First Principles Simulations of Phenol and Methanol Detector Based on Pristine Graphene Nanosheet and Armchair Graphene Nanoribbons
Abstract
:1. Introduction
2. Materials and Methods
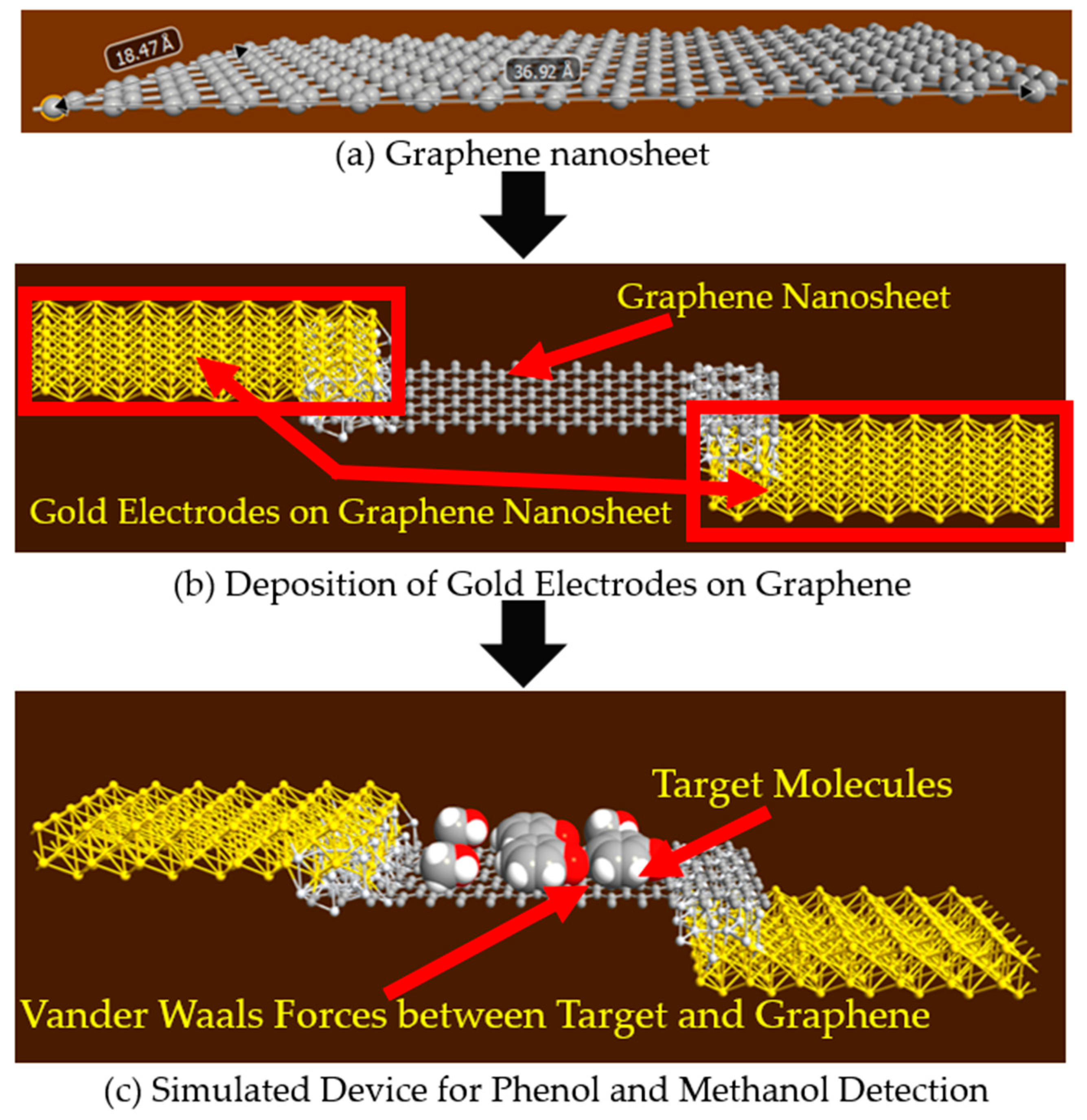
2.1. Graphene Nanosheet for the Detection of Phenol and Methanol Molecules
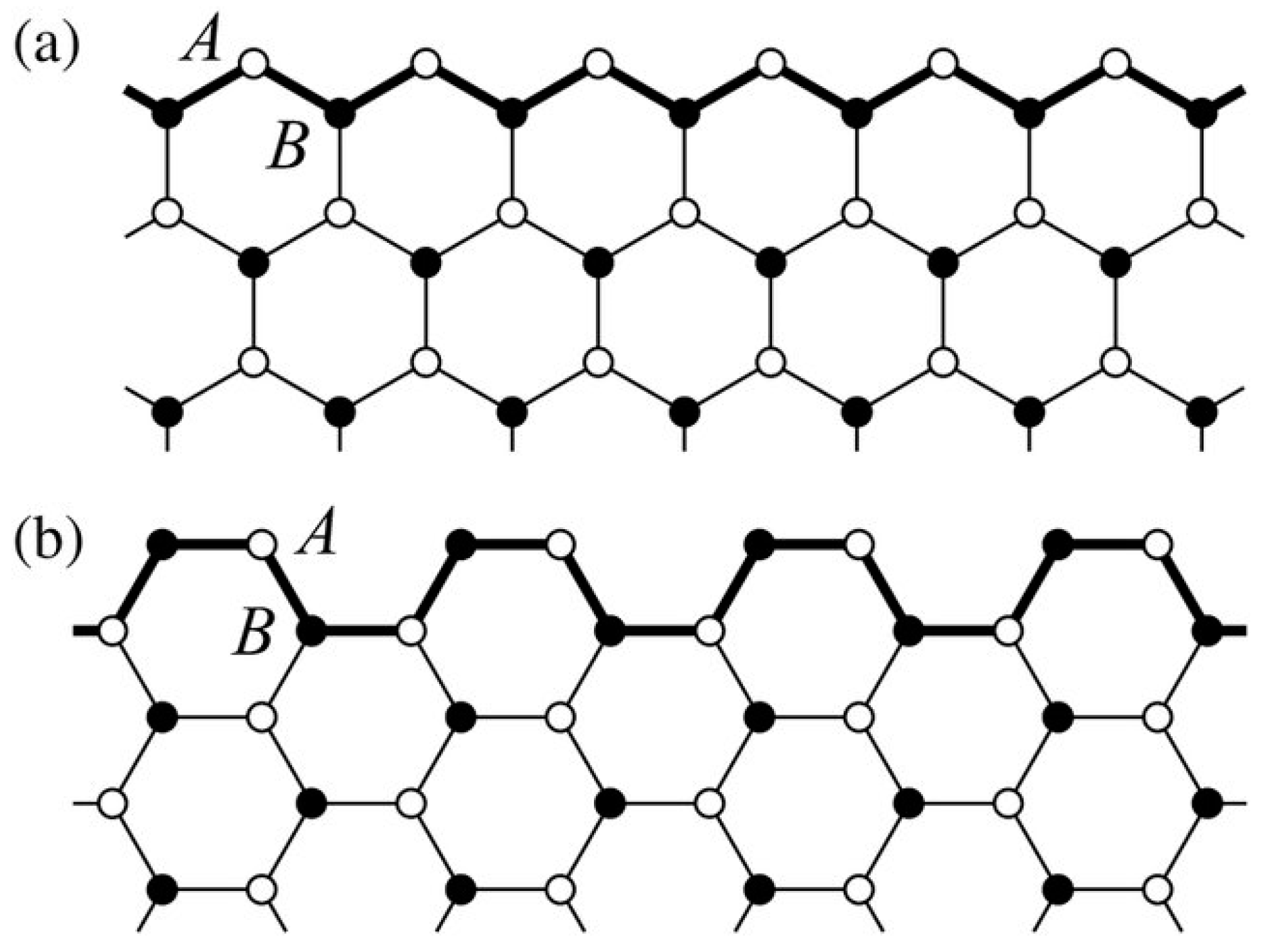
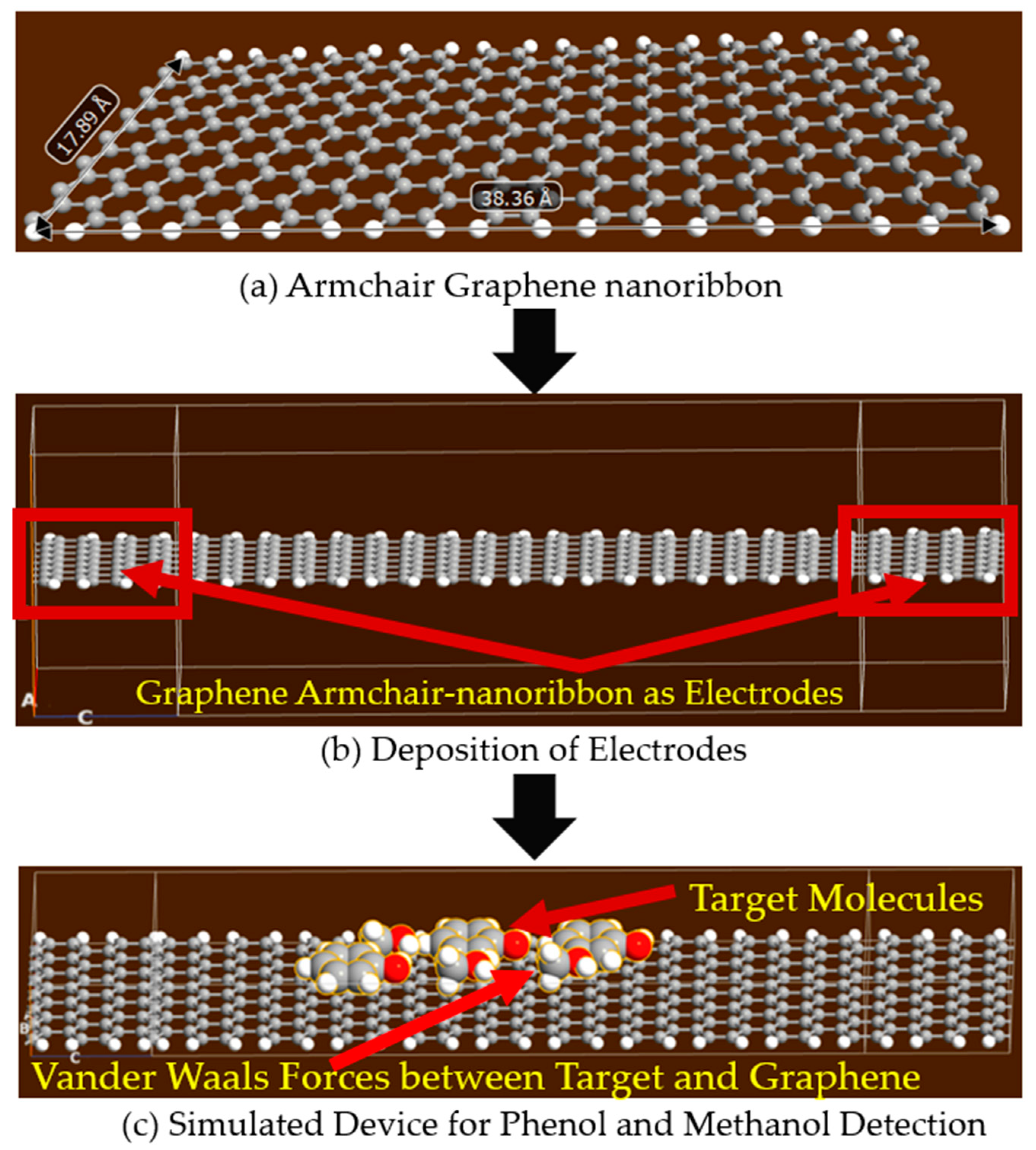
2.2. Armchair Graphene Nanoribbon (AGNR) for the Detection of Phenol and Methanol Molecules
2.3. Methodology for Simulated Graphene Nanosheet and Armchair Graphene Nanoribbon Based Devices
3. Results and Discussions
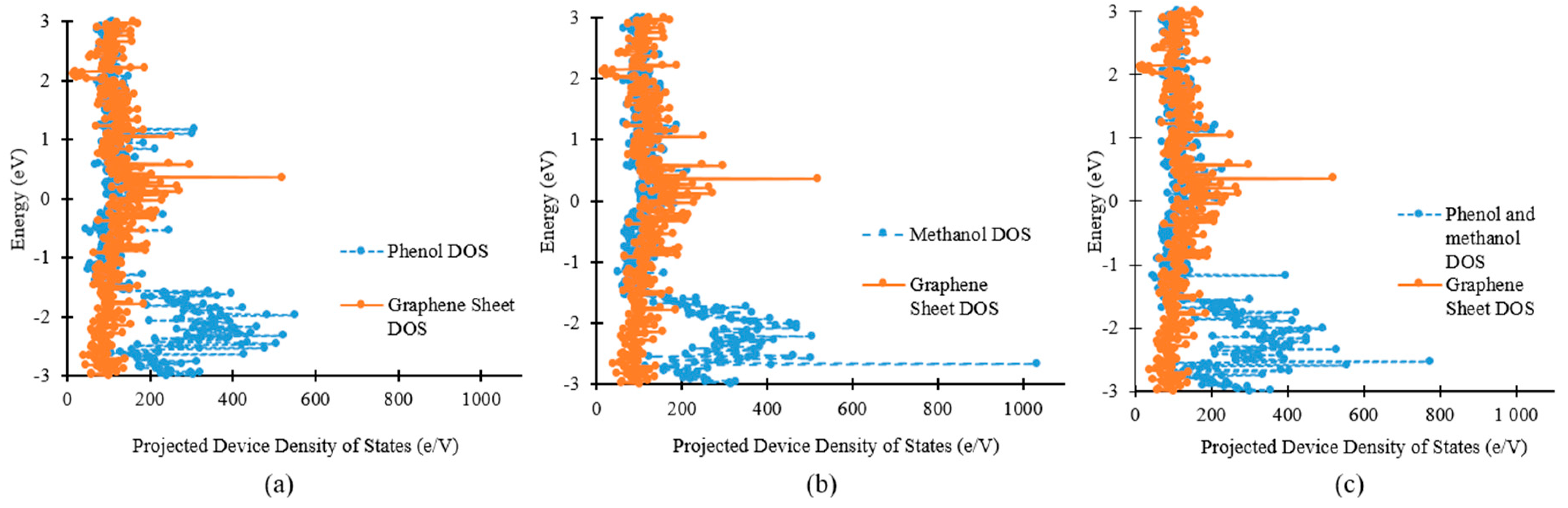
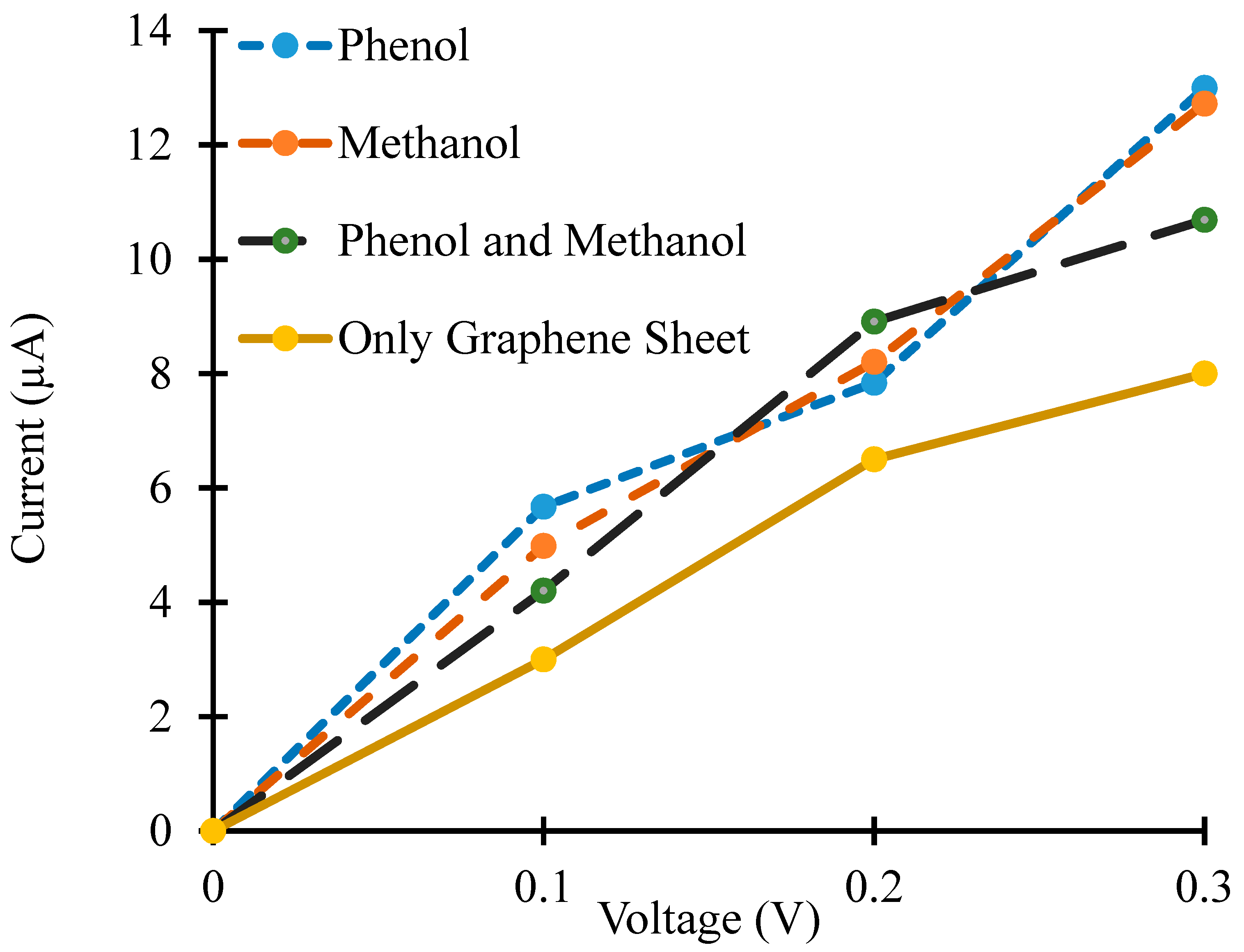
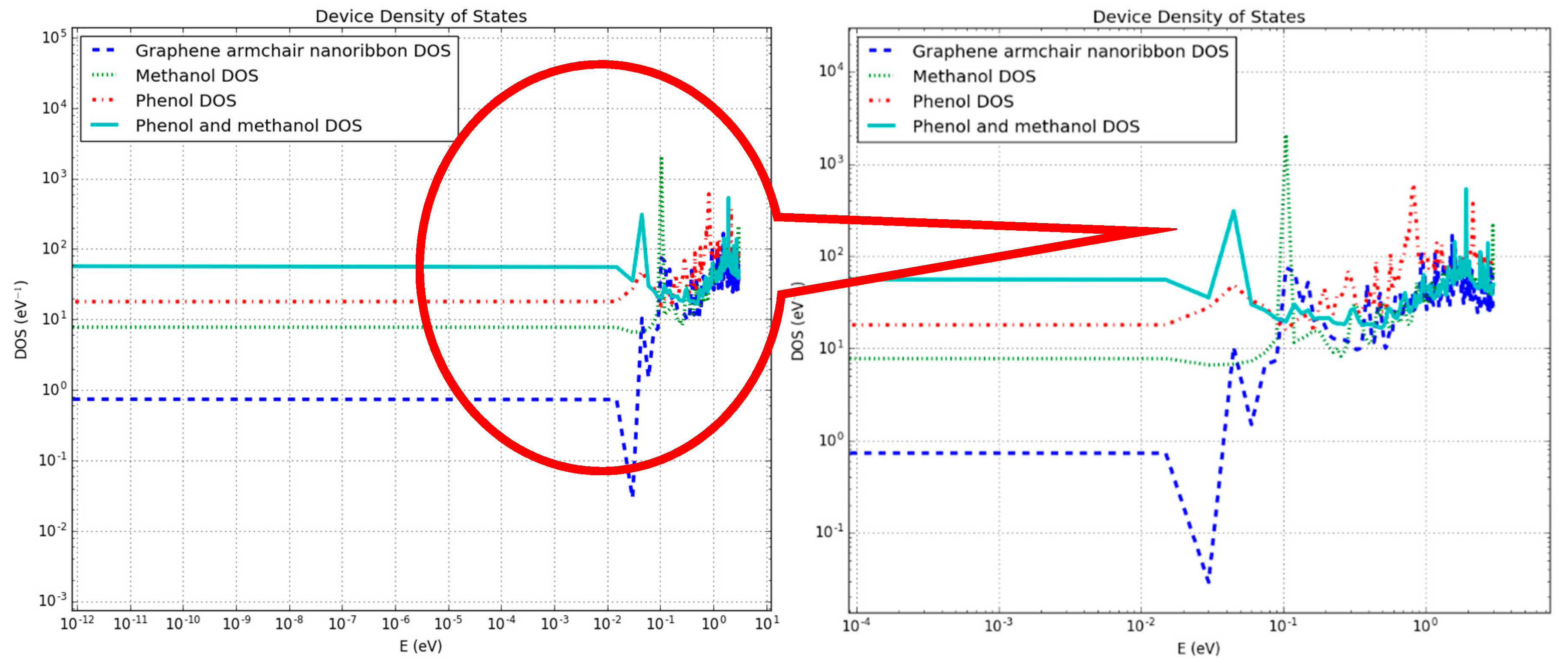
3.1. Density of States and Current–voltage Characteristics Analysis of the Graphene Nanosheet Based Device
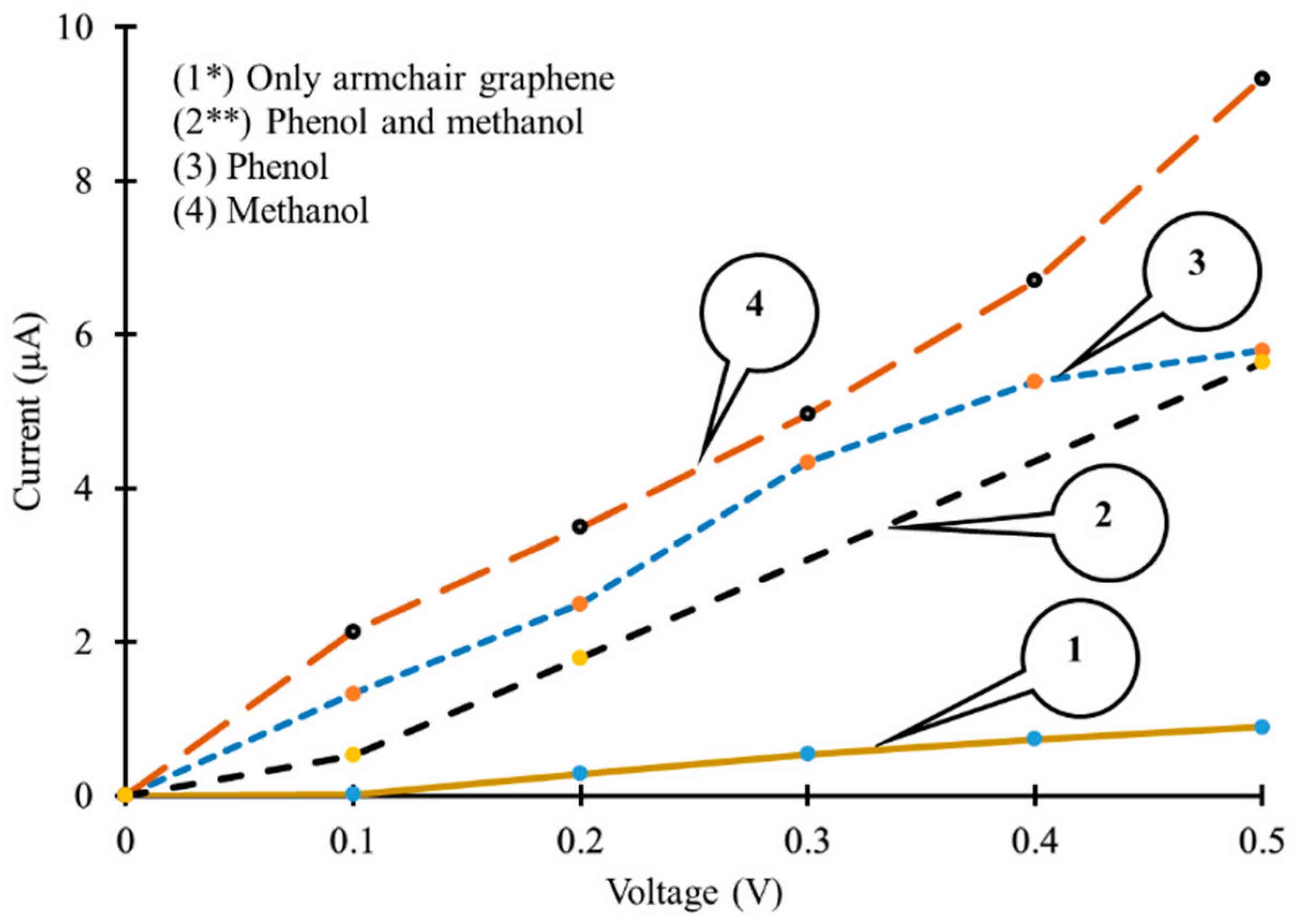
3.2. Density of States and Current–Voltage Characteristics Analysis of Armchair Graphene Nanoribbon Based Device
PhotoCurrent Analysis of an Armchair Graphene Nanoribbon Based Device in the Proximity of Different Target Molecules
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Andre, R.S.; Mercante, L.A.; Facure, M.H.; Mattoso, L.H.C. Enhanced and selective amonia detection. Appl. Surf. Sci. 2019, 473, 133–140. [Google Scholar] [CrossRef]
- Costa, S.; Ferreira, J.; Silveira, C.; Costa, C. Integrating health on air quality assessment―Review report on health risks of two major european outdoor air pollutants: PM and NO2. J. Toxicol. Environ. Heal. Part B Crit. Rev. 2014, 17, 307–340. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.G.; Chatterjee, S.; Ray, A.K.; Chakraborty, A.K. Graphene-metal oxide nanohybrids for toxic gas sensor: A review. Sens. Actuators B Chem. 2015, 221, 1170–1181. [Google Scholar] [CrossRef]
- Hong, X.; Wang, J.; Hai, Z. Discrimination and prediction of multiple beef freshness indexes based on electronic nose. Sens. Actuators B Chem. 2012, 161, 381–389. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, J.; Jin, W. Evaluation of varieties of set yogurts and their physical properties using a voltammetric electronic tongue based on various potential waveforms. Sens. Actuators B Chem. 2013, 177, 684–694. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Ye, S.; Chang, M. Application of electronic nose and statistical analysis to predict quality indices of peach. Food Bioprocess Technol. 2012, 5, 65–72. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, J. Tracing floral and geographical origins of honeys by potentiometric and voltammetric electronic tongue. Comput. Electron. Agric. 2014, 108, 112–122. [Google Scholar] [CrossRef]
- Marinho, B.; Ghislandi, M.; Tkalya, E.; Koning, C.E.; de With, G. Electrical conductivity of compacts of graphene, multi-wall carbon nanotubes, carbon black, and graphite powder. Powder Technol. 2012, 221, 351–358. [Google Scholar] [CrossRef]
- Wright, A.R.; Xu, X.G.; Cao, J.C.; Zhang, C. Strong nonlinear optical response of graphene in the terahertz regime. Appl. Phys. Lett. 2009, 95, 93–96. [Google Scholar] [CrossRef]
- Chai, G.; Chen, Q. Characterisation study of the thermal conductivity of carbon nanotube copper nanocomposites. J. Compos. Mater. 2010, 44, 2863–2873. [Google Scholar] [CrossRef]
- Yavari, F.; Koratkar, N. Graphene-based chemical sensors. J. Phys. Chem. Lett. 2012, 3, 1746–1753. [Google Scholar] [CrossRef] [PubMed]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselvo, K.S. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Lahiri, I.; Seelaboyina, R.; Kang, Y.S. Synthesis of graphene and its applications: A review. Crit. Rev. Solid State Mater. Sci. 2010, 35, 52–71. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, T.; Girit, C.; Hao, Z.; Martin, M.C.; Zettl, A.; Crommie, M.F.; Shen, Y.R.; Wang, F. Direct observation of a widely tunable bandgap in bilayer graphene. Nature 2009, 459, 820–823. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Zhang, P.; Liu, F.; Luo, S. Laser-induced freestanding graphene papers: A new route of scalable fabrication with tunable morphologies and properties for multifunctional devices and structures. Nano Micro Small 2018, 14. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Liu, T. Graphite Nanoplatelet enabled embeddable fibre sensor for in-situ curing monitoring and structural health monitoring of polymeric composites. Appl. Mater. Interfaces 2014, 6, 9314–9320. [Google Scholar] [CrossRef]
- Brigida, A.; Alfe, M.; Gargiulo, V.; Polichetti, T.; Massera, E.; Miglietta, M.; Francia, G.D. A new chemical sensing material for ethanol detection: Graphene-like film. In Proceedings of the 3rd National Conference on Sensors, Rome, Italy, 23–25 February 2016. [Google Scholar]
- Eric, S.; Meyya, M.; Hari, S.N. Flexible graphene-based wearable gas and chemical sensors. ACS Appl. Mater. Interfaces 2017, 9, 34544–34586. [Google Scholar]
- Pearce, R.; Iakimov, T. Epitaxially grown graphene based gas sensors for ultra sensitive NO2, detection. Sens. Actuators B Chem. 2010, 155, 451–455. [Google Scholar] [CrossRef]
- Sun, F.; Xu, S. Application of graphene material in gas-sensor. J. South China Norm. Univ. 2013, 6, 93–98. [Google Scholar]
- Ohno, Y.; Maehashi, K. Chemical and biological sensing applications based on graphene field-effect transistors. Biosens. Bioelectron. 2010, 26, 1727–1730. [Google Scholar] [CrossRef] [PubMed]
- Syu, Y.-C.; Hsu, W.-E.; Lin, C.-T. Review―Field effect transistor biosensing: Devices and clinical application. ECS J. Solid-State SC. 2018, 7, Q3196–Q3207. [Google Scholar] [CrossRef]
- Arsat, R.; Breedon, M. Graphene-like nano-sheets for surface acoustic wave gas sensor applications. Chem. Phys. Lett. 2009, 467, 344–347. [Google Scholar] [CrossRef]
- Lin, Y.M.; Avouris, P. Strong suppression of electrical noise in bi-layer graphene nanodevices. Nano Lett. 2008, 8, 2119–2125. [Google Scholar] [CrossRef] [PubMed]
- Condensed Concepts. Available online: http://condensedconcepts.blogspot.com/2015/08/conical-intersections-vs-dirac-cones.html (accessed on 1 February 2019).
- Owens, F.J. Electronic and magnetic properties of armchair and zigzag graphene nanoribbons. J. Chem. Phys. 2008, 128. [Google Scholar] [CrossRef]
- Sukang Bae, X.X.; Kim, H.; Lee, Y. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotechnol. 2010, 5, 574–578. [Google Scholar] [Green Version]
- Paupitz, R.; Autreto, PA.; Legoas, S.B.; Srinivasan, S.G.; Van Duin, A.C.; Galvao, D.S. Graphene to fluorographene and fluorographane: A theoretical study. Nanotechnology 2013, 24. [Google Scholar] [CrossRef]
- Pajooheshpour, N.; Rezaei, M.; Hajian, A.; Afkhami, A.; Sillanpää, M.; Arduini, F.; Bagheri, H. Protein templated Au-Pt nanoclusters-graphene nanoribbons as a high-performance sensing layer for the electrochemical determination of diazinon. Sens. Actuators B Chem. 2018, 275, 180–189. [Google Scholar] [CrossRef]
- Yan, P.; Jiang, D.; Li, H.; Bao, J.; Xu, Li.; Qian, J.; Chen, C.; Xia, J. BiPO4 nanocrystal/BiOCl nanosheet heterojunction as the basis for a photoelectrochemical 4-chlorophenol sensor. Sens. Actuators B Chem. 2018, 279, 466–475. [Google Scholar] [CrossRef]
- Budavari, S. The Merck Index: An Encyclopedia of Chemical, Drugs, and Biologicals; Merck: Hoboken, NJ, USA, 1996. [Google Scholar]
- Methanol. Available online: www.ilo.org/dyn/icsc/showcard.display?p_lang=en&p_card_id=0057&p_version=2 (accessed on 15 February 2019).
- Tian, W.; Liu, X.; Yu, W. Research progress of gas sensor based on graphene and its derivatives: A review. Appl. Sci. 2018, 8, 1118. [Google Scholar] [CrossRef]
- Rashid, M.H.; Koel, A.; Rang, T. Phenol and methanol detector based on pristine graphene nano-sheet: A first principles study. In Proceedings of the 16th Biennial Baltic Electronics Conference, Tallinn, Estonia, 8–10 October 2018. [Google Scholar]
- Poklonski, N.A.; Kislyakov, E.F.; Vyrko, S.A.; Bubel, O.N.; Ratkevich, S.V. Electronic band structure and magnetic states of zigzag graphene nanoribbons: Quantum chemical calculations. J. Nanophotonics 2012, 6, 61712. [Google Scholar] [CrossRef]
- Atomic-scale Modelling for Semiconductor & Materials Research. Available online: www.synopsys.com/silicon/quantumatk.html (accessed on 28 February 2019).
- ATK-DFT. Available online: https://docs.quantumwise.com/v2017/manuals/ATKDFT.html (accessed on 1 March 2019).
- High Power Computing. Available online: https://www.ttu.ee/support-structure/it-services/services-4/high-performance-computing-2/ (accessed on 13 March 2019).
- Dau, T.N.N.; Vu, V.H.; Cao, T.T.; Nguyen, V.C.; Ly, C.T.; Tran, D.L.; Pham, T.T.N.; Loc, N.T.; Piro, B.; Vu, T.T. In-situ electrochemically deposited Fe3O4 nanoparticles onto graphene nanosheets as amperometric amplifier for electrochemical biosensing applications. Sens. Actuators B Chem. 2019, 283, 52–60. [Google Scholar] [CrossRef]
- Zhang, H.F.; Wu, D.P.; Ning, X.J. Atomistic mechanism for graphene based gaseous sensor working. Appl. Surf. Sci. 2019, 470, 448–453. [Google Scholar] [CrossRef]
- Ni, J.; Yang, N.; Liang, Q.; Jiang, J.; Meng, R.; Huang, Y.; Chen, X. Gas adsorption on graphene with different layers: A first-principles study. In Proceedings of the 16th International Conference on Electronic Packaging Technology, Changsha, China, 11–14 August 2015. [Google Scholar]
- National Renewable Energy Laboratory (N.R.E. Lab.). Solar Spectra. Available online: https://www.nrel.gov/grid/solar-resource/spectra.html (accessed on 2 April 2019).
- Palsgaard, M.; Markussen, T.; Gunst, T.; Brandbyge, M.; Stokbro, K. Efficient first principles calculation of phonon-assisted photocurrent in large-scale solar-cell devices. Phys. Rev. Appl. 2018, 10, 1–5. [Google Scholar] [CrossRef]
- Xia, F.; Mueller, T.; Lin, Y.; Valdes-Garcia, A.; Avouris, P. Ultrafast graphene photodetector. Nat. Nanotechnol. 2009, 4, 839–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabor, N.M.; Song, J.C.W.; Ma, Q.; Nair, N.L.; Taychatanapat, T.; Watanabe, K.; Taniguchi, T.; Levitov, L.S.; Jarillo-Herrero, P. Hot Carrier–Assisted Intrinsic Photoresponse in graphene. Science 2011, 334, 648–651. [Google Scholar] [CrossRef] [PubMed]
- Freitag, M.; Low, T.; Zhu, W.; Yan, H.; Xia, F.; Avouris, P. Photocurrent in graphene harnessed by tunable intrinsic plasmons. Nat. Commun. 2013, 4, 1–8. [Google Scholar] [CrossRef]
- Ojarand, J.; Min, M.; Koel, A. Multichannel electrical impedance spectroscopy analyser with microfluidic sensors. Sensors 2019, 19. [Google Scholar] [CrossRef]
- Halder, S.; Amorim, R.G.; Sanyal, B.; Scheicher, R.H.; Rocha, A. Energetic stability, STM fingerprints and electronic transport properties of defects in graphene and silicone. RSC Adv. 2016, 6, 6702–6708. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haroon Rashid, M.; Koel, A.; Rang, T. First Principles Simulations of Phenol and Methanol Detector Based on Pristine Graphene Nanosheet and Armchair Graphene Nanoribbons. Sensors 2019, 19, 2731. https://doi.org/10.3390/s19122731
Haroon Rashid M, Koel A, Rang T. First Principles Simulations of Phenol and Methanol Detector Based on Pristine Graphene Nanosheet and Armchair Graphene Nanoribbons. Sensors. 2019; 19(12):2731. https://doi.org/10.3390/s19122731
Chicago/Turabian StyleHaroon Rashid, Muhammad, Ants Koel, and Toomas Rang. 2019. "First Principles Simulations of Phenol and Methanol Detector Based on Pristine Graphene Nanosheet and Armchair Graphene Nanoribbons" Sensors 19, no. 12: 2731. https://doi.org/10.3390/s19122731




