Lessons Learned: Gastric Motility Assessment During Driving Simulation
Abstract
:1. Introduction
- Is it possible to reliably acquire slow-wave activity using a custom-made EGG sensing system during driving simulation?
- What EGG parameters are suitable for the analysis of recorded signals and, is there a correlation between them and subjective sickness assessment?
- Is there a clear difference in signals for resting, and the motion and no-motion drive?
2. Methods
2.1. Participants
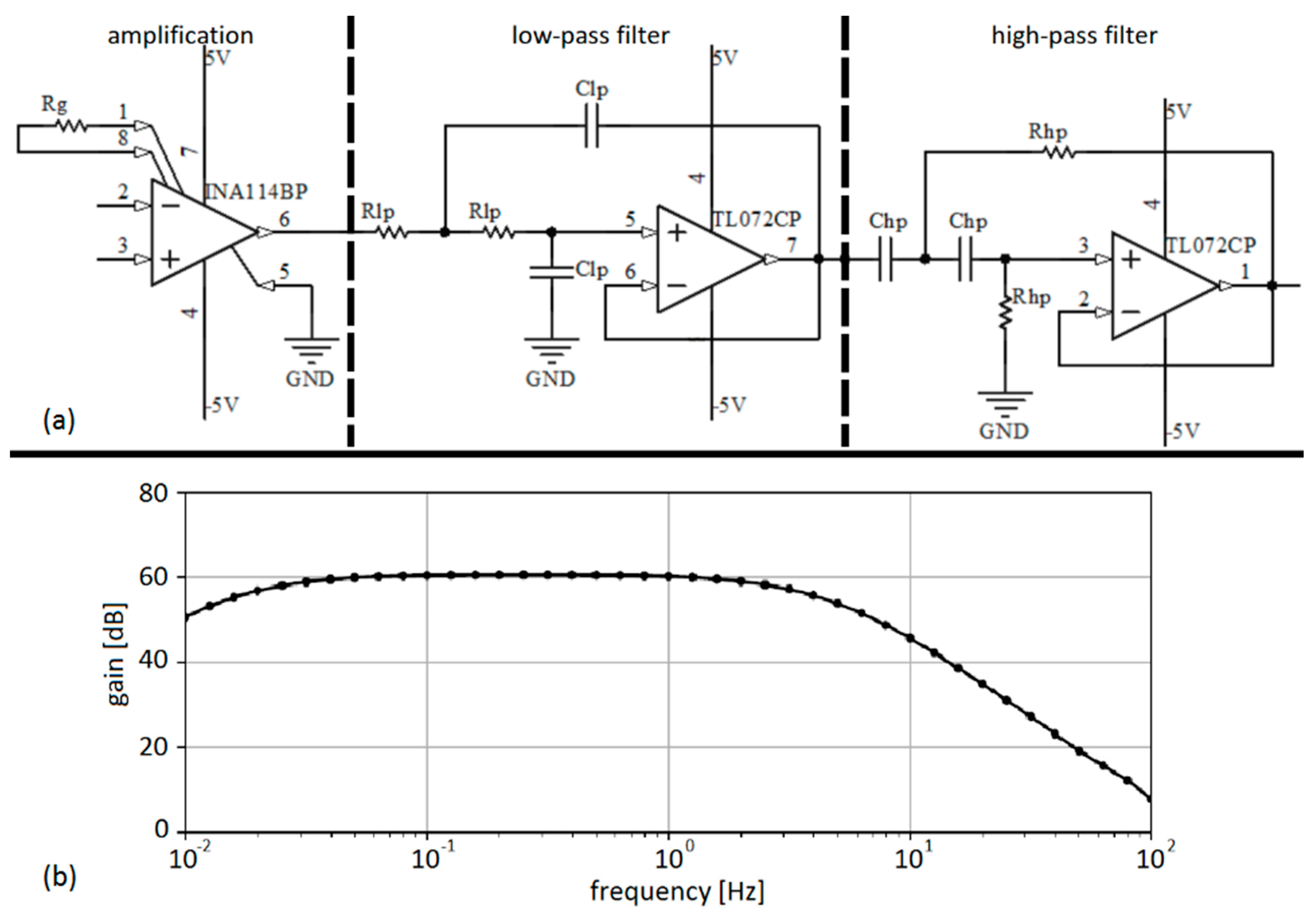
2.2. EGG Sensing System
2.3. Driving Simulator
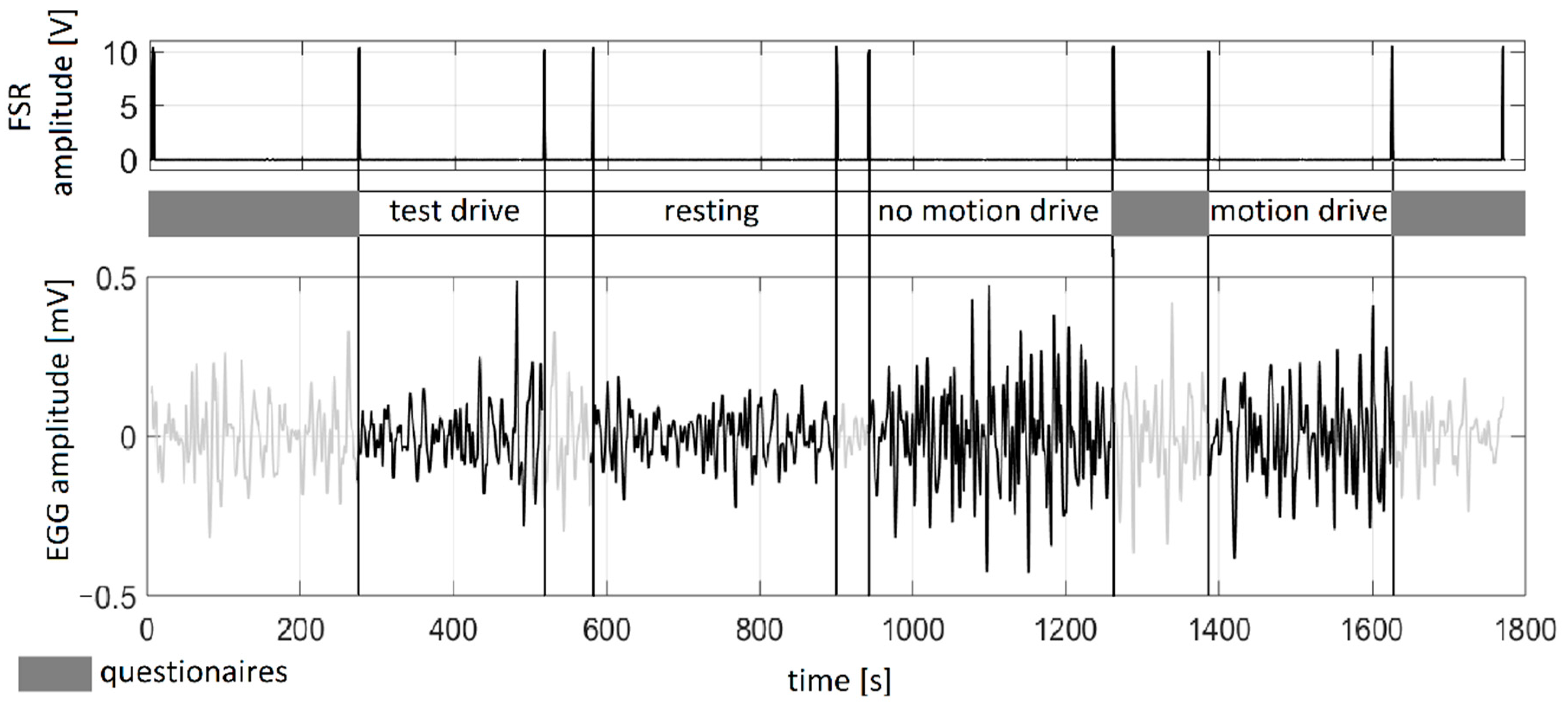
2.4. Protocol
- reference electrode—tissue covering the iliac crest;
- common electrode—on the stomach, 8 cm straight above the navel;
- channel 1 electrode—8 cm left of the common electrode inclined by 20 degrees from the line that connects the navel and the sternum;
- channel 2 electrode—8 cm left of the common electrode inclined by 55 degrees from the line that connects the navel and the sternum;
- channel 3 electrode—8 cm left of the common electrode inclined by 90 degrees from the line that connects the navel and the sternum.
- Test drive—in order to enable participants to become familiar with the driving simulator operation (~ 5 min);
- Resting sequence—baseline slow-wave activity before driving simulation was recorded (~ 5 min);
- Drive with motion—driving simulation with haptic feedback included (~ 5 min);
- Drive without motion—driving simulation with no haptic feedback (~ 5 min).
2.5. EGG Analysis
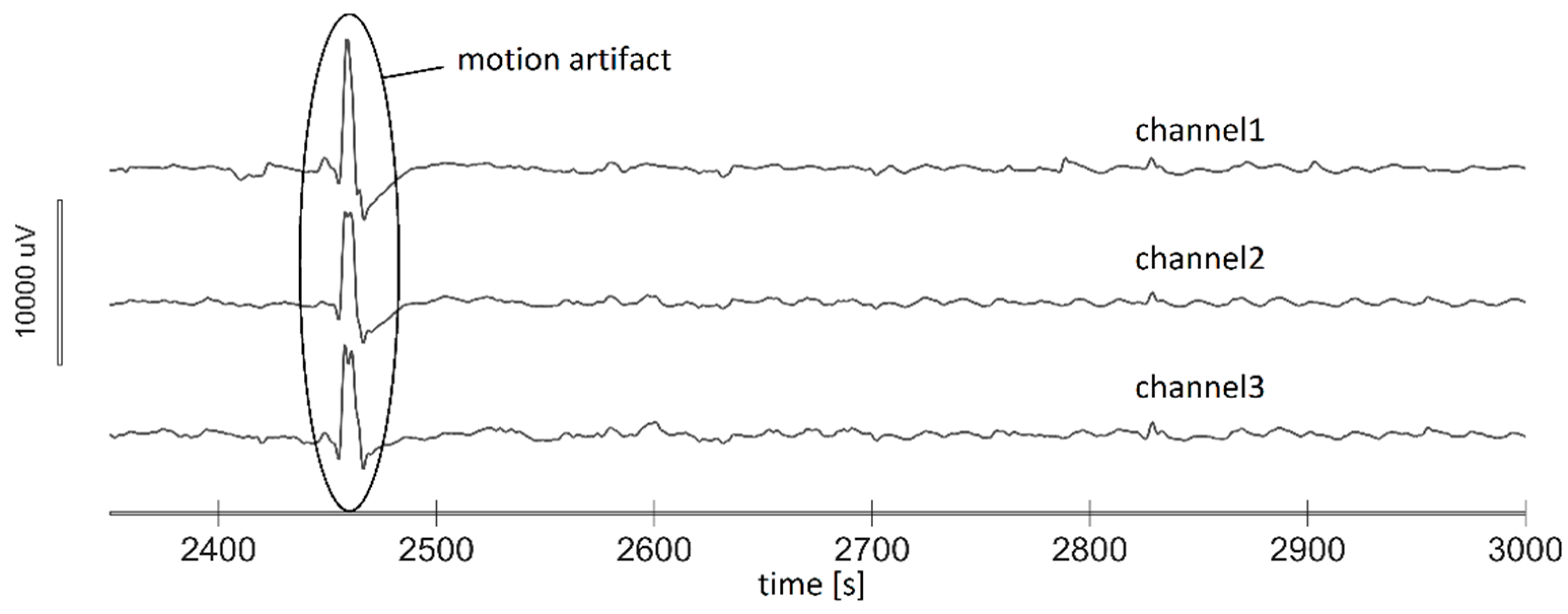
2.5.1. Motion Artifact Cancellation
- automatic selection of a channel that was least affected by artifacts, and
- manual cancellation of the remaining artifacts on the chosen channel.
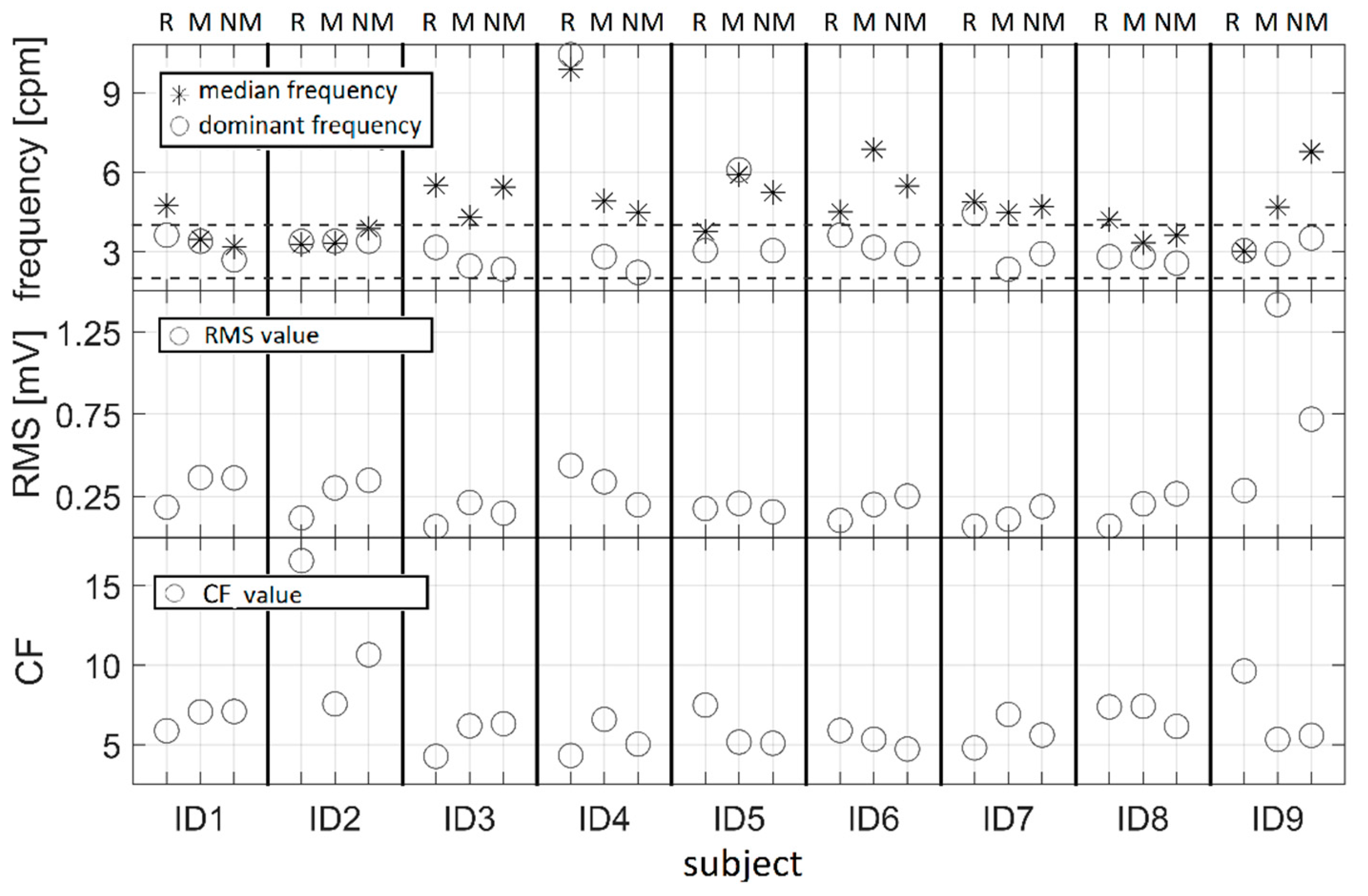
2.5.2. Feature Extraction
3. Results
- Drop-out due to severe nausea and anxiety symptoms (in one subject);
- Severe artifacts present in the signal, most probably due to the electrodes detachment and movements (in three subjects).
4. Discussion
4.1. Lessons Learned: Open-Source EGG
4.2. Lessons Learned: Protocol
- Firstly, subject’s movements can cause erroneous EGG, so body movements should be carefully controlled when recording EGG in a dynamic environment, such as driving simulation with haptic feedback.
- Secondly, EGG could be affected by the posture as stated in [26]. Although the supine position is more preferable than the sitting position, the results presented in [18] showed that successful EGG assessment can be performed in the sitting position. We assumed that the posture did not significantly affect our recordings.
- Thirdly, we faced a problem with electrode detachment in two study participants, which caused the drop-out of those two subjects. Hence, we propose the application of an additional protective layer of adhesive bandage over the surface electrodes.
4.3. Lessons Learned: Channel Selection
4.4. Lessons Learned: Artifact Cancellation
4.5. Lessons Learned: Feature Extraction
4.6. Lessons Learned: Haptic Feedback in Relation to SS
4.7. Lessons Learned: Qualitative and Quantitative Nausea Assessment
- ID4 after driving with motion,
- ID5 after driving with motion, and
- ID9 after both driving with and without motion.
5. Conclusions
- Recording of EGG during driving simulation is possible using our custom-made open-source device with careful considerations regarding recording setup and the protocol. Despite that, its effectiveness for SS assessment is yet to be shown.
- RMS values might be used for the estimation of amplitude variations in EGG signals. Correlation between RMS and nausea should be examined in a future study. MF, CF, and DF could be used for the assessment of the EGG spectrum. In order to confirm these findings, future study should be performed on a larger sample.
- There was no clear difference between resting, motion, and no-motion sequences, except for an increase in RMS for driving sessions compared to resting.
- EGG under dynamic conditions should be recorded by carefully following protocol recommendations and the application of more than one EGG channel.
- The resting sequence of EGG recording should be obtained prior to any simulator activity.
- EGG signals should be visually examined in order to detect and manually extract motion artifacts.
- Features for a description of frequency content (DF, MF, and CF) should be carefully examined prior to any conclusions.
- Further improvement of the EGG device, primarily realization on a PCB with consideration regarding filter design.
- Assessment of nausea on a larger study group with higher statistical power in order to divide subjects into nausea and non-nausea groups. For the selection of target population, it could be beneficial to use a Motion Sickness Susceptibility Questionnaire to estimate susceptibility to suffer from SS.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Classen, S.; Bewernitz, M.; Shechtman, O. Driving simulator sickness: An evidence-based review of the literature. Am. J. Occup. Ther. 2011, 65, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Blana, E. Driving Simulator Salidation Studies: A Literature Review; Institute of Transport Studies, University of Leeds: Leeds, UK, 1996. [Google Scholar]
- Hoesch, A.; Poeschl, S.; Weidner, F.; Walter, R.; Doering, N. The relationship between visual attention and simulator sickness: A driving simulation study. In Proceedings of the 25th IEEE Conference on Virtual Reality 3D User Interfaces, Reutlingen, Germany, 12–22 March 2018; pp. 1–2. [Google Scholar]
- Klüver, M.; Herrigel, C.; Preuß, S.; Schöner, H.P.; Hecht, H. Comparing the incidence of simulator sickness in five different driving simulators. In Proceedings of the Driving Simulation Conference, Tübingen, Germany, 16–18 September 2015; pp. 1–8. [Google Scholar]
- Ebenholtz, S.M. Oculomotor Systems and Perception; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]
- Keshavarz, B.; Hecht, H. Validating an efficient method to quantify motion sickness. Hum. Factors. 2011, 53, 415–526. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, R.S.; Lane, E.N.; Berbaum, K.S.; Lilienthal, M.G. Simulator sickness questionnaire: An enhanced method for quantifying simulator sickness. Int. J. Aviat. Psychol. 1993, 3, 203–220. [Google Scholar] [CrossRef]
- Min, B.C.; Chung, S.C.; Min, K.Y.; Sakamodo, K. Psychophysiological evaluation of simulator sickness evoked by a graphic simulator. Appl. Ergon. 2004, 35, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, W.C. Action currents in stomach and intestine. Am. J. Physiol-Legacy. Content. 1922, 58, 476–493. [Google Scholar] [CrossRef]
- Murakami, H. Current status of multichannel electrogastrography and examples of its use. J. Smooth. Muscle. Res. 2013, 49, 78–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bortolotti, M. Electrogastrography: A seductive promise, only partially kept. Am. J. Gastroenterol. 1998, 93, 1791–1794. [Google Scholar] [CrossRef] [PubMed]
- Koch, K.L.; Stern, R.M. Handbook of Electrogastrography; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Riezzo, G.; Russo, F.; Indrio, F. Electrogastrography in adults and children: The strength, pitfalls, and clinical significance of the cutaneous recording of the gastric electrical activity. Biomed. Res. Int. 2013, 2013, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Tokumaru, O.; Mizumoto, C.; Takada, Y.; Tatsuno, J.; Ashida, H. Vector analysis of electrogastrography during motion sickness. Dig. Dis. Sci. 2003, 48, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Koch, K.L. Gastric dysrhythmias: A potential objective measure of nausea. Exp. Brain. Res. 2014, 232, 2553–2561. [Google Scholar] [CrossRef] [PubMed]
- Stern, R.M. The psychophysiology of nausea. Acta. Biol. Hung. 2002, 53, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.D.Z.; Zou, X.; Lin, X.; Ouyang, S.; Liang, J. Detection of gastric slow wave propagation from the cutaneous electrogastrogram. Am. J. Physiol. 1999, 277, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Miljković, N.; Popović, N.B.; Prodanov, M.; Sodnik, J. Assessment of sickness in virtual environments. In Proceedings of the 9th International Conference on Information Society and Technology (ICIST 2019), Kopaonik, Serbia, 10–13 March 2019. In print. [Google Scholar]
- Shupak, A.; Gordon, C.R. Motion sickness: Advances in pathogenesis, prediction, prevention, and treatment. Aviat. Space. Environ. Med. 2006, 77, 1213–1223. [Google Scholar] [PubMed]
- Golding, J.F. Motion sickness susceptibility. Auton. Neurosci. 2006, 129, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Popović, N.B.; Miljković, N.; Popović, M.B. Simple gastric motility assessment method with a single-channel electrogastrogram. Biomed. Tech. 2018, 64, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Pejovic, P. An automated system for frequency response measurement based on free software tools. In Proceedings of the International symposium of industrial electronics, Banja Luka, Bosnia and Herzegovina, 1–3 November 2018; pp. 1–5. [Google Scholar]
- Vengust, M.; Možina, D.; Pušenjak, N.; Zevnik, L.; Sodnik, J.; Kaluža, B.; Tavčar, A. NERVteh 4DOF motion car driving simulator. In Proceedings of the 6th International Conference on Automotive User Interfaces and Interactive Vehicular Applications, Seattle, WA, USA, 17–19 September 2014; pp. 1–6. [Google Scholar]
- O’Grady, G.; Timothy, R.A.; Paskaranandavadivel, N.; Erickson, J.C.; Wells, C.I. Methods for high-resolution electrical mapping in the gastrointestinal tract. IEEE. Rev. Biomed. Eng. 2019, 12, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Komorowski, D. EGG DWPack: System for multi-channel electrogastrographic signals recording and analysis. J. Med. Syst. 2018, 42, 201. [Google Scholar] [CrossRef] [PubMed]
- Jonderko, K.; Kasicka-Joderko, A.; Blonska-Fajfrowska, B. Does body posture affect the parameters of a cutaneous electrogastrogram? J. Smooth. Muscle. Res. 1998, 41, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Popović, N.B.; Miljković, N.; Djordjevic, O.; Šekara, T.B. Artifact cancellation using median filter, moving average filter, and fractional derivatives in biomedical signals. In Proceedings of the International Conference on Fractional Differentiation and its Applications (ICFDA 2016), Novi Sad, Serbia, 18–20 July 2016; pp. 150–161. [Google Scholar]
- Himi, N.; Koga, T.; Nakamura, E.; Kobashi, M.; Yamane, M.; Tsujioka, K. Differences in autonomic responses between subjects with and without nausea while watching an irregularly oscillating video. Auton. Neurosci. 2004, 116, 46–53. [Google Scholar] [CrossRef] [PubMed]




| Subject | Age [years] | Sex [F-Female, M-Male] | Height [cm] | Weight [kg] | Driving Experience [years] | Driving Simulator Experience [Yes/No] |
|---|---|---|---|---|---|---|
| ID1 | 23 | F | 173 | 60 | 5 | Yes |
| ID2 | 23 | M | 172 | 60 | 5 | No |
| ID3 | 26 | F | 169 | 56 | 8 | No |
| ID4 | 23 | M | 180 | 88 | 4 | No |
| ID5 | 32 | M | 192 | 115 | 14 | Yes |
| ID6 | 47 | M | 182 | 87 | 29 | No |
| ID7 | 23 | M | 173 | 65 | 5 | Yes |
| ID8 | 40 | F | 160 | 49 | 15 | Yes |
| ID9 | 25 | F | 169 | 59 | 6 | Yes |
| IDN1 | 26 | M | 183 | 97 | 6 | Yes |
| IDN2 | 27 | M | 181 | 75 | 9 | Yes |
| IDN3 | 33 | M | 177 | 60 | 15 | Yes |
| IDN4 | 35 | M | 186 | 78 | 17 | No |
| Amplification | LP Filtering | HP Filtering | |||||
|---|---|---|---|---|---|---|---|
| Instrumentation amplifier | Rg | Operational amplifier | Rlp | Clp | Operational amplifier | Rhp | Chp |
| INA114BP | 50 Ω | TL072CP | 15 kΩ | 2.2 µF | TL072CP | 10 MΩ | 1 µF |
| Subject | Resting | No-Motion Drive | Motion Drive | |||
|---|---|---|---|---|---|---|
| Nausea | Total | Nausea | Total | Nausea | Total | |
| ID1 | 28.6 | 49.2 | 0.0 | 19.0 | 0.0 | 34.2 |
| ID2 | 28.6 | 30.0 | 28.6 | 18.8 | / | / |
| ID3 | 9.5 | 15.0 | 9.5 | 31.5 | 19.1 | 22.7 |
| ID4 | 19.1 | 11.3 | 28.6 | 37.6 | 38.2 | 68.0 |
| ID5 | 38.2 | 18.7 | 38.2 | 15.1 | 57.2 | 68.1 |
| ID6 | 0.0 | 7.6 | 19.1 | 15.0 | 19.1 | 7.5 |
| ID7 | 28.6 | 22.6 | 28.6 | 7.5 | 9.5 | 7.5 |
| ID8 | 0.0 | 11.2 | 9.5 | 11 | 0.0 | 0.0 |
| ID9 | 9.5 | 26.4 | 76.3 | 117.4 | 47.7 | 71.8 |
| IDN1 | 47.7 | 68.1 | 57.2 | 83.2 | 57.2 | 56.5 |
| IDN2 | 0.0 | 0.0 | 19.0 | 18.9 | 0.0 | 0.0 |
| IDN3 | 9.5 | 7.5 | 38.2 | 49.2 | / | / |
| IDN4 | 0.0 | 0.0 | 0.0 | 0.0 | 9.5 | 3.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popović, N.B.; Miljković, N.; Stojmenova, K.; Jakus, G.; Prodanov, M.; Sodnik, J. Lessons Learned: Gastric Motility Assessment During Driving Simulation. Sensors 2019, 19, 3175. https://doi.org/10.3390/s19143175
Popović NB, Miljković N, Stojmenova K, Jakus G, Prodanov M, Sodnik J. Lessons Learned: Gastric Motility Assessment During Driving Simulation. Sensors. 2019; 19(14):3175. https://doi.org/10.3390/s19143175
Chicago/Turabian StylePopović, Nenad B., Nadica Miljković, Kristina Stojmenova, Grega Jakus, Milana Prodanov, and Jaka Sodnik. 2019. "Lessons Learned: Gastric Motility Assessment During Driving Simulation" Sensors 19, no. 14: 3175. https://doi.org/10.3390/s19143175






