Real-Time Smart-Digital Stethoscope System for Heart Diseases Monitoring
Abstract
:1. Introduction
2. Experiment Details and Methods
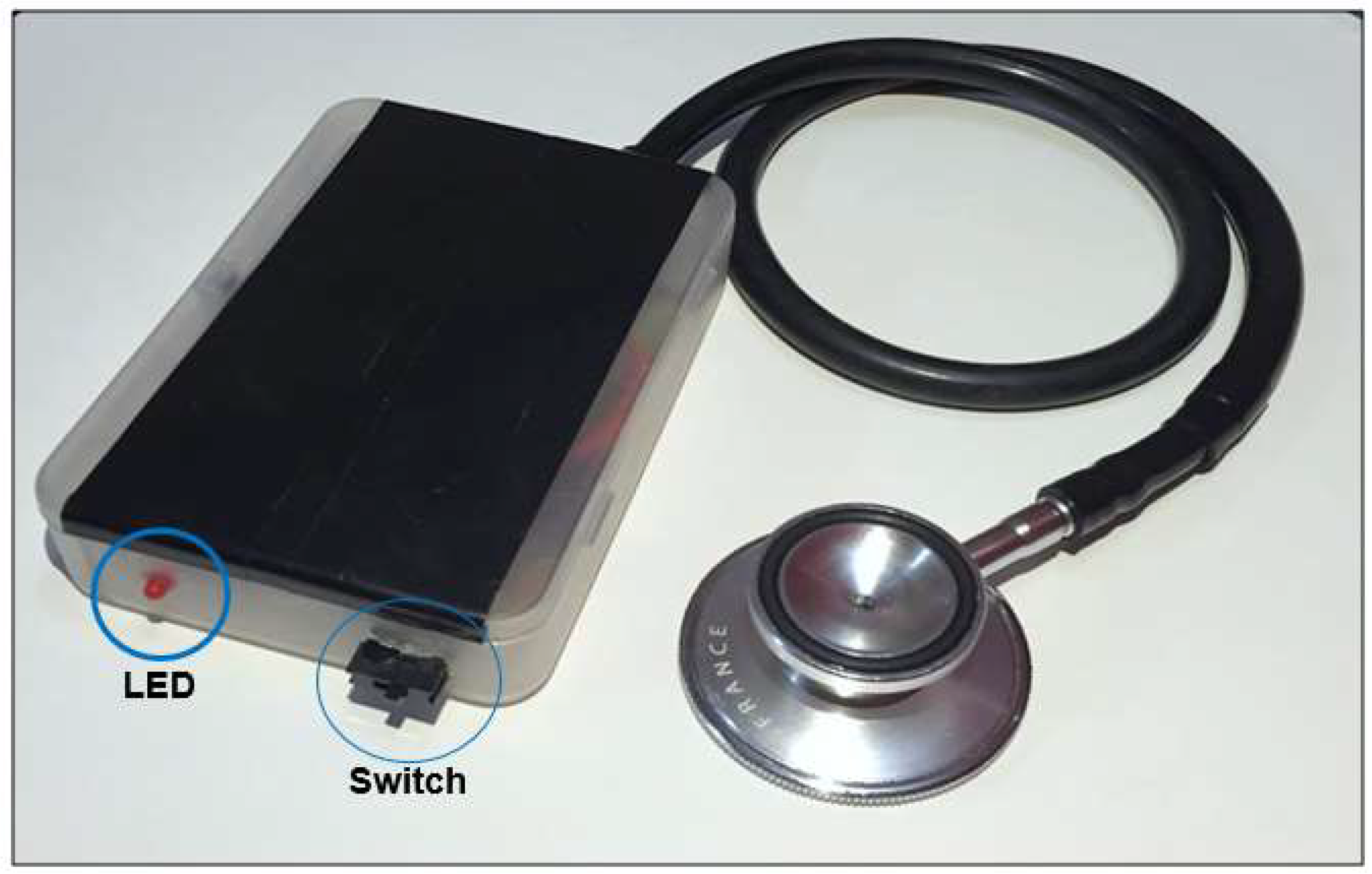
2.1. Evaluation of the Signal Fidelity of Prototype Sensor Subsystem
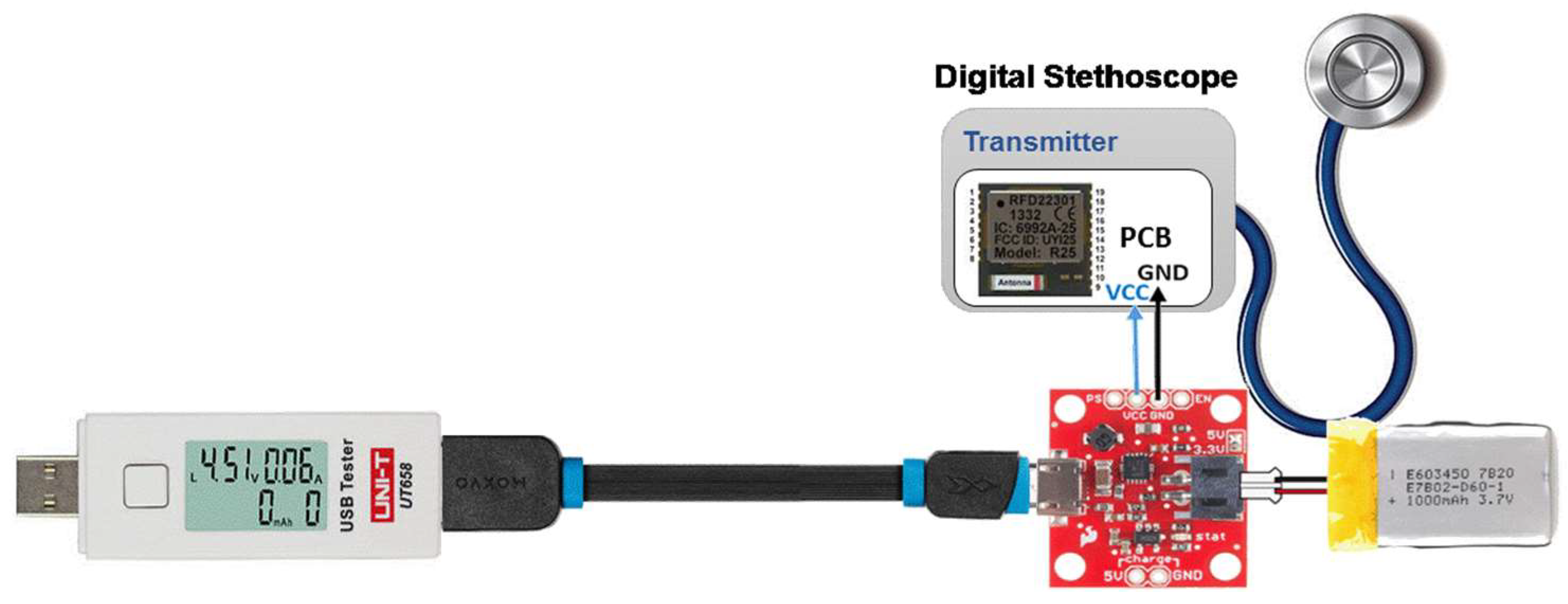
2.2. Evaluation of the Reliability of the BLE Transmission System
2.3. Evaluation of Battery Life of the Sensor Subsystem
2.4. Performance Evaluation of Machine Learning Abnormality Detection Algorithms
2.4.1. Database Description
2.4.2. Optimized Classification Model Selection
2.5. Real-Time Classification of Heart Sound Signals
3. Analysis
3.1. Pre-Processing Steps
3.1.1. Filtering and Spikes Removal
3.1.2. Segmentation
3.2. Feature Extraction
3.3. Classification
Performance Evaluation Matrix
3.4. Feature Reduction
3.5. Hyperparameter Optimization of the Best-Performing Algorithm
3.6. Unequal Misclassification Costs
4. Results and Discussion
4.1. Evaluation of the Signal Fidelity of Prototype Sensor Subsystem
4.2. Evaluation of the Reliability of the BLE Transmission System
4.3. Evaluation of Battery Life of the Sensor Subsystem
4.4. Performance Eevaluation of Machine Learning Abnormality Detection Algorithm
4.5. Real-Time Classification of Heart Sound Signals
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Després, J.P.; Fullerton, H.J. Heart disease and stroke statistics—2016 update a report from the American Heart Association. Circulation 2016, 133, e38–e48. [Google Scholar] [PubMed]
- Bhatnagar, P.; Wickramasinghe, K.; Williams, J.; Rayner, M.; Townsend, N. The epidemiology of cardiovascular disease in the UK 2014. Heart 2015, 101, 1182–1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nichols, M.; Peterson, K.; Herbert, J.; Alston, L.; Allender, S. Australian Heart Disease Statistics 2015; National Heart Foundation of Australia: Melbourne, Australia, 2016. [Google Scholar]
- Wilkins, E.; Wilson, L.; Wickramasinghe, K.; Bhatnagar, P.; Leal, J.; Luengo-Fernandez, R.; Burns, R.; Rayner, M.; Townsend, N. European Cardiovascular Disease Statistics 2017; University of Bath: Bath, UK, 2017. [Google Scholar]
- Reed, T.R.; Reed, N.E.; Fritzson, P. Heart sound analysis for symptom detection and computer-aided diagnosis. Simul. Model. Pract. Theory 2004, 12, 129–146. [Google Scholar] [CrossRef]
- Kim, R.J.; Wu, E.; Rafael, A.; Chen, E.-L.; Parker, M.A.; Simonetti, O.; Klocke, F.J.; Bonow, R.O.; Judd, R.M. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N. Engl. J. Med. 2000, 343, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Huang, J.; Zhang, S.; Qian, Z.; Voros, S.; Metaxas, D.; Axel, L. 4D cardiac reconstruction using high resolution CT images. In Proceedings of the International Conference on Functional Imaging and Modeling of the Heart, New York, NY, USA, 25–27 May 2011; pp. 153–160. [Google Scholar]
- Canè, F.; Verhegghe, B.; De Beule, M.; Bertrand, P.B.; Van der Geest, R.J.; Segers, P.; De Santis, G. From 4D Medical Images (CT, MRI, and Ultrasound) to 4D Structured Mesh Models of the Left Ventricular Endocardium for Patient-Specific Simulations. Biomed Res. Int. 2018, 2018, 7030718. [Google Scholar] [CrossRef]
- Gaziano, T.A.; Bitton, A.; Anand, S.; Abrahams-Gessel, S.; Murphy, A. Growing epidemic of coronary heart disease in low-and middle-income countries. Curr. Probl. Cardiol. 2010, 35, 72–115. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.K.; Roy, T.S.; Mukhopadhyay, S.C. Heart Sound: Detection and Analytical Approach Towards Diseases. In Modern Sensing Technologies; Springer: Berlin/Heidelberg, Germany, 2019; pp. 103–145. [Google Scholar]
- Hedayioglu, F.D.L. Heart Sound Segmentation for Digital Stethoscope Integration. Master’s Thesis, University of Porto, Porto, Portugal, 2011. [Google Scholar]
- Leng, S.; San Tan, R.; Chai, K.T.C.; Wang, C.; Ghista, D.; Zhong, L. The electronic stethoscope. Biomed. Eng. Online 2015, 14, 66. [Google Scholar] [CrossRef]
- Landge, K.; Kidambi, B.R.; Singhal, A.; Basha, A. Electronic stethoscopes: Brief review of clinical utility, evidence, and future implications. J. Pract. Cardiovasc. Sci. 2018, 4, 65. [Google Scholar] [CrossRef]
- Silverman, B.; Balk, M. Digital Stethoscope—Improved Auscultation at the Bedside. Am. J. Cardiol. 2019, 123, 984–985. [Google Scholar] [CrossRef]
- Reynolds, J.; Forero, J.; Botero, J.; Leguizamón, V.; Ramírez, L.; Lozano, C. Electronic Stethoscope with Wireless Communication to a Smart-phone, Including a Signal Filtering and Segmentation Algorithm of Digital Phonocardiography Signals. In Proceedings of the VII Latin American Congress on Biomedical Engineering CLAIB 2016, Bucaramanga, Colombia, 26–28 October 2016; pp. 553–556. [Google Scholar]
- Swarup, S.; Makaryus, A.N. Digital stethoscope: Technology update. Med. Devices 2018, 11, 29. [Google Scholar] [CrossRef]
- Tschannen, M.; Kramer, T.; Marti, G.; Heinzmann, M.; Wiatowski, T. Heart sound classification using deep structured features. In Proceedings of the 2016 Computing in Cardiology Conference (CinC), Vancouver, BC, Canada, 11–14 September 2016; pp. 565–568. [Google Scholar]
- Gupta, C.N.; Palaniappan, R.; Swaminathan, S.; Krishnan, S.M. Neural network classification of homomorphic segmented heart sounds. Appl. Soft Comput. 2007, 7, 286–297. [Google Scholar] [CrossRef]
- Noponen, A.-L.; Lukkarinen, S.; Angerla, A.; Sepponen, R. Phono-spectrographic analysis of heart murmur in children. BMC Pediatr. 2007, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Lubaib, P.; Muneer, K.A. The heart defect analysis based on PCG signals using pattern recognition techniques. Procedia Technol. 2016, 24, 1024–1031. [Google Scholar] [CrossRef]
- Abbas, A.K.; Bassam, R. Phonocardiography signal processing. Synth. Lect. Biomed. Eng. 2009, 4, 1–194. [Google Scholar] [CrossRef]
- Marascio, G.; Modesti, P.A. Current trends and perspectives for automated screening of cardiac murmurs. Heart Asia 2013, 5, 213–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Springer, D.; Li, Q.; Moody, B.; Juan, R.A.; Chorro, F.J.; Castells, F.; Roig, J.M.; Silva, I.; Johnson, A.E. An open access database for the evaluation of heart sound algorithms. Physiol. Meas. 2016, 37, 2181. [Google Scholar] [CrossRef] [PubMed]
- Livanos, G.; Ranganathan, N.; Jiang, J. Heart sound analysis using the S transform. In Proceedings of the Computers in Cardiology 2000, Cambridge, MA, USA, 24–27 September 2000; pp. 587–590. [Google Scholar]
- Liang, H.; Lukkarinen, S.; Hartimo, I. Heart sound segmentation algorithm based on heart sound envelogram. In Proceedings of the Computers in Cardiology 1997, Lund, Sweden, 7–10 September 1997; pp. 105–108. [Google Scholar]
- Choi, S.; Jiang, Z. Comparison of envelope extraction algorithms for cardiac sound signal segmentation. Expert Syst. Appl. 2008, 34, 1056–1069. [Google Scholar] [CrossRef]
- Mondal, A.; Bhattacharya, P.; Saha, G. An automated tool for localization of heart sound components S1, S2, S3 and S4 in pulmonary sounds using Hilbert transform and Heron’s formula. SpringerPlus 2013, 2, 512. [Google Scholar] [CrossRef]
- Clifford, G.D.; Liu, C.; Moody, B.; Springer, D.; Silva, I.; Li, Q.; Mark, R.G. Classification of normal/abnormal heart sound recordings: The PhysioNet/Computing in Cardiology Challenge 2016. In Proceedings of the 2016 Computing in Cardiology Conference (CinC), Vancouver, BC, Canada, 11–14 September 2016; pp. 609–612. [Google Scholar]
- Schmidt, S.E.; Holst-Hansen, C.; Hansen, J.; Toft, E.; Struijk, J.J. Acoustic features for the identification of coronary artery disease. IEEE Trans. Biomed. Eng. 2015, 62, 2611–2619. [Google Scholar] [CrossRef]
- Davis, S.; Mermelstein, P. Comparison of parametric representations for monosyllabic word recognition in continuously spoken sentences. IEEE Trans. Acoust. Speech Signal Process. 1980, 28, 357–366. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, S.; Wang, P.; Sing Lim, C.; Anantharaman, V. A computer aided MFCC-based HMM system for automatic auscultation. Comput. Biol. Med. 2008, 38, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Vepa, J. Classification of heart murmurs using cepstral features and support vector machines. In Proceedings of the 31st Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009; pp. 2539–2542. [Google Scholar]
- Potes, C.; Parvaneh, S.; Rahman, A.; Conroy, B. Ensemble of Feature based and Deep learning-based Classifiers for Detection of Abnormal Heart Sounds. In Proceedings of the Computing in Cardiology 2016, Vancouver, BC, Canada, 11–14 September 2016. [Google Scholar]
- Zabihi, M.; Rad, A.B.; Kiranyaz, S.; Gabbouj, M.; Katsaggelos, A.K. Heart Sound Anomaly and Quality Detection using Ensemble of Neural Networks without Segmentation. In Proceedings of the Computing in Cardiology 2016, Vancouver, BC, Canada, 11–14 September 2016. [Google Scholar]

















| Feature | Definition | Equation |
|---|---|---|
| Mean | Sum of all data divided by the number of entries. | |
| Median | Value that is in the middle of ordered set of data. | Odd numbers of entries: Median = middle data entry. Even numbers of entries: Median = adding the two numbers in the middle and dividing the result by two. |
| Standard Deviation | Measure variability and consistency of the sample. | s = |
| Percentile | The data value at which the percent of the value in the data set are less than or equal to this value. | |
| Mean Absolute Deviation | Average distance between the mean and each data value. | MAD = |
| Inter Quartile Range | The measure of the middle 50% of a data set. | IQR = Q3 – Q1 Q3: third quartile, Q1: first quartile, Quartile: dividing the data set into four equal portions. |
| Skewness | The measure of the lack of symmetry from the mean of the dataset. | Y: mean, s: the standard deviation, N: number of the data. |
| Kurtosis | The pointedness of a peak in distribution curve, in other words it’s the measure of sharpness of the peak of distribution curve. | k = Y: mean, s: the standard deviation, N: the number of data. |
| Shannon’s Entropy | Entropy measures the degree of randomness in a set of data, higher entropy indicates a greater randomness, and lower entropy indicates a lower randomness. | H(x) = − |
| Spectral Entropy | The normalized Shannon’s entropy that is applied to the power spectrum density of the signal. | SEN = pk: the spectral power of the normalized frequency, N: the number of frequencies in binary |
| Maximum Frequency | The value of highest frequency in the signal spectrum | fmax |
| Magnitude at Fmax | Signal magnitude at highest Frequency | X(fmax) |
| Ratio of signal energy | Ratio of signal energy between fmax ± Δf and the whole spectrum | X (fmax ± Δf) |
| MFCC (13 features) | Mel-Frequency Cepstral Coefficients (MFCC): coefficients that collectively make up a Mel-Frequency Cepstral (MFC). | x = x − 0.95*[0; x (1: N-1)]; X = fft(x); |
| Categories | No. of Observation | |
|---|---|---|
| Training and Validation | Abnormal | 2505 |
| Normal | 7907 | |
| Testing | Abnormal | 653 |
| Normal | 1950 |
| Items | Fine KNN | Weighted KNN | Ensemble Subspace Discriminant |
|---|---|---|---|
| Accuracy | 94.63% | 93.72 | 93.17% |
| Accuracy: Abnormal | 88%,12% | 85%,15% | 87%, 13% |
| Accuracy: Normal | 96.6%, 3.4% | 97%,3% | 95%, 5% |
| Error | 5.37% | 6.28% | 6.83% |
| Sensitivity | 96.32% | 95.24% | 95.67% |
| Specificity | 89.34% | 88.72% | 85.49% |
| Precision | 96.62% | 96.54% | 95.29% |
| FPR | 10.66% | 11.28% | 14.51% |
| F_Score | 96.46% | 95.88% | 95.48% |
| MCC | 85.34% | 82.7% | 81.5% |
| Items | Fine KNN | Weighted KNN | Ensemble Subspace Discriminant |
|---|---|---|---|
| Accuracy | 92.36% | 92.02% | 92.89% |
| Accuracy: Abnormal | 84%,16% | 82%,18% | 83%, 17% |
| Accuracy: Normal | 95%, 5% | 95%,5% | 96%, 4% |
| Error | 7.64% | 7.98% | 7.11% |
| Sensitivity | 94.85% | 94.30% | 94.77% |
| Specificity | 84.52% | 84.62% | 86.71% |
| Precision | 95.08% | 95.22% | 95.90% |
| FPR | 15.48% | 15.38% | 13.29% |
| F_Score | 94.96% | 94.76% | 95.33% |
| MCC | 79.17% | 78.09% | 80.42% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chowdhury, M.E.H.; Khandakar, A.; Alzoubi, K.; Mansoor, S.; M. Tahir, A.; Reaz, M.B.I.; Al-Emadi, N. Real-Time Smart-Digital Stethoscope System for Heart Diseases Monitoring. Sensors 2019, 19, 2781. https://doi.org/10.3390/s19122781
Chowdhury MEH, Khandakar A, Alzoubi K, Mansoor S, M. Tahir A, Reaz MBI, Al-Emadi N. Real-Time Smart-Digital Stethoscope System for Heart Diseases Monitoring. Sensors. 2019; 19(12):2781. https://doi.org/10.3390/s19122781
Chicago/Turabian StyleChowdhury, Muhammad E.H., Amith Khandakar, Khawla Alzoubi, Samar Mansoor, Anas M. Tahir, Mamun Bin Ibne Reaz, and Nasser Al-Emadi. 2019. "Real-Time Smart-Digital Stethoscope System for Heart Diseases Monitoring" Sensors 19, no. 12: 2781. https://doi.org/10.3390/s19122781






