Hydrogen Sensing Using Paper Sensors with Pencil Marks Decorated with Palladium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sensor Fabrication
2.2. Sensor Testing
2.3. Characterization of Graphene Sheets
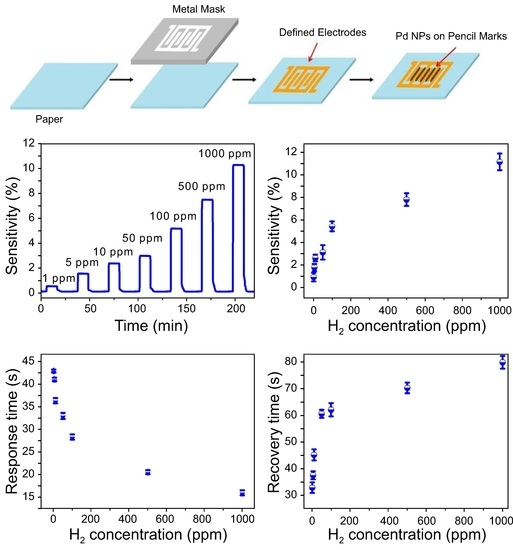
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sayago, I.; Terrado, E.; Lafuente, E.; Horrillo, M.C.; Maser, W.K.; Benito, A.M.; Navarro, R.; Urriolabeitia, E.P.; Martinez, M.T.; Gutierrez, J. Hydrogen sensors based on carbon nanotubes thin films. Synth. Met. 2005, 148, 15–19. [Google Scholar] [CrossRef]
- Krishna Kumar, M.; Ramaprabhu, S. Palladium dispersed multiwalled carbon nanotube based hydrogen sensor for fuel cell applications. Int. J. Hydrog. Energy 2007, 32, 2518–2526. [Google Scholar] [CrossRef]
- Hüberta, T.; Boon-Brettb, L.; Blackb, G.; Banacha, U. Hydrogen sensors—A review. Sens. Actuators B 2011, 157, 329–352. [Google Scholar] [CrossRef]
- Arya, S.K.; Krishnan, S.; Silva, H.; Jeana, S.; Bhansali, S. Advances in materials for room temperature hydrogen sensors. Analyst 2012, 137, 2743–2756. [Google Scholar] [CrossRef] [PubMed]
- Rýgera, I.; Vankoa, G.; Lalinskýa, T.; Kunzoa, P.; Valloa, M.; Vávraa, I.; Plecenikb, T. Pt/NiO ring gate based Schottky diode hydrogen sensors with enhanced sensitivity and thermal stability. Sens. Actuators B 2014, 202, 1–8. [Google Scholar] [CrossRef]
- Chena, D.; Wangb, J.J.; Lia, D.H.; Xua, Y. Hydrogen sensor based on Pd-functionalized film bulk acoustic resonator. Sens. Actuators B 2011, 159, 234–237. [Google Scholar] [CrossRef]
- Xianga, C.; She, Z.; Zoua, Y.; Chenga, J.; Chu, H.; Qiu, S.; Zhang, H.; Sun, L.; Xua, F. A room-temperature hydrogen sensor based on Pd nanoparticles doped TiO2 nanotubes. Ceram. Int. 2014, 128, 16343–16348. [Google Scholar] [CrossRef]
- Crespo, E.A.; Ruda, M.; de Debiaggi, S.R.; Bringa, E.M.; Braschi, F.U.; Bertolino, G. Hydrogen absorption in Pd nanoparticles of different shapes. Int. J. Hydrog. Energy. 2012, 37, 14831–14837. [Google Scholar] [CrossRef]
- Kim, J.Y.; Gila, B.P.; Abernathy, C.R.; Chung, G.Y.; Ren, R.; Pearton, S.J. Comparison of Pt/GaN and Pt/4H-SiC gas sensors. Solid State Electron. 2003, 47, 1487–1490. [Google Scholar] [CrossRef]
- Kim, J.Y.; Ren, F.; Gila, B.P.; Abernathy, C.R.; Pearton, S.J. Reversible barrier height changes in hydrogen-sensitive Pd/GaN and Pt/GaN diodes. Appl. Phys. Lett. 2003, 82, 739. [Google Scholar] [CrossRef]
- Varghese, O.K.; Gong, D.; Paulose, M.; Ong, K.G.; Grimes, C.A. Hydrogen sensing using titania nanotube. Sens. Actuators B 2003, 93, 338–344. [Google Scholar] [CrossRef]
- Rout, C.S.; Krishna, S.H.; Vivekchand, S.R.C.; Govindaraj, A.; Rao, C.N.R. Hydrogen and ethanol sensors based on ZnO nanorods, nanowires and nanotubes. Chem. Phys. Lett. 2006, 418, 586–590. [Google Scholar] [CrossRef]
- Wang, B.; Zhu, L.F.; Yang, Y.H.; Xu, N.S.; Yang, G.W. Fabrication of a SnO2 nanowire gas sensor and sensor performance for hydrogen. J. Phys. Chem. C 2008, 112, 6643–6647. [Google Scholar] [CrossRef]
- Qurashia, A.; El-Maghraby, E.M.; Yamazakia, T.; Kikuta, T. Catalyst supported growth of In2O3 nanostructures and their hydrogen gas sensing properties. Sens. Actuators B 2010, 147, 48–54. [Google Scholar] [CrossRef]
- Öztürk, S.; Kılınç, N.; Torun, I.; Kösemen, A.; Şahin, Y.; Öztürk, Z.Z. Hydrogen sensing properties of ZnO nanorods: Effects of annealing, temperature and electrode structure. Int. J. Hydrog. Energy 2014, 39, 5194–5201. [Google Scholar] [CrossRef]
- Chou, P.C.; Chen, H.I.; Liu, I.P.; Chen, C.C.; Liou, J.K.; Hsu, K.S.; Liu, W.C. Hydrogen sensing performance of a nickel oxide (NiO) thin film-based device. Int. J. Hydrog. Energy 2015, 40, 729–734. [Google Scholar] [CrossRef]
- Lu, C.; Chen, Z. High-temperature resistive hydrogen sensor based on thin nanoporous rutile TiO2 film on anodic aluminum oxide. Sens. Actuators B 2009, 140, 109–115. [Google Scholar] [CrossRef]
- Hughes, R.C.; Schubert, W.K.; Buss, R.J. Solid-state hydrogen sensors using palladium–nickel alloys: Effect of alloy composition on sensor response. J. Electrochem. Soc. 1995, 142, 249–254. [Google Scholar] [CrossRef]
- Goltsova, M.V.; Artemenko, Y.A.; Zaitsev, V.I. Kinetics and morphology of the reverse β → α hydride transformation in thermodynamically open Pd–H system. J. Alloys. Compd. 1999, 293–295, 379–384. [Google Scholar] [CrossRef]
- Pundt, A. Hydrogen in nano-sized metals. Adv. Eng. Mater. 2004, 6, 11–22. [Google Scholar] [CrossRef]
- Lee, E.; Lee, J.M.; Lee, E.; Noh, J.S.; Joe, J.H.; Jung, B.; Lee, W. Hydrogen gas sensing performance of Pd–Ni alloy thin films. Thin. Solid. Films 2010, 519, 880–884. [Google Scholar] [CrossRef]
- Lee, E.; Lee, J.M.; Koo, J.H.; Lee, W.; Lee, T. Hysteresis behavior of electrical resistance in Pd thin films during the process of absorption and desorption of hydrogen gas. Int. J. Hydrog. Energy. 2010, 35, 6984–6991. [Google Scholar] [CrossRef]
- Yang, F.; Taggart, D.K.; Penner, R.M. Joule heating a palladium nanowire sensor for accelerated response and recovery to hydrogen gas. Small 2010, 6, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, T.B.; Oates, W.A. The palladium–hydrogen system. Annu. Rev. Mater. Sci. 1991, 21, 269–304. [Google Scholar] [CrossRef]
- Kong, J.; Chapline, M.G.; Dai, H. Functionalized carbon nanotubes for molecular hydrogen sensors. Adv. Mater. 2001, 13, 1384–1386. [Google Scholar] [CrossRef]
- Sippel-Oakley, J.; Wang, H.T.; Kang, B.S.; Wu, Z.C.; Ren, F.; Rinzler, A.G.; Pearton, S.J. Carbon nanotube films for room temperature hydrogen sensing. Nanotechnology 2005, 16, 2218. [Google Scholar] [CrossRef] [PubMed]
- Mubeen, S.; Zhang, T.; Yoo, B.; Deshusses, M.A.; Myung, N.V. Palladium nanoparticles decorated single-walled carbon nanotube hydrogen sensor. J. Phys. Chem. C 2007, 11, 6321–6327. [Google Scholar] [CrossRef]
- Yang, F.; Taggart, D.K.; Penner, R.M. Fast, sensitive hydrogen gas detection using single palladium nanowires that resist fracture. Nano. Lett. 2009, 9, 2177–2182. [Google Scholar] [CrossRef]
- Offermans, P.; Tong, H.D.; van Rijn, C.J.M.; Merken, P.; Brongersma, S.H.; Crego-Calama, M. Ultralow-power hydrogen sensing with single palladium nanowires. Appl. Phys. Lett. 2009, 94, 223110. [Google Scholar] [CrossRef]
- Jeon, K.J.; Lee, J.M.; Lee, E.; Lee, W. Individual Pd nanowire hydrogen sensors fabricated by electron-beam lithography. Nanotechnology 2009, 20, 135502. [Google Scholar] [CrossRef]
- Favier, F.; Walter, E.C.; Zach, M.P.; Benter, T.; Penner, R.M. Hydrogen sensors and switches from electrodeposited palladium mesowire arrays. Science 2001, 293, 2227–2231. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Welp, U.; Hua, L.Z.; Rydh, A.; Kwok, W.K.; Wang, H.H. Fabrication of palladium nanotubes and their application in hydrogen sensing. Chem. Mater. 2005, 17, 3345–3450. [Google Scholar] [CrossRef]
- Lange, U.; Hirsch, T.; Mirsky, V.M.; Wolfbeis, O.S. Hydrogen sensor based on a graphene-palladium nanocomposite. Electrochim. Acta. 2011, 56, 3707–3712. [Google Scholar] [CrossRef]
- Johnson, J.L.; Behnam, A.; Pearton, S.J.; Ural, A. Hydrogen sensing using pd-functionalized multi-layer graphene nanoribbon networks. Adv. Mater. 2010, 22, 4877–4880. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Orozco, R.D.; Antaño-López, R.; Rodríguez-González, V. Hydrogen-gas sensors based on graphene functionalized palladium nanoparticles: Impedance response as a valuable sensor. New J. Chem. 2015, 39, 8044–8054. [Google Scholar] [CrossRef]
- Phan, D.T.; Chung, G.S. Reliability of hydrogen sensing based on bimetallic Ni–Pd/graphene composites. Int. J. Hydrog. Energy. 2014, 39, 20294–20304. [Google Scholar] [CrossRef]
- Phan, D.T.; Chung, G.S. Effects of Pd nanocube size of Pd nanocube-graphene hybrid on hydrogen sensing properties. Sens. Actuators B 2014, 204, 437–444. [Google Scholar] [CrossRef]
- Pak, Y.; Kim, S.M.; Jeong, H.; Kang, C.G.; Park, J.S.; Song, H.; Lee, R.; Myoung, N.S.; Lee, B.H.; Seo, S.; et al. Palladium-decorated hydrogen-gas sensors using periodically aligned graphene nanoribbons. ACS Appl. Mater. Interfaces 2014, 6, 13293–13298. [Google Scholar] [CrossRef]
- García-Aguilar, J.; Miguel-García, I.; Berenguer-Murcia, Á.; Cazorla-Amorós, D. Single wall carbon nanotubes loaded with Pd and NiPd nanoparticles for H2 sensing at room temperature. Carbon 2014, 66, 599–611. [Google Scholar] [CrossRef]
- Liao, X.; Liao, Q.; Yan, X.; Liang, Q.; Si, H.; Li, M.; Wu, H.; Cao, S.; Zhang, Y. Flexible and highly sensitive strain sensors fabricated by pencil drawn for wearable monitor. Adv. Funct. Mater. 2015, 25, 2395–2401. [Google Scholar] [CrossRef]
- Sun, S.; Duan, Z.; Wang, X.; Lai, G.; Zhang, X.; Wei, H.; Liu, L.; Ma, N. Cheap, flexible, and thermal-sensitive paper sensor through writing with ionic liquids containing pencil leads. ACS Appl. Mater. Inter. 2017, 9, 29140–29146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, L.; Lin, Y.; Chen, L.; Zeng, Z.; Shen, L.; Chen, Q.; Shi, W. Pencil-trace on printed silver interdigitated electrodes for paper-based NO2 gas sensors. Appl. Phys. Lett. 2015, 106, 143101. [Google Scholar] [CrossRef]
- Gadipelli, S.; Guo, Z.X. Graphene-based materials: Synthesis and gas sorption, storage and separation. Prog. Mater. Sci. 2015, 69, 1–60. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhou, H. To draw an air electrode of a Li–air battery by pencil. Energy Environ. Sci. 2011, 4, 1704–1707. [Google Scholar] [CrossRef]
- Yang, H.; Huang, L.; Chang, Q.H.; Ma, Z.J.; Xu, S.H.; Chen, Q.; Shi, W.Z. Direct growth of large-area graphene films onto oxygen plasma-etched quartz for nitrogen dioxide gas detection. J. Phys. D Appl. Phys. 2014, 47, 315101. [Google Scholar] [CrossRef]
- Brun, M.; Berther, A.; Bertolini, J.C. XPS, AES and Auger parameter of Pd and PdO. J. Electron. Spectrosc. Relat. Phenom. 1999, 104, 55–60. [Google Scholar] [CrossRef]
- Zheng, G.; Hu, L.; Wu, H.; Xie, X.; Cui, Y. Paper supercapacitors by a solvent-free drawing method. Energy. Environ. Sci. 2011, 4, 3368–3373. [Google Scholar] [CrossRef]
- Lin, C.W.; Zhao, Z.; Kim, J.; Huang, J. Pencil drawn strain gauges and chemiresistors on paper. Sci. Rep. 2014, 4, 3812. [Google Scholar] [CrossRef]
- Pumera, M. Graphene-based nanomaterials for energy storage. Energy. Environ. Sci. 2011, 4, 668–674. [Google Scholar] [CrossRef]
- Yuan, W.; Shi, G. Graphene-based gas sensors. J. Mater. Chem. A 2013, 1, 10078–10091. [Google Scholar] [CrossRef]
- Yu, P.Y.; Cardona, M. Fundamentals of Semiconductors: Physics and Materials Properties, 4th ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2005. [Google Scholar]
- Masel, R.I. Principles of Adsorption and Reaction on Solid Surfaces; Wiley: New York, NY, USA, 1996. [Google Scholar]
- Wu, W.; Liu, Z.; Jauregui, L.A.; Yu, Q.; Pillai, R.; Cao, H.; Bao, J.; Chen, Y.P.; Pei, S.S. Wafer-scale synthesis of graphene by chemical vapor deposition and its application in hydrogen sensing. Sens. Actuators B 2010, 150, 296–300. [Google Scholar] [CrossRef]
- Cabrera, A.L.; Aguayo-Soto, R. Hydrogen absorption in palladium films sensed by changes in their resistivity. Catal. Lett. 1997, 45, 79–83. [Google Scholar] [CrossRef]






| Materials | H2 Concentration (ppm) | Work Temperature | Sensitivity (%) | Response Time * | Recovery Time ** |
|---|---|---|---|---|---|
| SWCNT [27] | 100 (in air) | RT | ΔR/R0 = 0.4 | 18 min (90%) | 20 min (90%) |
| GNR network [34] | 40 (in air) | RT | ( − R)/R = 55 | 21 s (50%) | 23 s (50%) |
| GO [35] | 5000 (in air) | RT | (Rg − Ra)/Ra = 13 | 30 s (90%) | 7 min (90%) |
| GNR array [38] | 1000 (in N2) | RT | (Rs − Ri)/Ri = 3 | 60 s (90%) | 90 s (80%) |
| GLs (Our work) | 1 (in N2) | RT | (RH − Ri)/Ri = 1 | 42 s (90%) | 32 s (90%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, N.H.; Baek, U.-B.; Nahm, S.-H. Hydrogen Sensing Using Paper Sensors with Pencil Marks Decorated with Palladium. Sensors 2019, 19, 3050. https://doi.org/10.3390/s19143050
Lee NH, Baek U-B, Nahm S-H. Hydrogen Sensing Using Paper Sensors with Pencil Marks Decorated with Palladium. Sensors. 2019; 19(14):3050. https://doi.org/10.3390/s19143050
Chicago/Turabian StyleLee, Nam Hee, Un-Bong Baek, and Seung-Hoon Nahm. 2019. "Hydrogen Sensing Using Paper Sensors with Pencil Marks Decorated with Palladium" Sensors 19, no. 14: 3050. https://doi.org/10.3390/s19143050
APA StyleLee, N. H., Baek, U. -B., & Nahm, S. -H. (2019). Hydrogen Sensing Using Paper Sensors with Pencil Marks Decorated with Palladium. Sensors, 19(14), 3050. https://doi.org/10.3390/s19143050