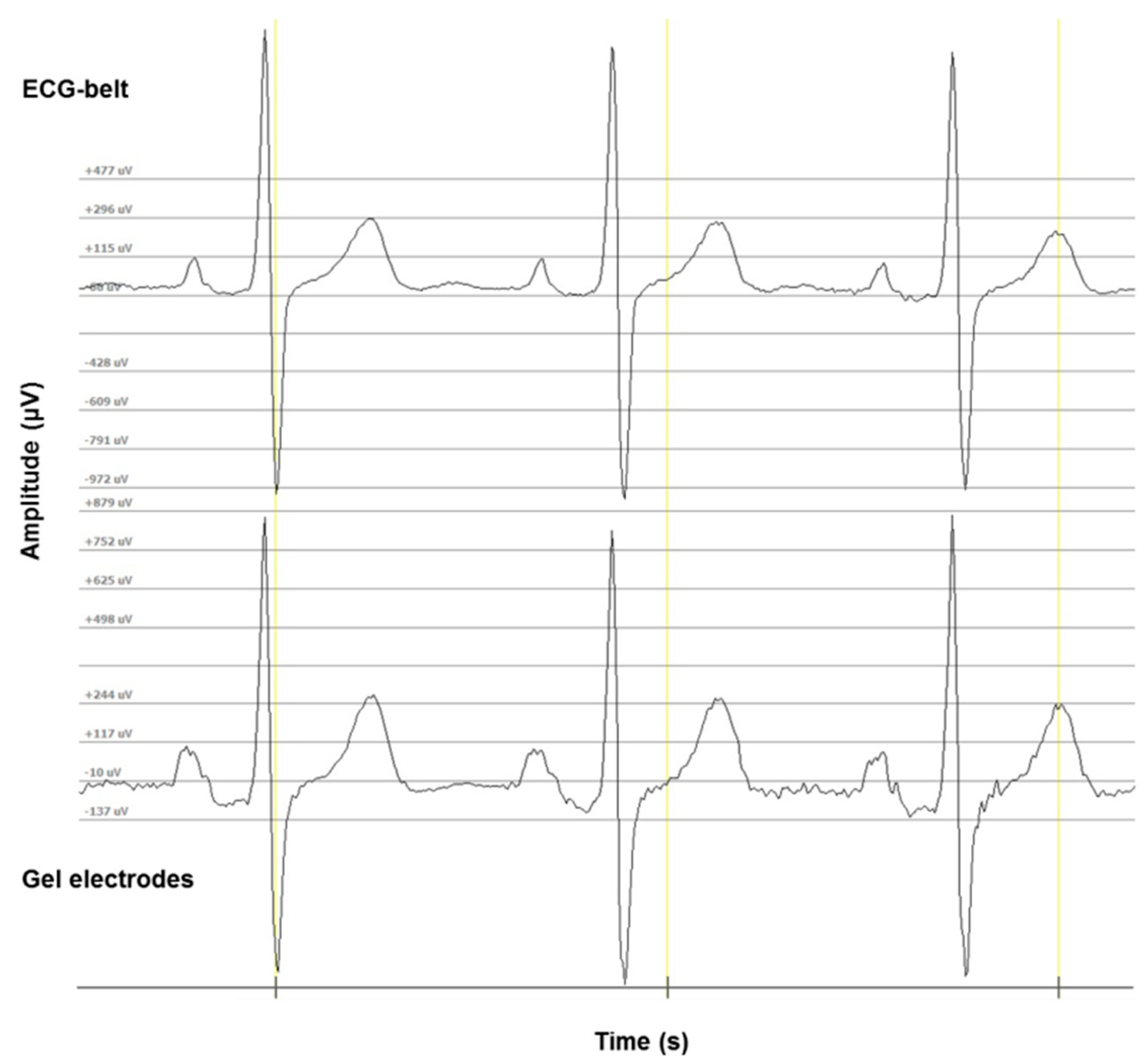
2.3. PSG and Conventional ECG (Gel Electrodes, Gold Standard)
2.4. ECG-Belt (Embroidered Electrodes)
2.5. Data Processing and Statistical Analysis
3. Results
4. Discussion
Clinical Relevance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hong, S.; Yang, Y.; Kim, S.; Shin, S.; Lee, I.; Jang, Y.; Kim, K.; Yi, H.; Lee, J. Performance Study of the Wearable One-Lead Wireless Electrocardiographic Monitoring System. Telemed. e-Health 2009, 15, 166–175. [Google Scholar] [CrossRef]
- Surrel, G.; Aminifar, A.; Rincón, F.; Murali, S.; Atienza, D. Online Obstructive Sleep Apnea Detection on Medical Wearable Sensors. IEEE Trans. Biomed. Circuits Syst. 2018, 12, 1–12. [Google Scholar] [CrossRef]
- Budidha, K.; Kyriacou, P.A. The human ear canal: Investigation of its suitability for monitoring photoplethysmographs and arterial oxygen saturation. Physiol. Meas. 2014, 35, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Weinschenk, S.W.; Beise, R.D.; Lorenz, J. Heart rate variability (HRV) in deep breathing tests and 5-min short-term recordings: Agreement of ear photoplethysmography with ECG measurements, in 343 subjects. Eur. J. Appl. Physiol. 2016, 116, 1527–1535. [Google Scholar] [CrossRef] [PubMed]
- Weder, M.; Hegemann, D.; Amberg, M.; Hess, M.; Boesel, L.F.; Abaecherli, R.; Meyer, V.R.; Rossi, R.M. Embroidered electrode with silver/titanium coating for long-term ECG monitoring. Sensors 2015, 15, 1750–1759. [Google Scholar] [CrossRef]
- Woodson, B.T.; Brusky, L.T.; Saurajen, A.; Jaradeh, S. Association of autonomic dysfunction and mild obstructive sleep apnea. Otolaryngol. Head Neck Surg. 2004, 130, 643–648. [Google Scholar] [CrossRef]
- Carlson, J.T.; Hedner, J.; Elam, M.; Ejnell, H.; Sellgren, J.; Wallin, B.G. Augmented Resting Sympathetic Activity in Awake Patients with Obstructive Sleep Apnea. Chest 1993, 103, 1763–1768. [Google Scholar] [CrossRef]
- Somers, V.K.; Dyken, M.E.; Clary, M.P.; Abboud, F.M. Sympathetic neural mechanisms in obstructive sleep apnea. J. Clin. Investig. 1995, 96, 1897–1904. [Google Scholar] [CrossRef]
- Rimoldi, O.; Pierini, S.; Ferrari, A.; Cerutti, S.; Pagani, M.; Malliani, A. Analysis of short-term oscillations of R-R and arterial pressure in conscious dogs. Am. J. Physiol. 1990, 258, H967–H976. [Google Scholar] [CrossRef] [PubMed]
- Persson, P.B. Modulation of cardiovascular control mechanisms and their interaction. Physiol. Rev. 1996, 76, 193–244. [Google Scholar] [CrossRef] [PubMed]
- Butler, G.C.; Naughton, M.T.; Rahman, M.A.; Bradley, T.D.; Floras, J.S. Continuous positive airway pressure increases heart rate variability in congestive heart failure. J. Am. Coll. Cardiol. 1995, 25, 672–679. [Google Scholar] [CrossRef]
- Narkiewicz, K.; Montano, N.; Cogliati, C.; van de Borne, P.J.; Dyken, M.E.; Somers, V.K. Altered cardiovascular variability in obstructive sleep apnea. Circulation 1998, 98, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.J.; Mietus, J.E.; Peng, C.-K.; Goldberger, A.L. An electrocardiogram-based technique to assess cardiopulmonary coupling during sleep. Sleep 2005, 28, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Health Quality Ontario Polysomnography in patients with obstructive sleep apnea: An evidence-based analysis. Ont. Health Technol. Assess. Ser. 2006, 6, 1–38. [PubMed]
- Lux, L.; Boehlecke, B.; Lohr, K. Effectiveness of Portable Monitoring Devices for Diagnosing Obstructive Sleep Apnea: Update of a Systematic Review; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2004.
- Qaseem, A.; Dallas, P.; Owens, D.K.; Starkey, M.; Holty, J.-E.C.; Shekelle, P. Diagnosis of Obstructive Sleep Apnea in Adults: A Clinical Practice Guideline from the American College of Physicians. Ann. Intern. Med. 2014, 161, 210. [Google Scholar] [CrossRef]
- Task force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology Heart rate variability standards of measurement, physiological interpretation, and clinical use. Eur. Heart J. 1996, 17, 354–381. [CrossRef]
- Piskorski, J.; Guzik, P. Geometry of the Poincaré plot of RR intervals and its asymmetry in healthy adults. Physiol. Meas. 2007, 28, 287–300. [Google Scholar] [CrossRef]
- Mao, M.; Chuo, Y.; Kaminska, B. Multi-functional Wearable Device for Heart Health and Position Assessment. NSTI Nanotech 2008, 2, 615–618. [Google Scholar]
- Georgiou, K.; Larentzakis, A.V.; Khamis, N.N.; Alsuhaibani, G.I.; Alaska, Y.A.; Giallafos, E.J. Can Wearable Devices Accurately Measure Heart Rate Variability? A Systematic Review. Folia Med. (Plovdiv.) 2018, 60, 7–20. [Google Scholar] [CrossRef] [Green Version]
- Akintola, A.A.; van de Pol, V.; Bimmel, D.; Maan, A.C.; van Heemst, D. Comparative Analysis of the Equivital EQ02 Lifemonitor with Holter Ambulatory ECG Device for Continuous Measurement of ECG, Heart Rate, and Heart Rate Variability: A Validation Study for Precision and Accuracy. Front. Physiol. 2016, 7, 391. [Google Scholar] [CrossRef]
- Lado, M.J.; Méndez, A.J.; Rodríguez-Liñares, L.; Otero, A.; Vila, X.A. Nocturnal evolution of heart rate variability indices in sleep apnea. Comput. Biol. Med. 2012, 42, 1179–1185. [Google Scholar] [CrossRef] [PubMed]





| n | 242 | |
| Age (y) | 52 [43–61] | |
| male / female | 186/56 | |
| BMI (kg·m−2) | 29 [25.5–32.5] | |
| ESS | 9 [6–12] | |
| ODI (h−1) | 17 [0.9–33.2] | |
| AHI (h−1) | 21 [4.4–37.6] | |
| Type of apnoea | Obstructive | 156 |
| Central | 5 | |
| Mixed | 35 | |
| Unspecified | 1 | |
| No apnoea detected | 44 | |
| Gel Electrodes | ECG-Belt | p | ||
|---|---|---|---|---|
| SNRhf | (dB) | 21 | 17 | <0.001 |
| SD | (dB) | 3 | 6 | |
| SNRlf | (dB) | 12 | 0 | <0.001 |
| SD | (dB) | 5 | 5 | |
| BLW | (mV) | 0.03 | 0.30 | <0.001 |
| SD | (mV) | 0.02 | 0.43 |
| Mean | p | RMS | SEE | Pearson r | ||||
|---|---|---|---|---|---|---|---|---|
| A | B | Diff | ||||||
| RRmean | (ms) | 944.84 | 941.90 | −2.94 | 0.429 | 57.79 | 0.39% | 0.91 |
| SD | (ms) | 133.20 | 138.87 | 57.84 | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fontana, P.; Martins, N.R.A.; Camenzind, M.; Rossi, R.M.; Baty, F.; Boesch, M.; Schoch, O.D.; Brutsche, M.H.; Annaheim, S. Clinical Applicability of a Textile 1-Lead ECG Device for Overnight Monitoring. Sensors 2019, 19, 2436. https://doi.org/10.3390/s19112436
Fontana P, Martins NRA, Camenzind M, Rossi RM, Baty F, Boesch M, Schoch OD, Brutsche MH, Annaheim S. Clinical Applicability of a Textile 1-Lead ECG Device for Overnight Monitoring. Sensors. 2019; 19(11):2436. https://doi.org/10.3390/s19112436
Chicago/Turabian StyleFontana, Piero, Neusa R. Adão Martins, Martin Camenzind, René M. Rossi, Florent Baty, Maximilian Boesch, Otto D. Schoch, Martin H. Brutsche, and Simon Annaheim. 2019. "Clinical Applicability of a Textile 1-Lead ECG Device for Overnight Monitoring" Sensors 19, no. 11: 2436. https://doi.org/10.3390/s19112436
APA StyleFontana, P., Martins, N. R. A., Camenzind, M., Rossi, R. M., Baty, F., Boesch, M., Schoch, O. D., Brutsche, M. H., & Annaheim, S. (2019). Clinical Applicability of a Textile 1-Lead ECG Device for Overnight Monitoring. Sensors, 19(11), 2436. https://doi.org/10.3390/s19112436







