1. Introduction
Gastrointestinal (GI) peristalsis is coordinated by an underlying bioelectrical activity, known as slow waves (SWs). Alvarez conducted pioneering studies in acquiring the SWs in 1920s [
1]. SWs either directly taken from the stomach or the abdominal skin have been proven to be an indicator of the peristalsis. Direct recording of the SWs demonstrated a robust acquisition, and acceptable signal to noise ratio compared to non-invasive recordings [
2]. The information that can be extracted from single channel recording of SWs is limited; as a result, high-resolution (HR) mapping has been employed in recent years [
3]. HR mapping of the SWs has been shown to be an effective tool for accurately defining functional motility disorders such as gastroparesis, chronic nausea, and functional dyspepsia [
4,
5,
6,
7]. Howsoever, current HR mapping methods are invasive, and the SWs are recorded directly from the serosa layer of a fasted subject undergoing abdominal surgery. Therefore, all the HR mapping studies have been based on short-term studies while the subject was under anesthesia. In addition to discomfort or risk of dislodgement or infection for the patient caused by current recording devices, a major limitation of these systems is their shortcoming for long-term studies.
Several studies have tried to employ wireless technologies to develop an implantable device suitable for HR mapping of the SWs. The first generations of such systems with limited number of channels (three to seven channels) were developed and validated by [
8,
9,
10]. The number of channels in these systems were sufficient to map the activity of the stomach while functioning normally; however, when the stomach activity was ectopic, seven channels were not enough to capture the direction of the SWs propagation [
11]. Using off-the-shelve components, we developed a 32-channel system to accommodate this shortcoming [
12,
13]. The power consumption of these systems was in the range 30 mW, making them unsuitable for long-term studies. Furthermore, none of these systems were equipped with a recharging mechanism, in case they need to be implanted.
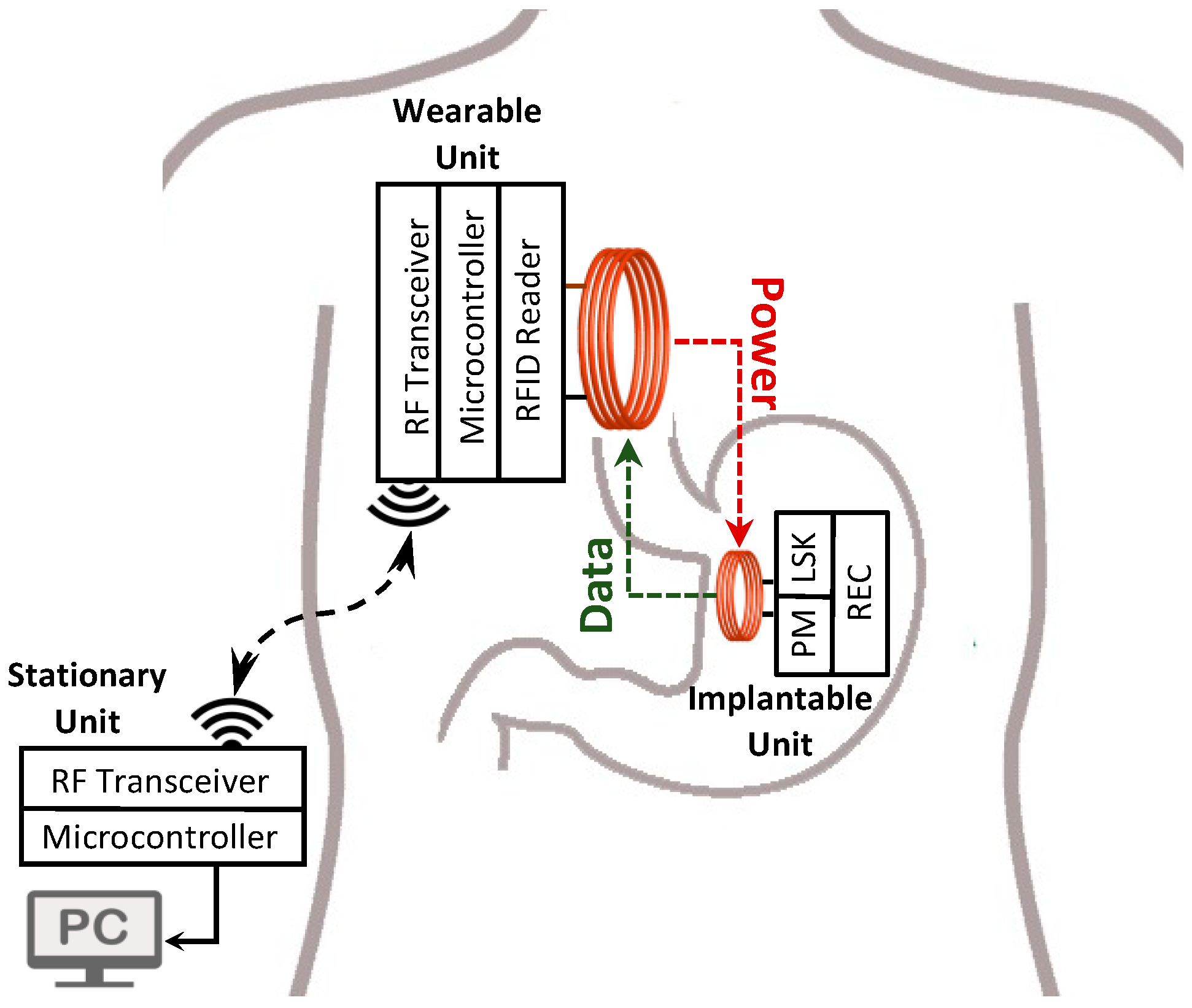
With the objective of designing a minimally invasive system for HR mapping, we have developed, and benchtop validated, a 64-channel recording system that is composed of an implantable unit (IU), a wearable unit (WU), and a stationary unit (SU). The IU conditions the SWs, and the WU reads the SWs from the IU through a radio frequency identification (RFID) -based near-field communication (NFC) system [
14,
15], and consequently, transmits the data to the SU. Furthermore, the WU can wirelessly transfer power to the IU through an inductive link. The low power consumption of the IU, and the recharging feature allows implantation of the IU for long-term studies.
In the following, we first describe the NFC methodology consisting of data encoding algorithm and data modulation method. Afterwards, the system architecture and implementation of the SU, the WU, and the IU devices are explained. At the end, the validation of the IU-WU wireless data communication and wireless power transfer (WPT) and the WU-SU RF link, followed by a discussion of the results, is presented.
3. System Architecture
The detailed block diagram of the NFC and WPT system is shown in
Figure 5, which consists of the IU, the WU, and the SU connected to a computer. The WPT feature is carried out by inductively transmitting the 13.56 MHz sinewave signal generated by the power amplifier of an RFID reader from the WU’s coil to the IU’s coil (L
1 to L
2). This signal is rectified and regulated at 3.3 V on the IU. For the NFC, an ADC embedded in a microcontroller at the IU reads the GEA signals from 64 electrodes consecutively which are multiplexed through a 64-to-1 multiplexing circuit and processed through an analog conditioning circuit, and digitizes the data with a sampling rate of 16 samples per second per channel. Afterward, the microcontroller applies the proposed DPP encoding algorithm to the data packet and drives the NMOS switch with the data pulse train of every 64-channel signals. At the WU, the data are demodulated by an envelope detector embedded in the RFID reader (TRF7970, Texas Instruments -TI-) and decodes them by the microcontroller. Although the system architecture was briefly described in our previous work [
18], the specific details are discussed here.
3.1. IU Data Packet
In addition to the digitized data of 64 signal acquisition channels, the instantaneous rectified voltage of received power by the IU (V
REC) and the IU’s battery voltage are concatenated in one data packet. As a result, based on the ADC sampling resolution of 10 bits, the data packet includes 640 bits for samples of 64 signal acquisition channels, 10 bits for the rectified voltage and 10 bits for the battery voltage. Furthermore, a start-of-frame of 8 bits is added as the header of the packet to make the decoder circuit capable of identifying the data stream sent to the WU. As a result, the final data packet consists of 668 bits per each round of acquiring 64 GEA signals. Speaking in terms of the time required to transmit the data through LSK modulation, the back-telemetry circuit is off for around 64 ms (1 ms is the multiplexing circuit delay time to let the ADC to acquire the input signal of 64 independent channels) and then takes 668 × 8 µs equal to 5.344 ms to send the NFC data. However, since the duty cycle of the DPP encoded data is 6.25%, the wireless power receiving at the IU is only off for around 0.334 ms during the NFC.
Table 1 presents the content of a data packet transmitted from the IU to the WU.
3.2. Closed-Loop WPT
Stomach motility or body movements of the subject undergoing the study can potentially result into misalignment and/or changes of distance between the primary and secondary coils. These, in turn, can significantly affect the amount of power received by the IU and decrease the efficiency of WPT. As a result, a closed-loop system for the WPT is designed to guarantee the minimum amount of power receiving by the IU and consequently, to adjust the transmitting power by the WU, accordingly. To implement the closed-loop WPT, instantaneous V
REC at the IU is read through a voltage divider and sampled by an ADC of the IU’s microcontroller (MSP430, TI) and as indicated in
Table 1, is sent to the WU as a part of the GEA data packet. At the WU, the DC bias voltage of the RF power amplifier is supplied through the output of a DC-DC buck-boost converter (TPS63000, TI). The feedback resistor of the buck-boost converter is a 200 kΩ digital potentiometer with 256 taps (MAX5424, Maxim Integrated) which is programmable through the WU’s microcontroller (MSP432, TI). At approximately every 70 ms, the WU receives the value of the instantaneous rectified voltage, the microcontroller compares it with a reference voltage (V
REF) equal to 3.7 V. For V
REC voltages higher than V
REF, the microcontroller increases the value of the digital potentiometer and consequently, decreases the output of the DC-DC buck-boost converter. As a result, the output power level of the RF power amplifier decreases until V
REC is decreased to V
REF. On the other hand, when V
REC is lower than V
REF, the digital potentiometer value decreases until the rectified voltage increases to V
REF. The resistive feedback loop of the DC-DC buck-boost converter is designed such that it gives an output voltage range from 2.75 V to 5 V.
The rectification bridge is a critical component in energy harvesting in wirelessly powered systems. Therefore, a Schottky diode array (BAS4002, Infineon, Neubiberg, Germany) employed at the IU is a low power and low forward voltage bridge with only 0.4 V voltage drop for 10–20 mA. As a result, the voltage decreases, in the worst case, 0.8 V in each direction.
3.3. Data Logging Modes
Once the GEA digitized signals are demodulated, and decoded at the WU, then the data can be either stored locally on a micro SD memory card or wirelessly transmitted to the SU through an ISM-band 2.4 GHz RF transceiver (nRF24L01+, Nordic Semiconductor). In the case of the former data logging mode (on the memory card), while the SU is out of the loop, the decoded data is saved in a text file on the memory card and can be extracted anytime. For the latter mode, data are received by the SU through the same RF transceiver and through a UART-to-USB bridge (FT232, FTDI Chip), are sent to the stationary computer.
3.4. Graphical User Interface (GUI)
An application-specific GUI is developed in LabVIEW, it allows the user to monitor the 64 channels GEA signals in real time and store them on computer for off-line analysis. Hence, 64 independent signal windows with modifiable time intervals are embedded in the GUI. Instantaneous rectified and battery voltages are also displayed in the GUI. Furthermore, the user can either continue receiving the data through the RF link or switch to micro SD memory card data logging mode, wirelessly. A snapshot of the developed GUI is shown in the results section.
4. Tests and Measurements Results
To validate the high-resolution NFC signal acquisition and WPT systems, the setup shown in
Figure 6 was implemented. The setup consists of the IU, the WU, the SU and a 3-D yellow-colored apparatus for the modification of the distances and the angles between the transmitter and receiver coils. The IU was fabricated on a 4-layer flex-rigid printed circuit board (PCB) with integrated flexible planar coil and integrated 64-channel flexible biocompatible electrode array. The IU (except the electrode array) was coated by a medical-grade epoxy (Epotek MED-301). The final size of the IU after coating was 13 × 11 × 40 mm
3. Besides, the WU (45 × 45 mm
2) and the SU (40 × 40 mm
2), and the WU’s planar coil (53 × 62 mm
2) were fabricated on regular 4-layer and 2-layer PCBs, respectively.
4.1. Measurements Results of WPT
Based on the analyses presented in
Section 2.2.1 and
Section 2.2.2, the 50 Ω capacitive matching network of the WU’s transmitter coil and the resonant network of the IU’s receiver coil were calculated, implemented and tuned at the RFID carrier frequency, i.e., 13.56 MHz. In this regard, we measured the inductances and internal resistances of the transmitter and receiver coils by a N9923A Vector Network Analyzer (VNA), independently. The inductance and outer diameter of the primary and secondary coils were measured as 3.02 µH and 490 nH, and 70 mm and 27 mm, respectively.
Figure 7 shows the impedances of the transmitter coil matched to 50 Ω and the resonant LC tank at the receiver coil measured by the VNA. In particular, the L
1 matched impedance of (49.5 + j0.3) Ω, provides an excellent return loss of around −30 dB, which guarantees the total amount of power generated by the RFID reader is delivered to the WU’s coil.
Furthermore, in order to verify the WPT, the WU was programmed to transmit constant power of 200 mW (23 dBm), and at the IU, the received power was measured for different distances and angles made between the pair of coils. For the medium between the primary and secondary coils, air and raw chicken were tested separately.
Figure 8 presents the amount of the power received at the IU along with the WPT efficiency when the distance and the angle between the transmitter and the receiver coils were modified from 2 cm to 5 cm with the steps of 0.5 cm and from 0° to 60° with the steps of 15°, respectively. As shown in this figure, when the distance between the transmitter and receiver coils is 2 cm, the received power is 107 mW and 89 mW, for air and raw chicken, which translates to 53.5% and 44.5% efficiency, respectively. Increasing the distance from 2 cm to 5 cm, with the steps of 0.5 cm, the received power and the WPT efficiency eventually decrease to 10 mW and 8 mW, and to 5% and 4% for the corresponding mediums, respectively. Furthermore, at a constant distance of 2 cm between the pair of coils, when the misalignment angle between the primary and secondary coils is 15°, the received power for the air and raw chicken is 54 mW and 46 mW, which translates to 27% and 23% efficiency, respectively. Increasing the angle from 15° to 60°, with the steps of 15°, the received power and the WPT efficiency ultimately decrease to 10.5 mW and 9 mW, and to 5.6% and 4.1% for the corresponding mediums, respectively.
Temperature increase and heat generation in human tissue is a major concern in WPT. While the maximum 200 mW power transmission is an order of magnitude lower than the allowed specific absorption rate (SAR) of the tissue which is 1.6 W/kg, we carried out three experiments to measure the power transfer and the heat generated in the two sides of the raw chicken at different alignments and distances. The first measurement was conducted for 36 h on 85 g of raw chicken, approximately 117 cm3, for the Tx-Rx aligned coils distance of 3.5 cm. The temperature on the outside edge of the chicken was 77.5 °F, and the ambient temperature was 76.5 °F. The second measurement was carried out for 14 h on 49.3 g of raw chicken, approximately 66 cm3, for the Tx-Rx aligned coils distance of 3.5 cm. The temperature on the outside edge of the raw chicken was 75.5 °F, and the ambient temperature was 75 °F. Finally, the third measurement was conducted for 4 h on 64 g of raw chicken, approximately 82 cm3, for the Tx-Rx misaligned coils distance of 3.5 cm and 45° misalignment angle. The temperature on the outside edge of the chicken was 78 °F, and the ambient temperature was 77.5 °F.
4.2. Measurement Results of NFC Slow Waves Recording
For validation of the NFC recording system, the IU was drawn in a container of saline solution. In this test, 5-min sample SWs recorded previously
in-vivo by our group were used [
19]. Sample signals were loaded into a digital to analog converter (DAQ device USB-6218, National Instrument) through a custom-made application and were streamed into the solution. The data were verified in two steps. First, the consistency between the DPP-encoded data at the IU and the demodulated data which were recovered at the WU were verified. Then, the data received at the SU and monitored in the GUI in real time were observed to be identical with the amplified original signals recorded at the IU. The benchtop setup for the NFC verification is shown in
Figure 9.
At the WU, the received modulated data over the RF inductive link and the demodulated data at the output of the RFID reader were probed (using an oscilloscope, Tektronix MDO4104C) and simultaneously compared with the encoded data at the IU and the modulated data at the secondary coil. As presented in
Figure 10, the power level at the IU’s coil drops to zero when the high-pulses of the encoded data are transmitted through the back-telemetry circuit. This is because the secondary coil is shorted to ground when the NMOS switch is closed. On the other side, when the modulated data is seen by the primary coil, the power level slightly increases. This is due to the fact that the reflected impedance to the WU’s coil is zero and as discussed in
Section 2.1.2. in detail, the power level at the transmitter coil reaches its maximum possible value. Furthermore, it can be seen that the demodulated data at the output of the RFID reader successfully follows the exact time intervals in DPP-encoded data at the IU.
In the second test, the GEA data which were received in the 64 channels of the GUI in real time were monitored.
Figure 11 shows the first two channels of the GUI received signals which are identical with the original ones; except that the received signal is amplified with the gain of 2000. Signals in other 62 channels of the GUI followed an identical pattern. Eight slow wave events can be counted in the period of 160 s which translates to 3 cycles per minute.
The power consumption of the IU, WU, and SU were measured as 6.3 mW, 823 mW (at maximum power), and 80 mW, respectively. When the IU and WU coils are well-aligned and separated at 3 cm, the received power is enough to allow the IU to run without the need for a battery. However, because obtaining the perfect alignment of the coils in vivo is not easily achieved, the use of a battery for the IU seems necessary. Considering a 105 mAh Li-polymer battery (30 × 12 × 4 mm3) for the IU, the system can theoretically work for more than 15 continuous hours without the need for recharging. However, with the WPT charging capability when the coils were separated for 2.5 cm from each other (with perfect alignment), while the IU was in the container of saline solution and the WU’s coil was outside, the battery was recharged for 60 mV per hour, while working at maximum load.
5. Discussion and Conclusions
A wireless miniature implantable NFC system for high-resolution acquisition of SWs has been developed. The system is featured with a 13.56 MHz inductive RFID-based closed-loop NFC between the IU and the WU. The NFC works based on LSK modulation of data through a back-telemetry circuit. In addition, the system is equipped with WPT which assures a stable and solid source of power for the IU, and as a result, makes it an excellent candidate for long-term studies. Since the NFC and WPT are carried out simultaneously on one shared inductive link, an application-specific self-clocking DPP data encoding algorithm was developed. This algorithm utilizes very narrow width of the high-pulses with only 0.5 µs pulse width and duty cycle of 6.25% for encoding data to minimize the disruption of the WPT link. Furthermore, the proposed DPP encoding method provides the data rate of 125 kb/s, which is enough for our application.
Two independent data logging modes were implemented in the system. The first mode is based on a 2.4 GHz RF link between the WU and the SU. Hence, the demodulated decoded data at the WU are transmitted to the SU connected to a computer. Through a GUI, the data can be monitored in real time and simultaneously stored in a text file for offline analysis. This method is preferred when the patient is under direct monitoring in a clinical facility. The second mode of data logging, which is wirelessly configurable through the GUI, is to store the data locally on the WU on a micro SD memory card. The data can be extracted from the memory card, anytime during or at the end of the GEA monitoring period.
The closed-loop feature of the system significantly improves the stability of the WPT. In case of any misalignment and change of distance between the primary and secondary coils due to body movement and stomach motility of the patient, the power transfer is instantaneously adjusted to keep the IU’s rectified voltage at a constant level. In addition, the range of 2 to 5 cm distance between the pair of coils is very reasonable for most of the subjects undergoing GI study, and the closed-loop WPT is capable of transmitting enough power to meet rectified voltage equal to reference voltage. The closed-loop WPT was intentionally limited to 200 mW that can be generated by the RFID reader. This amount is an order of magnitude lower than the specific absorption rate value allowed by the IEEE standard C95.1-2005 and International Commission on Non-Ionizing Radiation Protection (ICNIRP) guideline [
20,
21]. Furthermore, unnecessary higher amounts of power can generate noise that can reduces the fidelity of the signal transmission in the NFC link.
The developed system with 64 signal acquisition channels covers an approximate area of 30 × 30 mm2. However, one might be interested to simultaneously study different areas of the gastrointestinal tract. In this regard, up to four systems can be employed simultaneously by modifying the carrier frequency of the RFID link. The default 13.56 MHz oscillator frequency of the RFID reader can be divided by 2 and 4, which means we can modify the clock frequency to 6.78 MHz and 3.39 MHz through the firmware. Moreover, we can replace the 13.56 MHz crystal oscillator of the RFID reader at the WU with the 27.12 MHz one. As a result, with minor modifications of the firmware of the IU and WU, we can concurrently run four systems with modulation frequencies of 3.39 MHz, 6.78 MHz, 13.56 MHz and 27.12 MHz.
For the closed-loop WPT, we considered the initial power transfer of 150 mW to the IU. The rectified voltage of the IU was measured continuously, and the algorithm in the WU was set to increase (or decrease) the transmitted power with 10 mW steps, in case the rectified voltage decreases (or increases) below (or above) the 3.7 V. This implementation is simple and does not consider various battery charging methods to enhance the performance of the battery. For instance, we can consider methods such as “constant current, constant voltage charging” or “multi-state current charging” algorithms, each of which have their advantageous and disadvantageous [
22]. Using the closed-loop mechanism, more complicated algorithms for transmitting power to the IU can be developed in the future.
Furthermore, the heating concern as a consequence of WPT through human tissue was studied in different distances and misalignment angles of the Tx-Rx coils. The measurements with maximum power transmission of 200 mW from the WU to the IU through raw chicken showed worst case temperature rise of 1 °F referenced to ambient, which is insignificant. In addition, regarding the blood circulation in a human’s stomach muscle, we are expecting to observe less temperature increase in practical study.
The power consumption of the NFC-based IU was measured as 6.3 mW that is far smaller than the counterpart 32-channel device (measured 48 mW), which utilizes a RF transceiver for data communication [
12]. To even improve the power consumption further, one can consider developing the IU using application specific integrated circuit technology. Examples of such work can be found in [
23,
24]; however, this system is still in simulation phase.
The IU’s dimension is 13 × 11 × 40 mm
3. The IU has been designed to have a narrow width. The main reason is that we aspire to implant the device through a minimally-invasive procedure. We recently developed an endoscopic procedure for implantation of the device [
25]. In this procedure, an endoscopic overtube is placed through the esophagus of the subject and the IU is then pushed inside the stomach through the tube to be implanted. Therefore, the diameter of the overtube, which corresponds to the diameter of the esophagus, is the determining factor of the width and thickness of the IU. The implant was designed such that the flexible electrode to be folded around the coated IU and the total diameter of the cross-section of the cylinder-shaped IU not to be greater than 18 mm.
Finally, In vitro studies demonstrated that the system can successfully record the signals akin to gastric SWs from 64 independent channels with a sampling rate of 16 samples per second per channel through the inductive NFC and recharge the IU’s local battery, simultaneously. We will conduct in vivo validation in pigs in the future, as their digestive system is similar to human.