A New Sensor for Methyl Paraben Using an Electrode Made of a Cellulose Nanocrystal–Reduced Graphene Oxide Nanocomposite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Instrumentation
2.3. Preparation of the Nanocomposite and Modified Electrode
2.4. Electroanalytical Characterization
2.5. Electrochemical Sensing of the MP
2.6. Application of the Sensor to the Real Sample Analysis
3. Results and Discussion
3.1. Characterization of the CNC, rGO, and CNC–rGO Nanocomposite
3.2. Electroanalytical Characterization of the Modified Electrode
3.3. Electroanalytical Behavior of the MP Sensor
3.4. Optimization of the Nanocomposite CNC-rGO Modified Electrode
3.4.1. Reproducibility, Repeatability, and Stability Studies
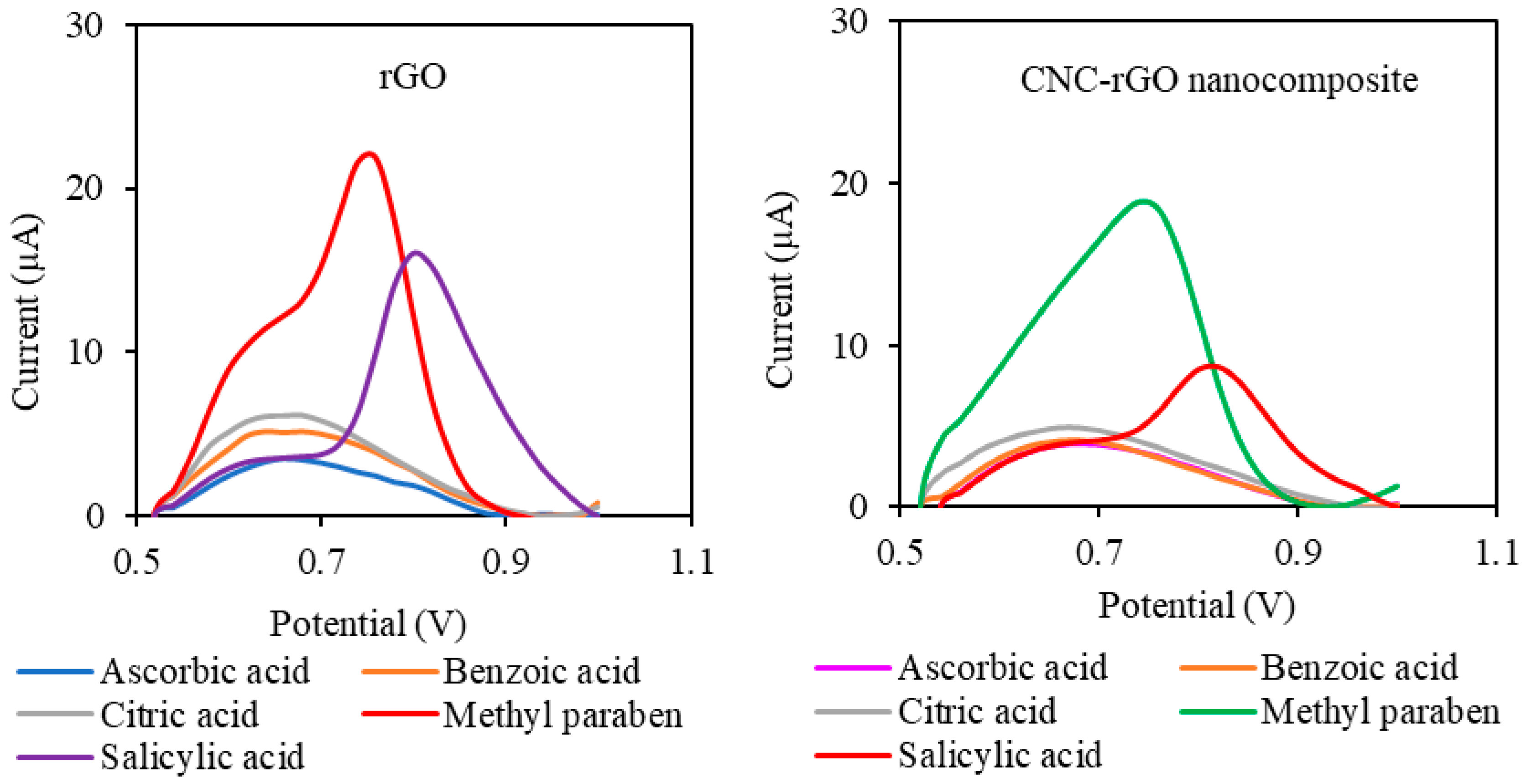
3.4.2. Selectivity Study and Real Sample Analysis
3.5. Comparison of the Developed Sensor with Other Carbon-Based Paraben Sensors
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Vela-Soria, F.; Rodríguez, I.; Ballesteros, O.; Zafra-Gómez, A.; Ballesteros, L. Simplified matrix solid phase dispersion procedure for the determination of parabens and benzophenone-ultraviolet filters in human placental tissue samples. J. Chromatogr. A 2014, 1371, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.; Basheer, C.; Narasimhan, K.; Choolani, M.; Lee, H.K. Application of microwave-assisted micro-solid-phase extraction for determination of parabens in human ovarian cancer tissues. J. Chromatogr. B 2015, 1000, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Darbre, P.D.; Aljarrah, A.; Miller, W.R.; Coldham, N.G.; Sauer, M.J.; Pope, G.S. Concentrations of parabens in human breast tumours. J. Appl. Toxicol. 2004, 24, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Rodas, M.; Portugal, L.A.; Avivar, J.; Estela, J.M.; Cerda, V. Parabens determination in cosmetic and personal care products exploiting a multi-syringe chromatographic (MSC) system and chemiluminescent detection. Talanta 2015, 143, 254–262. [Google Scholar] [CrossRef]
- Farajzadeh, M.A.; Djozan, D.; Bakhtiyari, R.F. Use of a capillary tube for collecting an extraction solvent lighter than water after dispersive liquid-liquid microextraction and its application in the determination of parabens in different samples by gas chromatography-Flame ionization detection. Talanta 2010, 81, 1360–1367. [Google Scholar] [CrossRef]
- Ye, X.; Bishop, A.M.; Needham, L.L.; Calafat, A.M. Automated on-line column-switching HPLC-MS/MS method with peak focusing for measuring parabens, triclosan, and other environmental phenols in human milk. Anal. Chim. Acta 2008, 622, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Sako, A.V.F.; Dolzan, M.D.; Micke, G.A. Fast and sensitive method to determine parabens by capillary electrophoresis using automatic reverse electrode polarity stacking mode: Application to hair samples. Anal. Bioanal. Chem. 2015, 407, 7333–7339. [Google Scholar] [CrossRef]
- Gholivand, M.B.; Shamsipur, M.; Dehdashtian, S.; Rajabi, H.R. Development of a selective and sensitive voltammetric sensor for propylparaben based on a nanosized molecularly imprinted polymer-carbon paste electrode. Mater. Sci. Eng. C 2014, 36, 102–107. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, Y.; Fang, C.; Gong, Q. Electrochemical sensor for parabens based on molecular imprinting polymers with dual-templates. Anal. Chim. Acta 2010, 673, 145–150. [Google Scholar] [CrossRef]
- Rather, J.A.; Al Harthi, A.J.; Khudaish, E.A.; Qurashi, A.; Munam, A.; Kannan, P. An electrochemical sensor based on fullerene nanorods for the detection of paraben, an endocrine disruptor. Anal. Methods 2016, 8, 5690–5700. [Google Scholar] [CrossRef]
- Qurashi, A.; Rather, J.A.; Yamazaki, T.; Sohail, M.; De Wael, K.; Merzougui, B.; Hakeem, A.S. Swift electrochemical detection of paraben an endocrine disruptor by In2O3 nanobricks. Sens. Actuators B Chem. 2015, 221, 167–171. [Google Scholar] [CrossRef]
- Hajian, A.; Ghodsi, J.; Afraz, A.; Yurchenko, O.; Urban, G. Nanomolar detection of methylparaben by a cost-effective hemoglobin-based biosensor. Mater. Sci. Eng. C 2016, 69, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Kajornkavinkul, S.; Punrat, E.; Siangproh, W.; Rodthongkum, N.; Praphairaksit, N.; Chailapakul, O. Graphene/polyvinylpyrrolidone/polyaniline nanocomposite-modified electrode for simultaneous determination of parabens by high performance liquid chromatography. Talanta 2016, 148, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Piovesan, J.V.; Santana, E.R.; Spinelli, A. Reduced graphene oxide/gold nanoparticles nanocomposite-modified glassy carbon electrode for determination of endocrine disruptor methylparaben. J. Electroanal. Chem. 2018, 813, 163–170. [Google Scholar] [CrossRef]
- Sasseville, D.; Alfalah, M.; Lacroix, J.-P. “Parabenoia” debunked, or “Who’s afraid of parabens?”. Dermatitis 2015, 26, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Sadasivuni, K.K.; Kafy, A.; Zhai, L.; Ko, H.-U.; Mun, S.; Kim, J. Supporting—Transparent and Flexible Cellulose Nanocrystal/Reduced Graphene Oxide Film for Proximity Sensing. Small 2014, 11, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Kafy, A.; Akther, A.; Shishir, M.I.R.; Kim, H.C.; Yun, Y.; Kim, J. Cellulose nanocrystalgraphene oxide composite film as humidity sensor. Sens. Actuators A Phys. 2016. [Google Scholar] [CrossRef]
- Di Mauro, A.; Cantarella, M.; Nicotra, G.; Pellegrino, G.; Gulino, A.; Brundo, M.V.; Privitera, V.; Impellizzeri, G. Novel synthesis of ZnO/PMMA nanocomposites for photocatalytic applications. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Wang, J.; Gao, W.; Zhang, H.; Zou, M.; Chen, Y.; Zhao, Y. Programmable wettability on photocontrolled graphene film. Sci. Adv. 2018, 4, 1–6. [Google Scholar] [CrossRef]
- Zainuddin, N.; Ahmad, I.; Kargarzadeh, H.; Ramli, S. Hydrophobic kenaf nanocrystalline cellulose for the binding of curcumin. Carbohydr. Polym. 2017, 163, 261–269. [Google Scholar] [CrossRef]
- Zaman, M.; Xiao, H.; Chibante, F.; Ni, Y. Synthesis and characterization of cationically modified nanocrystalline cellulose. Carbohydr. Polym. 2012, 89, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cao, W.; Grishkewich, N.; Berry, R.M.; Tam, K.C. Synthesis and characterization of pH-responsive and fluorescent poly (amidoamine) dendrimer-grafted cellulose nanocrystals. J. Colloid Interface Sci. 2015, 450, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.-G.; Yan, D.-X.; Ji, X.; Chen, T.; Li, Z.-M. Temperature dependence of graphene oxide reduced by hydrazine hydrate. Nanotechnology 2011, 22, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.-J.; Kong, F.-Y.; Chen, Z.-D.; Fan, D.-H.; Fang, H.-L.; Wang, W. Reduced graphene oxide-Hemin-Au nanohybrids: Facile one-pot synthesis and enhanced electrocatalytic activity towards the reduction of hydrogen peroxide. Biosens. Bioelectron. 2016, 78, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cheng, Y.; Zhou, Y.; Li, B.; Gu, W.; Shi, X.; Xian, Y. Electrochemical sensor for bisphenol A based on magnetic nanoparticles decorated reduced graphene oxide. Talanta 2013, 107, 211–218. [Google Scholar] [CrossRef]
- Yu, L.; Wang, L.; Xu, W.; Chen, L.; Fu, M.; Wu, J.; Ye, D. Adsorption of VOCs on reduced graphene oxide. J. Environ. Sci. 2018, 67, 171–178. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Mariano, M.; Gopakumar, D.; Ahmad, I.; Thomas, S.; Dufresne, A.; Huang, J.; Lin, N. Advances in cellulose nanomaterials. Cellulose 2018, 25, 2151–2189. [Google Scholar] [CrossRef]
- Nia, P.M.; Meng, W.P.; Lorestani, F.; Mahmoudian, M.R.; Alias, Y. Electrodeposition of copper oxide/polypyrrole/reduced graphene oxide as a nonenzymatic glucose biosensor. Sens. Actuators B Chem. 2015, 209, 100–108. [Google Scholar]
- Li, X.; Zhao, H.; Shi, L.; Zhu, X.; Lan, M.; Zhang, Q.; Fan, Z.H. Electrochemical sensing of nicotine using screen-printed carbon electrodes modified with nitrogen-doped graphene sheets. J. Electroanal. Chem. 2017, 784, 77–84. [Google Scholar] [CrossRef]
- Gai, P.-P.; Zhao, C.-E.; Wang, Y.; Abdel-Halim, E.S.; Zhang, J.-R.; Zhu, J.-J. NADH dehydrogenase-like behavior of nitrogen-doped graphene and its application in NAD+-dependent dehydrogenase biosensing. Biosens. Bioelectron. 2014, 62, 170–176. [Google Scholar] [CrossRef]
- El Miri, N.; El Achaby, M.; Fihri, A.; Larzek, M.; Zahouily, M.; Abdelouahdi, K.; Barakat, A.; Solhy, A. Synergistic effect of cellulose nanocrystals/graphene oxide nanosheets as functional hybrid nanofiller for enhancing properties of PVA nanocomposites. Carbohydr. Polym. 2016, 137, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Mariano, M.; El Kissi, N.; Dufresne, A. Cellulose nanocrystals and related nanocomposites: Review of some properties and challenges. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 791–806. [Google Scholar] [CrossRef]
- Rezaei, B.; Khalili Boroujeni, M.; Ensafi, A.A. Caffeine electrochemical sensor using imprinted film as recognition element based on polypyrrole, sol-gel, and gold nanoparticles hybrid nanocomposite modified pencil graphite electrode. Biosens. Bioelectron. 2014, 60, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Zhang, X.; Ren, S.; Lei, T.; Sun, X.; Qi, Y.; Wu, Q. Poly(diallyldimethylammonium chloride)–cellulose nanocrystals supported Au nanoparticles for nonenzymatic glucose sensing. RSC Adv. 2016, 6, 6436–6442. [Google Scholar] [CrossRef]
- Sun, G.; Zhang, L.; Zhang, Y.; Yang, H.; Ma, C.; Ge, S.; Yan, M.; Yu, J.; Song, X. Multiplexed enzyme-free electrochemical immunosensor based on ZnO nanorods modified reduced graphene oxide-paper electrode and silver deposition-induced signal amplification strategy. Biosens. Bioelectron. 2015, 71, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, M.; Tomar, M.; Gupta, V. Glad assisted synthesis of NiO nanorods for realization of enzymatic reagentless urea biosensor. Biosens. Bioelectron. 2014, 52, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Azzouzi, S.; Rotariu, L.; Benito, A.M.; Maser, W.K.; Ali, M.B.; Bala, C. A novel amperometric biosensor based on gold nanoparticles anchored on reduced graphene oxide for sensitive detection of L-lactate tumor biomarker. Biosens. Bioelectron. 2015, 69, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.; Scontri, M.; Materon, E.M.; Lanza, M.R.V.; Sotomayor, M.D.P.T. Development and application of an electrochemical sensor modified with multi-walled carbon nanotubes and graphene oxide for the sensitive and selective detection of tetracycline. J. Electroanal. Chem. 2015, 757, 250–257. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, J.; Céspedes, F.; Baeza, M. Modified multiwalled carbon nanotube/epoxy amperometric nanocomposite sensors with CuO nanoparticles for electrocatalytic detection of free chlorine. Microchem. J. 2015, 122, 189–196. [Google Scholar] [CrossRef]
- Cinti, S.; Minotti, C.; Moscone, D.; Palleschi, G.; Arduini, F. Fully integrated ready-to-use paper-based electrochemical biosensor to detect nerve agents. Biosens. Bioelectron. 2017, 93, 46–51. [Google Scholar] [CrossRef]
- Gomes, F.E.R.; de Souza, N.E.; Galinaro, C.A.; Arriveti, L.O.R.; de Assis, J.B.; Tremiliosi-Filho, G. Electrochemical degradation of butyl paraben on platinum and glassy carbon electrodes. J. Electroanal. Chem. 2016, 769, 124–130. [Google Scholar] [CrossRef]
- Niu, L.M.; Lian, K.Q.; Shi, H.M.; Wu, Y.B.; Kang, W.J.; Bi, S.Y. Characterization of an ultrasensitive biosensor based on a nano-Au/DNA/nano-Au/poly(SFR) composite and its application in the simultaneous determination of dopamine, uric acid, guanine, and adenine. Sens. Actuators B Chem. 2013, 178, 10–18. [Google Scholar] [CrossRef]
- Pan, D.; Gu, Y.; Lan, H.; Sun, Y.; Gao, H. Functional graphene-gold nano-composite fabricated electrochemical biosensor for direct and rapid detection of bisphenol A. Anal. Chim. Acta 2015, 853, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Govindhan, M.; Amiri, M.; Chen, A. Au nanoparticle/graphene nanocomposite as a platform for the sensitive detection of NADH in human urine. Biosens. Bioelectron. 2015, 66, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Sha, R.; Puttapati, S.K.; Srinkanth, V.V.; Badhulika, S. Ultra-sensitive phenol sensor based on overcoming surface fouling of reduced graphene oxide-zinc oxide composite electrode. J. Electroanal. Chem. 2017, 785, 26–32. [Google Scholar] [CrossRef]
- Fotouhi, L.; Fatollahzadeh, M.; Heravi, M.M. Electrochemical behavior and voltammetric determination of sulfaguanidine at a glassy carbon electrode modified with a multi-walled carbon nanotube. Int. J. Electrochem. Sci. 2012, 7, 3919–3928. [Google Scholar]
- Li, Y.; Wang, H.; Liu, X.; Guo, L.; Ji, X.; Wang, L.; Tian, D.; Yang, X. Nonenzymatic nitrite sensor based on a titanium dioxide nanoparticles/ionic liquid composite electrode. J. Electroanal. Chem. 2014, 719, 35–40. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Q.; Zhang, M.; Zhang, S.; Zhang, L. An electrochemical fungicide pyrimethanil sensor based on carbon nanotubes/ionic-liquid construction modified electrode. Food Chem. 2015, 187, 1–6. [Google Scholar] [CrossRef]
- Ghanbari, K.; Hajian, A. Electrochemical characterization of Au/ZnO/PPy/RGO nanocomposite and its application for simultaneous determination of ascorbic acid (AA), epinephrine (EP) and uric acid (UA). J. Electroanal. Chem. 2017. [Google Scholar] [CrossRef]
- Shetti, N.P.; Nayak, D.S.; Malode, S.J.; Kulkarni, R.M. Electrochemical sensor based upon ruthenium doped TiO2 nanoparticles for the determination of flufenamic acid. J. Electrochem. Soc. 2017, 164, 3036–3042. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Li, G.; Ye, B. A new strategy for enhancing electrochemical sensing from MWCNTs modified electrode with Langmuir-Blodgett film and used in determination of methylparaben. Sens. Actuators B Chem. 2015, 211, 332–338. [Google Scholar] [CrossRef]
- Muhammad, A.; Yusof, N.A.; Hajian, R.; Abdullah, J. Construction of an electrochemical sensor based on carbon nanotubes/gold nanoparticles for trace determination of amoxicillin in bovine milk. Sensors 2016, 16, 56. [Google Scholar] [CrossRef] [PubMed]
- Feier, B.; Blidar, A.; Pusta, A.; Carciuc, P.; Cristea, C. Electrochemical sensor based on molecularly imprinted polymer for the detection of Cefalexin. Biosensors 2019, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Gualandi, I.; Tonelli, D.; Mariani, F.; Scavetta, E.; Marzocchi, M.; Fraboni, B. Selective detection of dopamine with an all PEDOT:PSS organic electrochemical transistor. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hassaninejad-Darzi, S.K.; Rahimnejad, M.; Mirzababaei, S.N. Electrocatalytic oxidation of glucose onto carbon paste electrode modified with nickel hydroxide decorated NaA nanozeolite. Microchem. J. 2016, 128, 7–17. [Google Scholar] [CrossRef]
- Tang, J.; Song, Y.; Berry, R.M.; Tam, K.C. Polyrhodanine coated cellulose nanocrystals as optical pH indicators. RSC Adv. 2014, 60249–60252. [Google Scholar] [CrossRef]
- Wang, F.; Drzal, L.T.; Qin, Y.; Huang, Z. Multifunctional graphene nanoplatelets/cellulose nanocrystals composite paper. Compos. Part B 2015, 79, 521–529. [Google Scholar] [CrossRef] [Green Version]
- Ulianas, A.; Yook Heng, L.; Ahmad, M.; Lau, H.-Y.; Ishak, Z.; Ling Ling, T. A regenerable screen-printed DNA biosensor based on acrylic microsphere-gold nanoparticle composite for genetically modified soybean determination. Sens. Actuators B Chem. 2014, 190, 694–701. [Google Scholar] [CrossRef]
- Behpour, M.; Masoum, S.; Lalifar, A.; Khoobi, A. A novel method based on electrochemical approaches and multivariate calibrations for study and determination of methylparaben in the presence of unexpected interference in cosmetics. Sens. Actuators B Chem. 2015, 214, 10–19. [Google Scholar] [CrossRef]
- Noorashikin, M.S.; Raoov, M.; Mohamad, S.; Abas, M.R. Extraction of parabens from water samples using cloud point extraction with a non-ionic surfactant with β-cyclodextrin as modifier. J. Surfactants Deterg. 2014, 17, 747–758. [Google Scholar] [CrossRef]
- Noorashikin, M.S.; Mohamad, S.; Abas, M.R. Extraction and determination of parabens in water samples using an aqueous two-phase system of ionic liquid and salts with beta-cyclodextrin as the modifier coupled with high performance liquid chromatography. Anal. Methods 2014, 6, 419–425. [Google Scholar] [CrossRef]
- Dai, H.; Xu, G.; Gong, L.; Yang, C.; Lin, Y.; Tong, Y.; Chen, J.; Chen, G. Electrochemical detection of triclosan at a glassy carbon electrode modifies with carbon nanodots and chitosan. Electrochim. Acta 2012, 80, 362–367. [Google Scholar] [CrossRef]
- Haldorai, Y.; Kim, J.Y.; Vilian, A.T.E.; Heo, N.S.; Huh, Y.S.; Han, Y.-K. An enzyme-free electrochemical sensor based on reduced graphene oxide/Co3O4 nanospindle composite for sensitive detection of nitrite. Sens. Actuators B Chem. 2016, 227, 92–99. [Google Scholar] [CrossRef]
- Ruecha, N.; Rangkupan, R.; Rodthongkum, N.; Chailapakul, O. Novel paper-based cholesterol biosensor using graphene/polyvinylpyrrolidone/polyaniline nanocomposite. Biosens. Bioelectron. 2014, 52, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Wu, H.; Li, K.; Wang, Y.; Tao, X.; Yang, H.; Li, A.; Cheng, R. Influence of the surface structure of graphene oxide on the adsorption of aromatic organic compounds from water. ACS Appl. Mater. Interfaces 2015, 7, 6690–6697. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Tripathy, N.; Jang, N.K.; Khang, G.; Hahn, Y.-B. Fabrication of highly sensitive uric acid biosensor based on directly grown ZnO nanosheets on electrode surface. Sens. Actuators B Chem. 2015, 206, 146–151. [Google Scholar] [CrossRef]
- Cha, N.-R.; Lee, J.-K.; Jeong, H.-J.; Cho, J.-C.; Kim, M.-J.; Lee, S.-Y. Determination of 19 preservatives in various matrices by high-performance liquid chromatography. Anal. Lett. 2012, 45, 2148–2160. [Google Scholar] [CrossRef]
- Marengo, E.; Gennaro, M.C.; Gianotti, V. A simplex-optimized chromatographic separation of fourteen cosmetic preservatives: Analysis of commercial products. J. Chromatogr. Sci. 2001, 339–344. [Google Scholar] [CrossRef]
- Torriero, A.A.J.; Luco, J.M.; Sereno, L.; Raba, J. Voltammetric determination of salicylic acid in pharmaceuticals formulations of acetylsalicylic acid. Talanta 2004, 62, 247–254. [Google Scholar] [CrossRef]
- Naik, K.M.; Nandibewoor, S.T. Electroanalytical method for the determination of methylparaben. Sens. Actuators A Phys. 2014, 212, 127–132. [Google Scholar] [CrossRef]
- Peng, C.; Chow, A.H.L.; Chan, C.K. Hygroscopic study of glucose, citric acid, and sorbitol using an electrodynamic balance: Comparison with UNIFAC predictions. Aerosol Sci. Technol. 2001, 35, 753–758. [Google Scholar] [CrossRef]
- He, B.S.; Zhang, J.X. Rapid detection of ascorbic acid based on a dual-electrode sensor system using a powder microelectrode embedded with carboxyl multi-walled carbon nanotubes. Sensors 2017, 17, 1549. [Google Scholar]
- Baytak, A.K.; Duzmen, S.; Teker, T.; Aslanoglu, M. Voltammetric determination of methylparaben and its DNA interaction using a novel platform based on carbon nanofibers and cobalt-nickel-palladium nanoparticles. Sens. Actuators B Chem. 2017, 239, 330–337. [Google Scholar] [CrossRef]
- Mendonça, C.D.; Prado, T.M.; Cincotto, F.H.; Verbinnen, R.T.; Machado, S.A.S. Methylparaben quantification via electrochemical sensor based on reduced graphene oxide decorated with ruthenium nanoparticles. Sens. Actuators B Chem. 2017, 251, 739–745. [Google Scholar] [CrossRef]
- Baytak, A.K.; Teker, T.; Duzmen, S.; Aslanoglu, M. A novel electrochemical platform based on carbon nanofibers and tri-metallic nanoparticles of gold, nickel and cobalt for the quantification of ethyl paraben. Mater. Sci. Eng. C 2017, 72, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Indrasti, D.; Lioe, H.N.; Adawiyah, D.R. Evaluation of methyl paraben content in sweet soy-sauce in commercial instant fried noodle. In Proceedings of the 13th ASEAN Food Conference, Singapore, 9–11 September 2013; pp. 1–7. [Google Scholar]
- Tsvetkova, B.; Peikova, L. HPLC method for simultaneous determination of ketoprofen and preservatives in gel formulation. Int. J. Pharm. Pharm. Sci. 2014, 6, 199–202. [Google Scholar]
- Soni, M.G.; Taylor, S.L.; Greenberg, N.A.; Burdock, G.A. Evaluation of the health aspects of methyl paraben: A review of the published literature. Food Chem. Toxicol. 2002, 40, 1335–1373. [Google Scholar] [CrossRef]









| Interference | Current (µA) | |||||||
|---|---|---|---|---|---|---|---|---|
| rGO | CNC–rGO Nanocomposite | |||||||
| 1:1 | tcalculated | 1:10 | tcalculated | 1:1 | tcalculated | 1:10 | tcalculated | |
| MP | 23.43 ± 1.36 | - | 36.10 ± 1.41 | - | 18.11 ± 2.99 | - | 18.81 ± 1.65 | - |
| MP + ascorbic acid | 29.22 ± 1.58 | 1.000 | 29.59 ± 2.40 * | 2.97 | 17.03 ± 1.20 | 1.05 | 11.83 ± 1.44 | 1.33 |
| MP + salicylic acid | 25.32 ± 6.30 | 0.590 | 26.86 ± 3.56 * | 3.24 | 17.22 ± 0.55 | 0.47 | 20.51 ± 1.21 | 1.18 |
| MP + benzoic acid | 23.41 ± 4.12 | 0.005 | 33.10 ± 0.62 * | 5.59 | 17.34 ± 1.74 | 0.30 | 15.47 ± 1.35 | 1.41 |
| MP + citric acid | 23.61 ± 2.29 | 0.086 | 34.81 ± 3.22 | 0.74 | 16.82 ± 0.38 | 0.36 | 16.85 ± 1.92 | 2.35 |
| Linear Range without Any Addition of Salicylic Acid | Linear Range with Addition of Salicylic Acid | *tcalculated | ||
|---|---|---|---|---|
| Concentration | Current (µA) ± SD | Concentration | Current (µA) ± SD | |
| 3 × 10−4 M MP | 12.27 ± 1.00 | 3 × 10−4 M MP + 3 × 10−4 M SA | 10.56 ± 1.28 | 1.32 |
| 5 × 10−4 M MP | 17.93 ± 1.78 | 5 × 10−4 M MP + 5 × 10−4 M SA | 15.80 ± 2.88 | 0.84 |
| Analyte | Detection Method (V) | Sensing Materials | Linear Range (mM) | LOD (mM) | Reference |
|---|---|---|---|---|---|
| MP | DPV (+0.75) | Nanocomposite CNC–rGO | 2.00 × 10−4 to 9.00 × 10−4 | 1.00 × 10−4 | This work |
| MP | Square wave voltammetry (SWV) (+0.768) | Carbon nanofibers (CNFs) and nickel–cobalt–palladium nanoparticles, (Co-Ni-Pd)NPs-CNFs/GC | 3.00 × 10−9 to 3.00 × 10−7 | 1.20 × 10−9 | [73] |
| EP | SWV (+0.7) | Fullerene nanorod modified glassy carbon electrode (C60NRs–NH–Ph–GCE) | 1.00 × 10−8 to 5.20 × 10−7 | 3.80 × 10−9 | [10] |
| MP | DPV (+0.78) | rGO decorated with ruthenium nanoparticles | 5.00 × 10−7 to 3.00 × 10−6 | 2.40 × 10−7 | [74] |
| MP | SWV (+1.0) | rGO/gold nanoparticle (AuNP) nanocomposite | 3.00 × 10−8 to 1.30 × 10−6 | 1.38 × 10−8 | [14] |
| EP | SWV (+0.76) | Composite of CNFs and tri-metallic nanoparticles of gold, cobalt, and nickel (Au-Ni-Co)NPs-CNFs/GCE | 1.00 × 10−9 to 1.00 × 10−7 | 3.50 × 10−10 | [75] |
| MP | Linear sweep voltammetry (LSV) (>+1.0) | Langmuir–Blodgett (LB) film of multi-walled carbon nanotubes (MWCNTs) perpendicularly modified glassy carbon electrode (GCE), (MWCNTs-LB/GCE), | 1.00 × 10−6 to 8.00 × 10−5 | 4.00 × 10−7 | [51] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan Khalid, W.E.F.; Mat Arip, M.N.; Jasmani, L.; Lee, Y.H. A New Sensor for Methyl Paraben Using an Electrode Made of a Cellulose Nanocrystal–Reduced Graphene Oxide Nanocomposite. Sensors 2019, 19, 2726. https://doi.org/10.3390/s19122726
Wan Khalid WEF, Mat Arip MN, Jasmani L, Lee YH. A New Sensor for Methyl Paraben Using an Electrode Made of a Cellulose Nanocrystal–Reduced Graphene Oxide Nanocomposite. Sensors. 2019; 19(12):2726. https://doi.org/10.3390/s19122726
Chicago/Turabian StyleWan Khalid, Wan Elina Faradilla, Mohamad Nasir Mat Arip, Latifah Jasmani, and Yook Heng Lee. 2019. "A New Sensor for Methyl Paraben Using an Electrode Made of a Cellulose Nanocrystal–Reduced Graphene Oxide Nanocomposite" Sensors 19, no. 12: 2726. https://doi.org/10.3390/s19122726





