Development of an Ultrasensitive Impedimetric Immunosensor Platform for Detection of Plasmodium Lactate Dehydrogenase
Abstract
:1. Introduction
2. Material and Methods
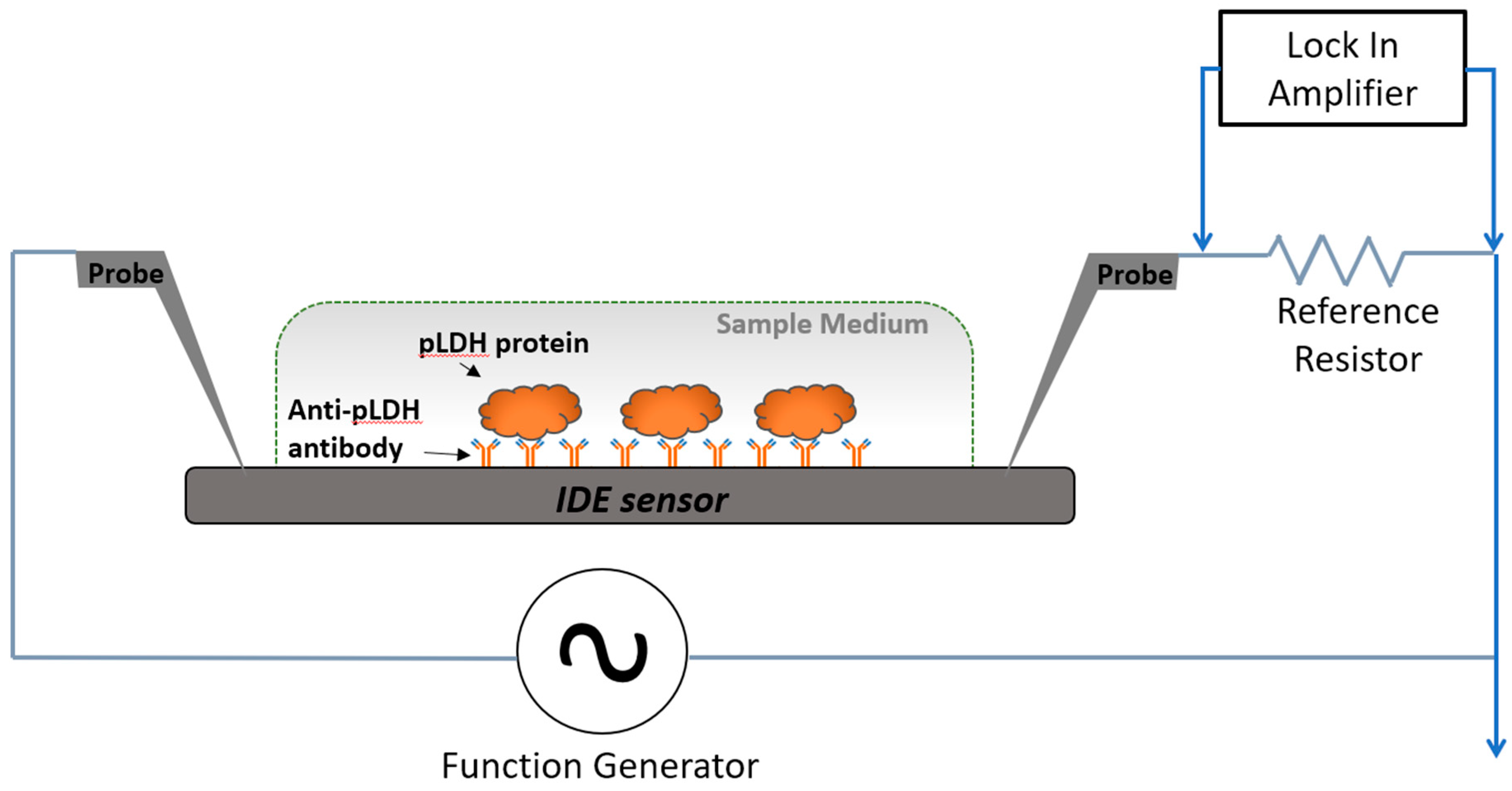
2.1. General Design
2.2. pLDH and Anti-pLDH Antibody
2.3. Fabrication and Functionalization of IDE Sensor
2.4. pLDH Detection Assay
2.5. Electrical Measurement
2.6. Statistical Analysis
3. Results
3.1. Feasibility of pLDH Detection on IDE Sensor
3.2. Detection Range of pLDH in the IDE Sensor
3.3. Detection of pLDH in Saliva by IDE Sensors
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Method | LLOD * | Reference |
|---|---|---|
| Light Microscopy | ~320 ng/mL | [10,14] |
| RDTs | 25 ng/mL | [13] |
| This work (in PBS) | 250 pg/mL |
References
- WHO. World Malaria Report. 2018. Available online: https://apps.who.int/iris/bitstream/handle/10665/275867/9789241565653-eng.pdf?ua=1. (accessed on 19 November 2018).
- WHO. World Malaria Report 2016; World Health Organization: Geneva, Switzerland, 2017; pp. xii–xvii. Available online: http://apps.who.int/iris/bitstream/10665/252038/1/9789241511711-eng.pdf?ua=1 (accessed on 19 November 2018).
- WHO Malaria. Available online: http://www.who.int/mediacentre/factsheets/fs094/en/ (accessed on 19 November 2018).
- malERA Consultative Group on Diagnoses and Diagnostics. A research agenda for malaria eradication: Diagnoses and diagnostics. PLoS Med. 2011, 8, e1000396. [Google Scholar] [CrossRef]
- WHO. WHO Evidence Review Group on Malaria Diagnosis in Low Transmission Settings. Available online: http://www.who.int/malaria/mpac/mpac_mar2014_diagnosis_low_transmission_settings_report.pdf (accessed on 19 November 2018).
- Arrow, K.J.; Panosian, C.; Gelband, H. Saving Lives, Buying Time: Economics of Malaria Drugs in an Age of Resistance; National Academies Press: Washington, DC, USA, 2004. Available online: https://www.ncbi.nlm.nih.gov/books/NBK215634/?report=reader (accessed on 19 November 2018).
- Collins, W.E.; Jeffery, G.M. A retrospective examination of the patterns of recrudescence in patients infected with Plasmodium falciparum. Am. J. Trop. Med. Hyg. 1999, 61, 44–48. [Google Scholar] [CrossRef]
- Chen, I.; Clarke, S.E.; Gosling, R.; Hamainza, B.; Killeen, G.; Magill, A.; O’Meara, W.; Price, R.N.; Riley, E.M. “Asymptomatic” malaria: A chronic and debilitating infection that should be treated. PLoS Med. 2016, 13, e1001942. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.T.; Saunders, D.L.; Meshnick, S.R. The role of submicroscopic parasitemia in malaria transmission: What is the evidence? Trends Parasitol. 2014, 30, 183–190. [Google Scholar] [CrossRef]
- Bell, D.; Fleurent, A.E.; Hegg, M.C.; Boomgard, J.D.; McConnico, C.C. Development of new malaria diagnostics: Matching performance and need. Malar. J. 2016, 15, 406. [Google Scholar] [CrossRef]
- Milne, L.; Kyi, M.; Chiodini, P.; Warhurst, D. Accuracy of routine laboratory diagnosis of malaria in the United Kingdom. J. Clin. Pathol. 1994, 47, 740–742. [Google Scholar] [CrossRef]
- WHO. Malaria diagnosis: Memorandum from a WHO meeting. Bull. World Health Organ. (WHO) 1988, 66, 575–594. [Google Scholar]
- Jimenez, A.; Rees-Channer, R.R.; Perera, R.; Gamboa, D.; Chiodini, P.L.; González, I.J.; Mayor, A.; Ding, X.C. Analytical sensitivity of current best-in-class malaria rapid diagnostic tests. Malar. J. 2017, 16, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, J.W.; Cho, C.H.; Han, E.T.; An, S.S.A.; Lim, C.S. pLDH level of clinically isolated Plasmodium vivax and detection limit of pLDH based malaria rapid diagnostic test. Malar. J. 2013, 12, 181. [Google Scholar] [CrossRef]
- WHO. Universal Access to Malar. Diagnostic Testing: An Operational Manual; World Health Organization: Geneva, Switzerland, 2011; pp. 23–26. [Google Scholar]
- Okell, L.C.; Ghani, A.C.; Lyons, E.; Drakeley, C.J. Submicroscopic infection in Plasmodium falciparum-endemic populations: A systematic review and meta-analysis. J. Infect. Dis. 2009, 200, 1509–1517. [Google Scholar] [CrossRef]
- Mwingira, F.; Genton, B.; Kabanywanyi, A.-N.M.; Felger, I. Comparison of detection methods to estimate asexual Plasmodium falciparum parasite prevalence and gametocyte carriage in a community survey in Tanzania. Malar. J. 2014, 13, 433. [Google Scholar] [CrossRef]
- Wanja, E.W.; Kuya, N.; Moranga, C.; Hickman, M.; Johnson, J.D.; Moseti, C.; Anova, L.; Ogutu, B.; Ohrt, C. Field evaluation of diagnostic performance of malaria rapid diagnostic tests in western Kenya. Malar. J. 2016, 15, 456. [Google Scholar] [CrossRef]
- Rock, E.; Marsh, K.; Saul, A.; Wellems, T.; Taylor, D.W.; Maloy, W.; Howard, R. Comparative analysis of the Plasmodium falciparum histidine-rich proteins HRP-I, HRP-II and HRP-III in malaria parasites of diverse origin. Parasitology 1987, 95, 209–227. [Google Scholar] [CrossRef] [PubMed]
- Piper, R.; Lebras, J.; Wentworth, L.; Hunt-Cooke, A.; Houze, S.; Chiodini, P.; Makler, M. Immunocapture diagnostic assays for malaria using Plasmodium lactate dehydrogenase (pLDH). Am. J. Trop. Med. Hyg. 1999, 60, 109–118. [Google Scholar] [CrossRef]
- Imwong, M.; Hanchana, S.; Malleret, B.; Rénia, L.; Day, N.P.; Dondorp, A.; Nosten, F.; Snounou, G.; White, N.J. High-throughput ultrasensitive molecular techniques for quantifying low-density malaria parasitemias. J. Clin. Microbiol. 2014, 52, 3303–3309. [Google Scholar] [CrossRef]
- Hopkins, H.; González, I.J.; Polley, S.D.; Angutoko, P.; Ategeka, J.; Asiimwe, C.; Agaba, B.; Kyabayinze, D.J.; Sutherland, C.J.; Perkins, M.D. Highly sensitive detection of malaria parasitemia in a malaria-endemic setting: Performance of a new loop-mediated isothermal amplification kit in a remote clinic in Uganda. J. Infect. Dis. 2013, 208, 645–652. [Google Scholar] [CrossRef]
- Kamau, E.; Alemayehu, S.; Feghali, K.C.; Juma, D.W.; Blackstone, G.M.; Marion, W.R.; Obare, P.; Ogutu, B.; Ockenhouse, C.F. Sample-ready multiplex qPCR assay for detection of malaria. Malar. J. 2014, 13, 158. [Google Scholar] [CrossRef]
- Mens, P.; de Bes, H.; Sondo, P.; Laochan, N.; Keereecharoen, L.; Van Amerongen, A.; Flint, J.; Sak, J.; Proux, S.; Tinto, H. Direct blood PCR in combination with nucleic acid lateral flow immunoassay for detection of Plasmodium species in settings where malaria is endemic. J. Clin. Microbiol. 2012, 50, 3520–3525. [Google Scholar] [CrossRef]
- Yugender Goud, K.; Sunil Kumar, V.; Hayat, A.; Vengatajalabathy Gobi, K.; Song, H.; Kim, K.-H.; Marty, J.L. A highly sensitive electrochemical immunosensor for zearalenone using screen-printed disposable electrodes. J. Electroanal. Chem. 2019, 832, 336–342. [Google Scholar] [CrossRef]
- Abeyrathne, C.D.; Huynh, D.H.; McIntire, T.W.; Nguyen, T.C.; Nasr, B.; Zantomio, D.; Chana, G.; Abbott, I.; Choong, P.; Catton, M.; et al. Lab on a chip sensor for rapid detection and antibiotic resistance determination of Staphylococcus aureus. Analyst 2016, 141, 1922–1929. [Google Scholar] [CrossRef]
- Soraya, G.V.; Chan, J.; Nguyen, T.C.; Huynh, D.H.; Abeyrathne, C.D.; Chana, G.; Todaro, M.; Skafidas, E.; Kwan, P. An interdigitated electrode biosensor platform for rapid HLA-B*15:02 genotyping for prevention of drug hypersensitivity. Biosens. Bioelectron. 2018, 111, 174–183. [Google Scholar] [CrossRef]
- Soraya, G.V.; Nguyen, T.C.; Abeyrathne, C.D.; Huynh, D.H.; Chan, J.; Nguyen, P.D.; Nasr, B.; Chana, G.; Kwan, P.; Skafidas, E. A label-free, quantitative fecal hemoglobin detection platform for colorectal cancer screening. Biosensors 2017, 7, 19. [Google Scholar] [CrossRef]
- Asav, E.; Sezgintürk, M.K. A novel impedimetric disposable immunosensor for rapid detection of a potential cancer biomarker. Int. J. Biol. Macromol. 2014, 66, 273–280. [Google Scholar] [CrossRef]
- Gatton, M.L.; Rees-Channer, R.R.; Glenn, J.; Barnwell, J.W.; Cheng, Q.; Chiodini, P.L.; Incardona, S.; González, I.J.; Cunningham, J. Pan-Plasmodium band sensitivity for Plasmodium falciparum detection in combination malaria rapid diagnostic tests and implications for clinical management. Malar. J. 2015, 14, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abeyrathne, C.D.; Huynh, D.H.; Lee, T.T.; Nguyen, T.C.; Nasr, B.; Chana, G.; Skafidas, E. GFAP Antibody Detection Using Interdigital Coplanar Waveguide Immunosensor. IEEE Sens. J. 2016, 16, 2898–2905. [Google Scholar] [CrossRef]
- Shaila, M.; Pai, G.P.; Shetty, P. Salivary protein concentration, flow rate, buffer capacity and pH estimation: A comparative study among young and elderly subjects, both normal and with gingivitis and periodontitis. J. Indian Soc. Periodontol. 2013, 17, 42. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.P. Biosensors: Sense and sensibility. Chem. Soc. Rev. 2013, 42, 3184–3196. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, K.; Nelakurthi, H.; Kumar, A.; Rudraraju, A. Oral fluid-based biosensors: A novel method for rapid and noninvasive diagnosis. Indian J. Dent. Sci. 2017, 9, 60–66. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Low, Y.K.; Chan, J.; Soraya, G.V.; Buffet, C.; Abeyrathne, C.D.; Huynh, D.H.; Skafidas, E.; Kwan, P.; Rogerson, S.J. Development of an Ultrasensitive Impedimetric Immunosensor Platform for Detection of Plasmodium Lactate Dehydrogenase. Sensors 2019, 19, 2446. https://doi.org/10.3390/s19112446
Low YK, Chan J, Soraya GV, Buffet C, Abeyrathne CD, Huynh DH, Skafidas E, Kwan P, Rogerson SJ. Development of an Ultrasensitive Impedimetric Immunosensor Platform for Detection of Plasmodium Lactate Dehydrogenase. Sensors. 2019; 19(11):2446. https://doi.org/10.3390/s19112446
Chicago/Turabian StyleLow, Yu Kong, Jianxiong Chan, Gita V. Soraya, Christelle Buffet, Chathurika D. Abeyrathne, Duc H. Huynh, Efstratios Skafidas, Patrick Kwan, and Stephen J. Rogerson. 2019. "Development of an Ultrasensitive Impedimetric Immunosensor Platform for Detection of Plasmodium Lactate Dehydrogenase" Sensors 19, no. 11: 2446. https://doi.org/10.3390/s19112446
APA StyleLow, Y. K., Chan, J., Soraya, G. V., Buffet, C., Abeyrathne, C. D., Huynh, D. H., Skafidas, E., Kwan, P., & Rogerson, S. J. (2019). Development of an Ultrasensitive Impedimetric Immunosensor Platform for Detection of Plasmodium Lactate Dehydrogenase. Sensors, 19(11), 2446. https://doi.org/10.3390/s19112446






