Recent Advances in Electric-Double-Layer Transistors for Bio-Chemical Sensing Applications
Abstract
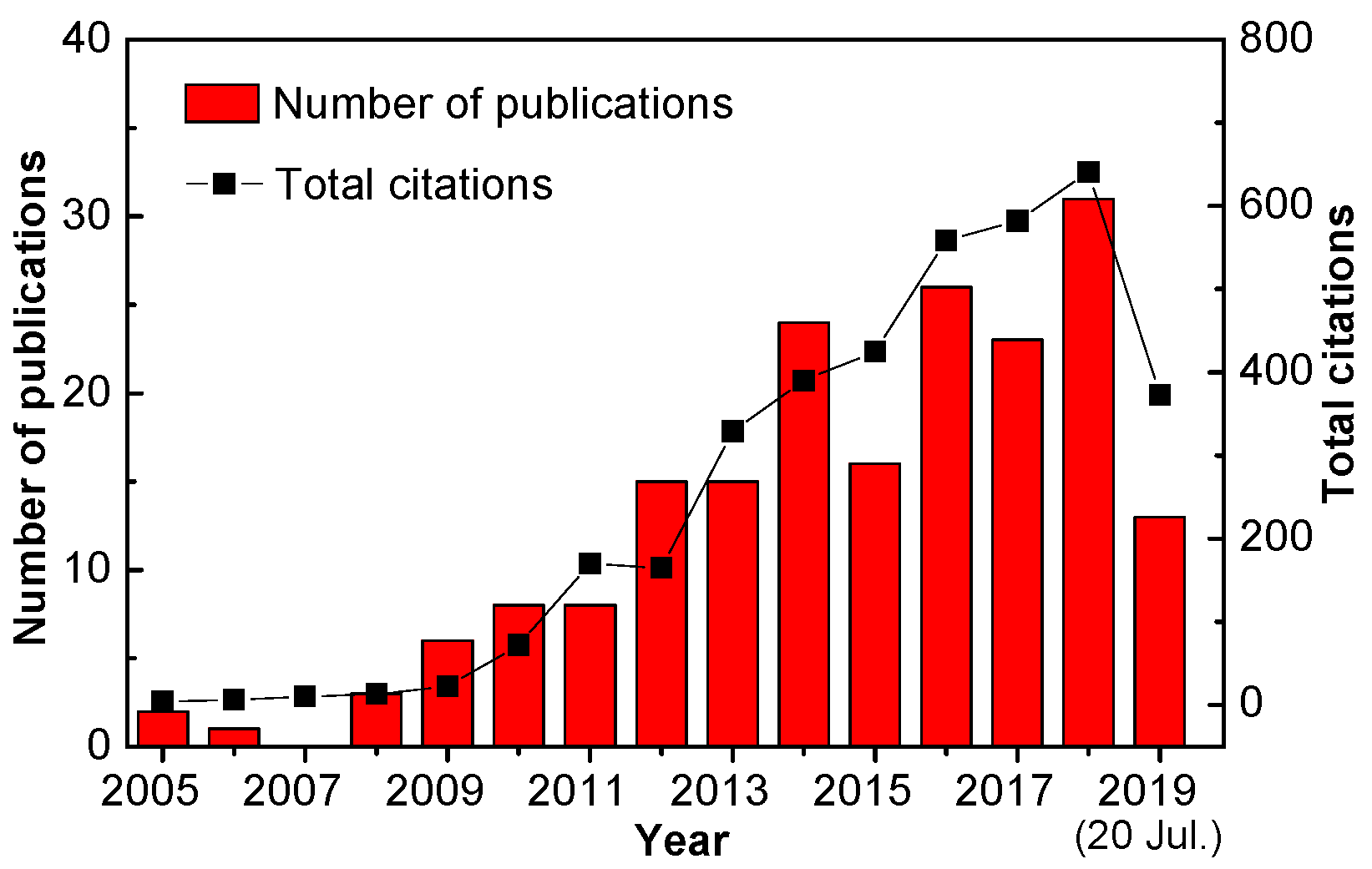
:1. Introduction
2. Physics of EDLTs: The Basics for Sensing
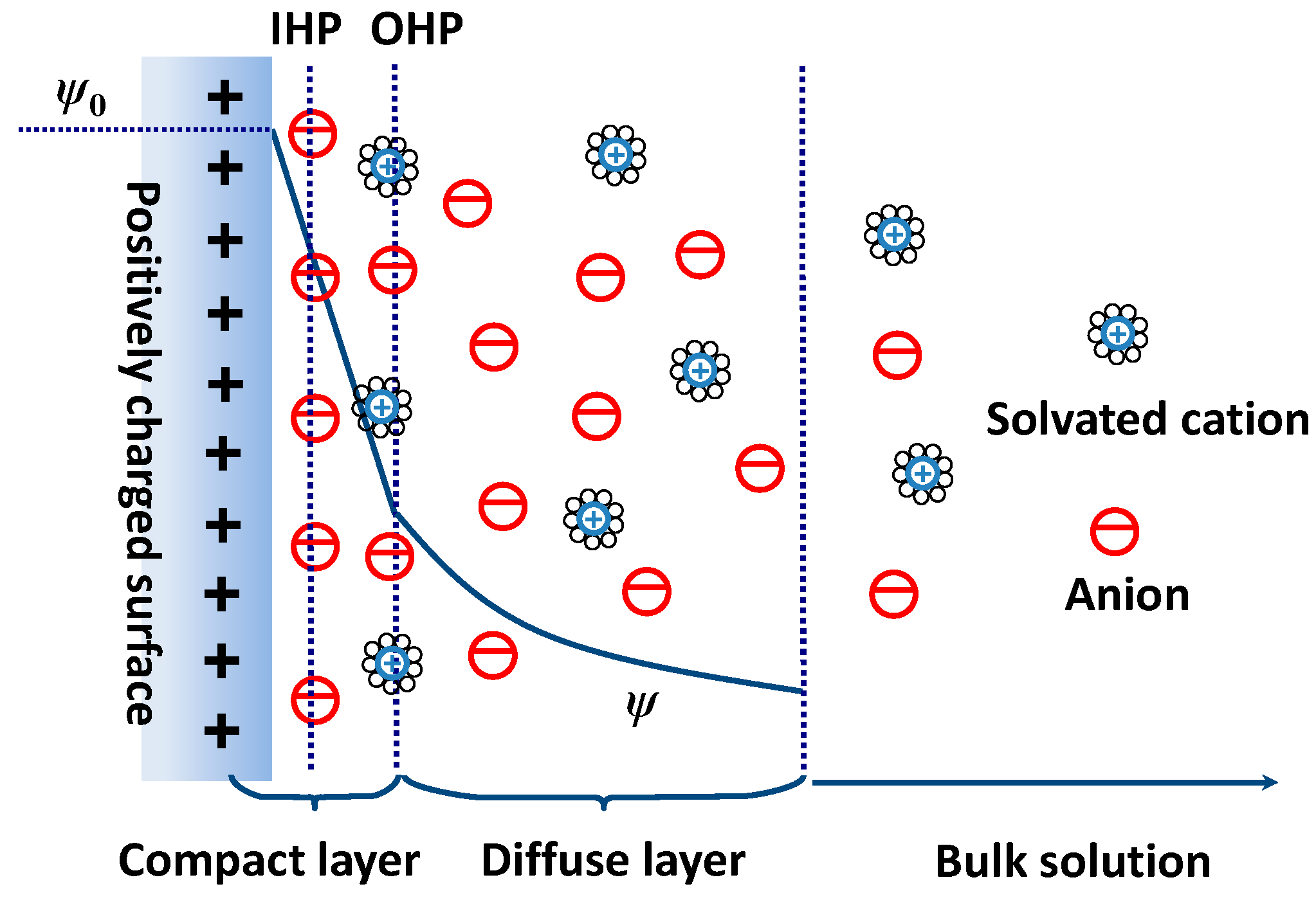
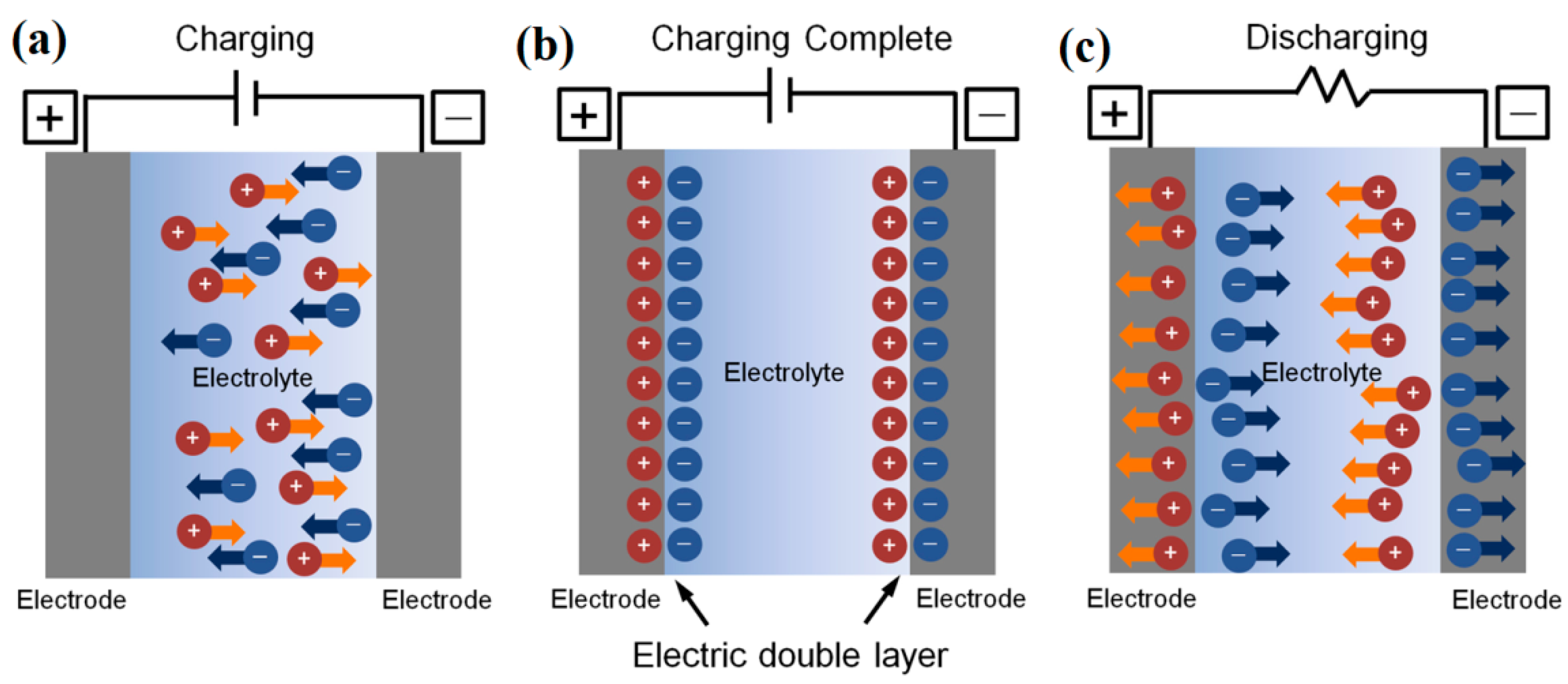
2.1. EDL Capacitance and EDLTs
2.2. Materials in EDLTs
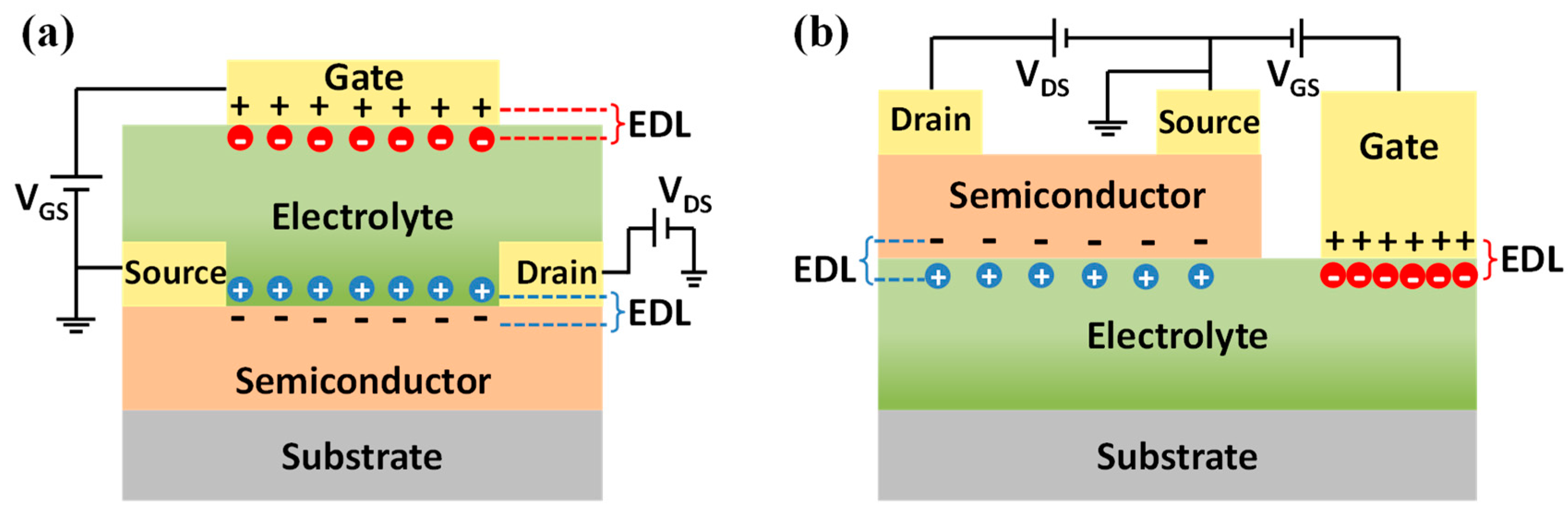
2.3. Ion-Modulation in EDLTs
2.4. Sensing Principle of EDLTs
3. EDLT Based Biochemical Sensors
3.1. Organic-Based EDLT Sensors
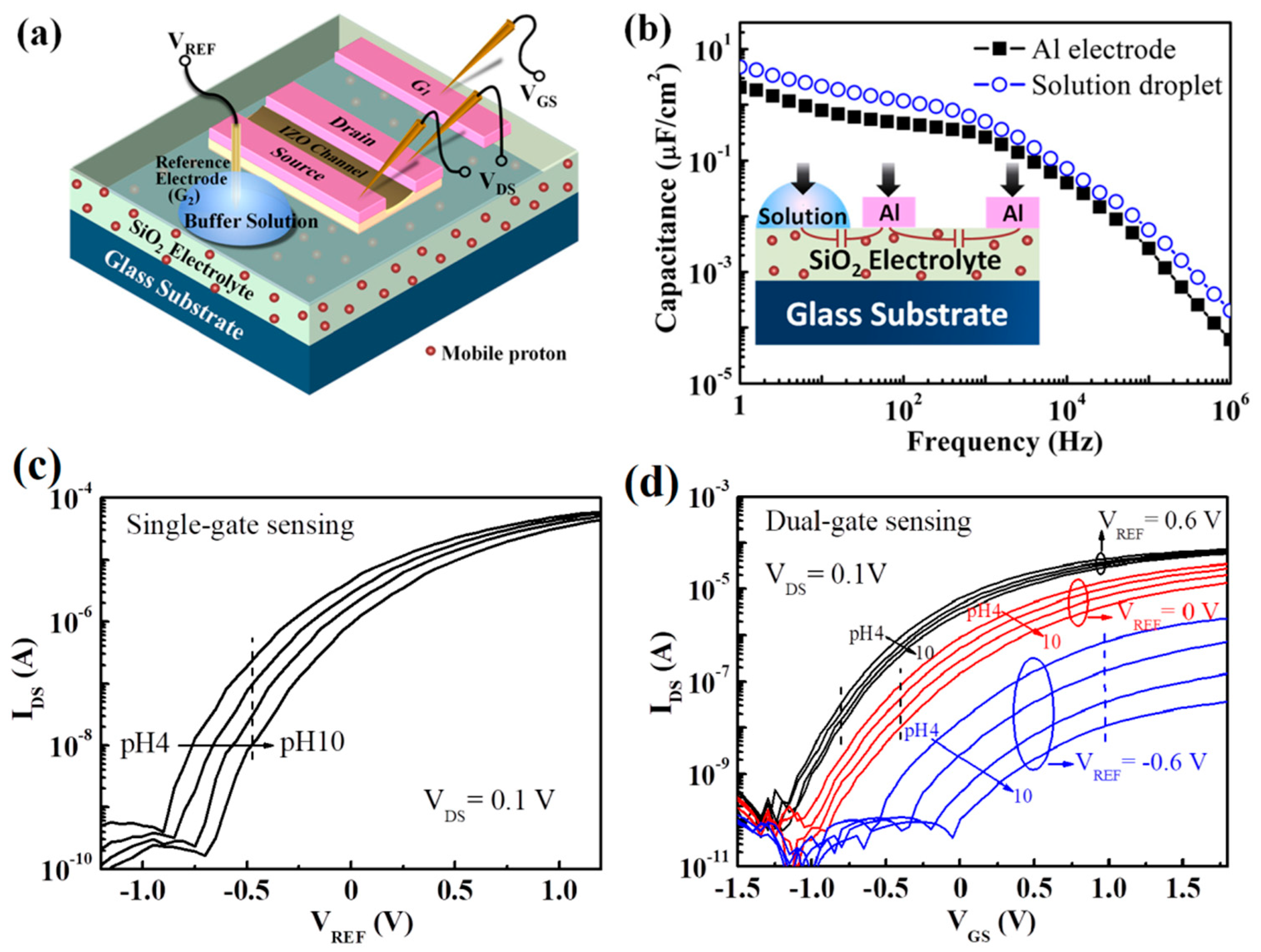
3.2. Oxide-Based EDLT Sensors
3.3. Nanomaterial-Based EDLT Sensors
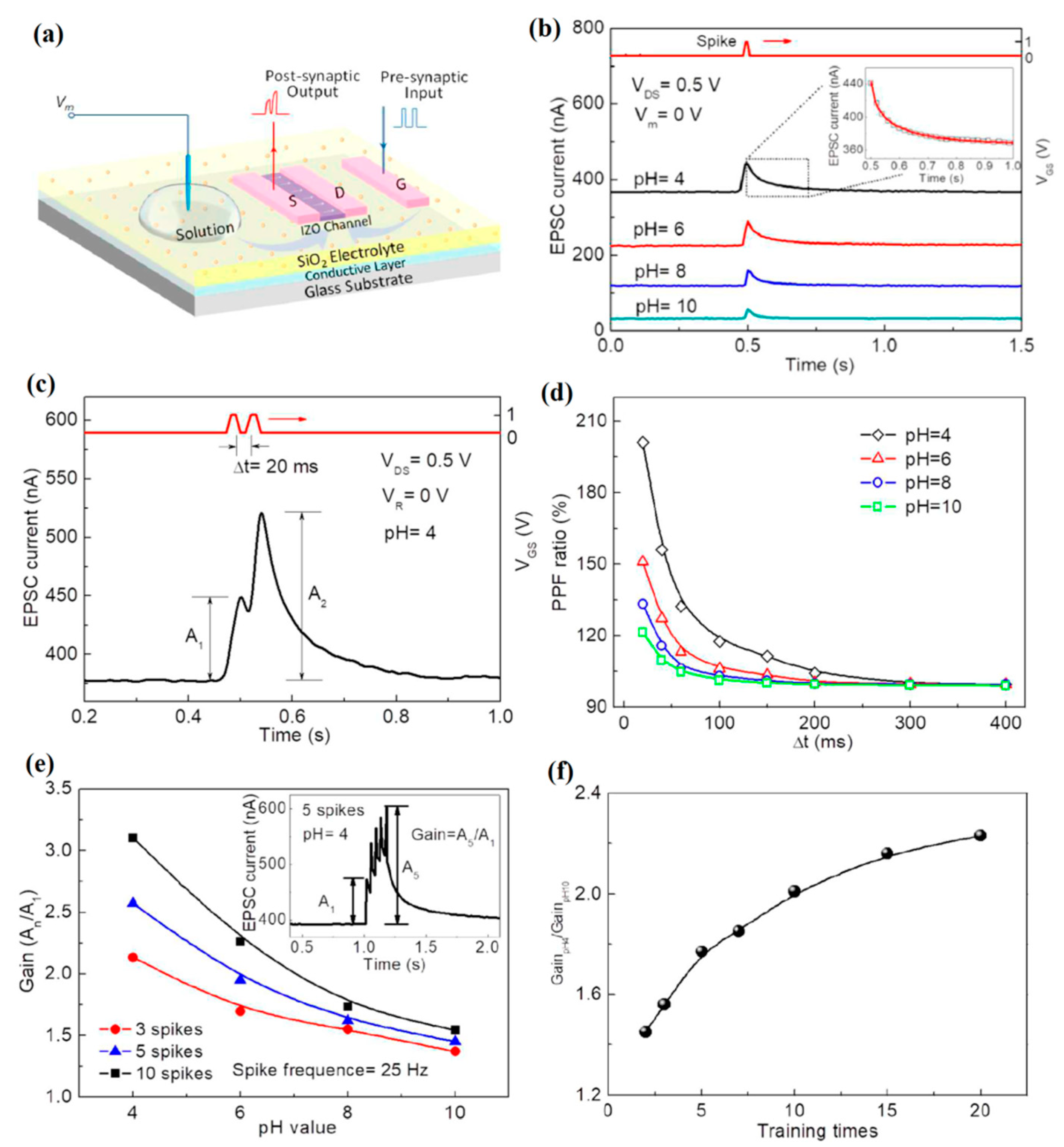
3.4. Neuromorphic EDLT Sensors
4. Conclusions and Outlook
Funding
Conflicts of Interest
References
- Fan, X.; White, I.M.; Shopova, S.I.; Zhu, H.; Suter, J.D.; Sun, Y. Sensitive optical biosensors for unlabeled targets: A review. Anal. Chim. Acta 2008, 620, 8–26. [Google Scholar] [CrossRef] [PubMed]
- Diculescu, V.C.; Paquim, A.-M.C.; Brett, A.M.O. Electrochemical DNA sensors for detection of DNA damage. Sensors 2005, 5, 377–393. [Google Scholar] [CrossRef]
- Roy, S.; Gao, Z.Q. Nanostructure-based electrical biosensors. Nano Today 2009, 4, 318–334. [Google Scholar] [CrossRef]
- Gabl, R.; Feucht, H.D.; Zeininger, H.; Eckstein, G.; Schreiter, M.; Primig, R.; Pitzer, D.; Wersing, W. First results on label-free detection of DNA and protein molecules using a novel integrated sensor technology based on gravimetric detection principles. Biosens. Bioelectron. 2004, 19, 615–620. [Google Scholar] [CrossRef]
- Lange, K.; Rapp, B.E.; Rapp, M. Surface acoustic wave biosensors: A review. Anal. Bioanal. Chem. 2008, 391, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Que, E.L.; Chang, C.J. Responsive magnetic resonance imaging contrast agents as chemical sensors for metals in biology and medicine. Chem. Soc. Rev. 2010, 39, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.C.; Zhang, W.D. Electrodeposition of TiO2 Nanoparticles on multiwalled carbon nanotube arrays for hydrogen peroxide sensing. Electroanalysis 2009, 21, 988–993. [Google Scholar] [CrossRef]
- Katz, E.; Alfonta, L.; Willner, I. Chronopotentiometry and Faradaic impedance spectroscopy as methods for signal transduction in immunosensors. Sens. Actuators B Chem. 2001, 76, 134–141. [Google Scholar] [CrossRef]
- Ion, A.C.; Moutet, J.C.; Pailleret, A.; Popescu, A.; St Aman, E.; Siebert, E.; Ungureanu, E.M. Electrochemical recognition of metal cations by poly (crown ether ferrocene) films investigated by cyclic voltammetry and electrochemical impedance spectroscopy. J. Electroanal. Chem. 1999, 464, 24–30. [Google Scholar] [CrossRef]
- Patolsky, F.; Zayats, M.; Katz, E.; Willner, I. Precipitation of an insoluble product on enzyme monolayer electrodes for biosensor applications: Characterization by faradaic impedance spectroscopy, cyclic voltammetry, and microgravimetric quartz crystal microbalance analyses. Anal. Chem. 1999, 71, 3171–3180. [Google Scholar] [CrossRef]
- Katz, H.E. Chemically sensitive field-effect transistors and chemiresistors: New materials and device structures. Electroanalysis 2004, 16, 1837–1842. [Google Scholar] [CrossRef]
- Bergveld, P. Thirty years of ISFETOLOGY—What happened in the past 30 years and what may happen in the next 30 years. Sens. Actuators B Chem. 2003, 88, 1–20. [Google Scholar] [CrossRef]
- Mabeck, J.T.; Malliaras, G.G. Chemical and biological sensors based on organic thin-film transistors. Anal. Bioanal. Chem. 2006, 384, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Seong, T.W.; Jeun, M.; Lee, K.H. Field-effect biosensors for on-site detection: Recent advances and promising targets. Adv. Healthc. Mater. 2017, 6, 1700796. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, X.; Tan, Y.; Yuan, Q. Recent progress in flexible and wearable bio-electronics based on nanomaterials. Nano Res. 2017, 10, 1560–1583. [Google Scholar] [CrossRef]
- Li, C.M.; Dong, H.; Cao, X.; Luong, J.H.T.; Zhang, X. Implantable electrochemical sensors for biomedical and clinical applications: Progress, problems, and future possibilities. Curr. Med. Chem. 2007, 14, 937–951. [Google Scholar] [PubMed]
- Bergveld, P. Development of an ion-sensitive solid-state device for neurophysiological measurements. IEEE Trans. Biomed. Eng. 1970, 17, 70–71. [Google Scholar] [CrossRef]
- Han, S.T.; Peng, H.; Sun, Q.; Venkatesh, S.; Chung, K.S.; Lau, S.C.; Zhou, Y.; Roy, V.A.L. An overview of the development of flexible sensors. Adv. Mater. 2017, 29, 1700375. [Google Scholar] [CrossRef]
- Hulea, I.N.; Fratini, S.; Xie, H.; Mulder, C.L.; Iossad, N.N.; Rastelli, G.; Ciuchi, S.; Morpurgo, A.F. Tunable Fröhlich polarons in organic single-crystal transistors. Nat. Mater. 2006, 5, 982–986. [Google Scholar] [CrossRef]
- Kim, S.H.; Hong, K.; Xie, W.; Lee, K.H.; Zhang, S.; Lodge, T.P.; Frisbie, C.D. Electrolyte-gated transistors for organic and printed electronics. Adv. Mater. 2013, 25, 1822–1846. [Google Scholar] [CrossRef]
- Xia, Y.; Xie, W.; Ruden, P.P.; Frisbie, C.D. Carrier localization on surfaces of organic semiconductors gated with electrolytes. Phys. Rev. Lett. 2010, 105, 036802. [Google Scholar] [CrossRef]
- Yuan, H.T.; Shimotani, H.; Ye, J.T.; Yoon, S.; Aliah, H.; Tsukazaki, A.; Kawasaki, M.; Iwasa, Y. Electrostatic and electrochemical nature of liquid-gated electric-double-layer transistors based on oxide semiconductors. J. Am. Chem. Soc. 2010, 132, 18402–18407. [Google Scholar] [CrossRef]
- Jiang, J.; Wan, Q.; Sun, J.; Lu, A. Ultralow-voltage transparent electric-double-layer thin-film transistors processed at room-temperature. Appl. Phys. Lett. 2009, 95, 152114. [Google Scholar] [CrossRef]
- Yuan, H.; Shimotani, H.; Tsukazaki, A.; Ohtomo, A.; Kawasaki, M.; Iwasa, Y. Hydrogenation-induced surface polarity recognition and proton memory behavior at protic-ionic-liquid/oxide electric-double-layer interfaces. J. Am. Chem. Soc. 2010, 132, 6672–6678. [Google Scholar] [CrossRef]
- Wang, D.J.; Noel, V.; Piro, B. Electrolytic gated organic field-effect transistors for application in biosensors—A review. Electronics 2016, 5, 9. [Google Scholar] [CrossRef]
- Wang, N.; Yang, A.; Fu, Y.; Li, Y.; Yan, F. Functionalized organic thin film transistors for biosensing. Acc. Chem. Res. 2019, 52, 277–287. [Google Scholar] [CrossRef]
- Liu, N.; Liu, Y.H.; Zhu, L.Q.; Shi, Y.; Wan, Q. Low-cost pH sensors based on low-voltage oxide-based electric-double-layer thin film transistors. IEEE Electron Device Lett. 2014, 35, 482–484. [Google Scholar] [CrossRef]
- Yan, F.; Zhang, M.; Li, J. Solution-gated graphene transistors for chemical and biological sensors. Adv. Healthc. Mater. 2014, 3, 313–331. [Google Scholar] [CrossRef]
- Du, H.; Lin, X.; Xu, Z.; Chu, D. Electric double-layer transistors: A review of recent progress. J. Mater. Sci. 2015, 50, 5641–5673. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef]
- Bisri, S.Z.; Shimizu, S.; Nakano, M.; Iwasa, Y. Endeavor of iontronics: From fundamentals to applications of ion-controlled electronics. Adv. Mater. 2017, 29, 1607054. [Google Scholar] [CrossRef]
- Ono, S.; Miwa, K.; Seki, S.; Takeya, J. A comparative study of organic single-crystal transistors gated with various ionic-liquid electrolytes. Appl. Phys. Lett. 2009, 94, 063301. [Google Scholar] [CrossRef]
- Ono, S.; Seki, S.; Hirahara, R.; Tominari, Y.; Takeya, J. High-mobility, low-power, and fast-switching organic field-effect transistors with ionic liquids. Appl. Phys. Lett. 2008, 92, 103313. [Google Scholar] [CrossRef] [Green Version]
- Ohta, H.; Sato, Y.; Kato, T.; Kim, S.; Nomura, K.; Ikuhara, Y.; Hosono, H. Field-induced water electrolysis switches an oxide semiconductor from an insulator to a metal. Nat. Commun. 2010, 1, 118. [Google Scholar] [CrossRef]
- Fujimoto, T.; Awaga, K. Electric-double-layer field-effect transistors with ionic liquids. Phys. Chem. Chem. Phys. 2013, 15, 8983–9006. [Google Scholar] [CrossRef]
- Ueno, K.; Shimotani, H.; Yuan, H.; Ye, J.; Kawasaki, M.; Iwasa, Y. Field-induced superconductivity in electric double layer transistors. J. Phys. Soc. Jpn. 2014, 83, 032001. [Google Scholar] [CrossRef]
- Leighton, C. Electrolyte-based ionic control of functional oxides. Nat. Mater. 2019, 18, 13–18. [Google Scholar] [CrossRef]
- Alam, A.U.; Qin, Y.; Nambiar, S.; Yeow, J.T.W.; Howlader, M.M.R.; Hu, N.X.; Deen, M.J. Polymers and organic materials-based pH sensors for healthcare applications. Prog. Mater. Sci. 2018, 96, 174–216. [Google Scholar] [CrossRef]
- Tarabella, G.; Mohammadi, F.M.; Coppede, N.; Barbero, F.; Iannotta, S.; Santato, C.; Cicoira, F. New opportunities for organic electronics and bioelectronics: Ions in action. Chem. Sci. 2013, 4, 1395–1409. [Google Scholar] [CrossRef]
- Yuen, J.D.; Dhoot, A.S.; Namdas, E.B.; Coates, N.E.; Heeney, M.; McCulloch, I.; Moses, D.; Heeger, A.J. Electrochemical doping in electrolyte-gated polymer transistors. J. Am. Chem. Soc. 2007, 129, 14367–14371. [Google Scholar] [CrossRef]
- Kergoat, L.; Piro, B.; Berggren, M.; Horowitz, G.; Minh-Chau, P. Advances in organic transistor-based biosensors: From organic electrochemical transistors to electrolyte-gated organic field-effect transistors. Anal. Bioanal. Chem. 2012, 402, 1813–1826. [Google Scholar] [CrossRef]
- Heller, I.; Chatoor, S.; Mannik, J.; Zevenbergen, M.A.G.; Dekker, C.; Lemay, S.G. Influence of electrolyte composition on liquid-gated carbon nanotube and graphene transistors. J. Am. Chem. Soc. 2010, 132, 17149–17156. [Google Scholar] [CrossRef]
- Panzer, M.J.; Frisbie, C.D. Polymer electrolyte-gated organic field-effect transistors: Low-voltage, high-current switches for organic electronics and testbeds for probing electrical transport at high charge carrier density. J. Am. Chem. Soc. 2007, 129, 6599–6607. [Google Scholar] [CrossRef]
- Ueno, K.; Nakamura, S.; Shimotani, H.; Ohtomo, A.; Kimura, N.; Nojima, T.; Aoki, H.; Iwasa, Y.; Kawasaki, M. Electric-field-induced superconductivity in an insulator. Nat. Mater. 2008, 7, 855–858. [Google Scholar] [CrossRef]
- Mondal, S.; Ghimire, R.R.; Raychaudhuri, A.K. Enhancing photoresponse by synergy of gate and illumination in electric double layer field effect transistors fabricated on n-ZnO. Appl. Phys. Lett. 2013, 103, 231105. [Google Scholar] [CrossRef]
- Panzer, M.J.; Frisbie, C.D. High carrier density and metallic conductivity in poly(3-hexylthiophene) achieved by electrostatic charge injection. Adv. Funct. Mater. 2006, 16, 1051–1056. [Google Scholar] [CrossRef]
- Dobrynin, A.V.; Rubinstein, M. Theory of polyelectrolytes in solutions and at surfaces. Prog. Polym. Sci. 2005, 30, 1049–1118. [Google Scholar] [CrossRef]
- Laiho, A.; Herlogsson, L.; Forchheimer, R.; Crispin, X.; Berggren, M. Controlling the dimensionality of charge transport in organic thin-film transistors. Proc. Natl. Acad. Sci. USA. 2011, 108, 15069–15073. [Google Scholar] [CrossRef] [Green Version]
- Ozel, T.; Gaur, A.; Rogers, J.A.; Shim, M. Polymer electrolyte gating of carbon nanotube network transistors. Nano Lett. 2005, 5, 905–911. [Google Scholar] [CrossRef]
- Cho, J.H.; Lee, J.; Xia, Y.; Kim, B.; He, Y.; Renn, M.J.; Lodge, T.P.; Frisbie, C.D. Printable ion-gel gate dielectrics for low-voltage polymer thin-film transistors on plastic. Nat. Mater. 2008, 7, 900–906. [Google Scholar] [CrossRef]
- Yuan, H.; Shimotani, H.; Tsukazaki, A.; Ohtomo, A.; Kawasaki, M.; Iwasa, Y. High-density carrier accumulation in ZnO field-effect transistors gated by electric double layers of ionic liquids. Adv. Funct. Mater. 2009, 19, 1046–1053. [Google Scholar] [CrossRef]
- Rehman, A.; Zeng, X.Q. Ionic liquids as green solvents and electrolytes for robust chemical sensor development. Acc. Chem. Res. 2012, 45, 1667–1677. [Google Scholar] [CrossRef]
- Zhu, L.Q.; Sun, J.; Wu, G.D.; Zhang, H.L.; Wan, Q. Self-assembled dual in-plane gate thin-film transistors gated by nanogranular SiO2 proton conductors for logic applications. Nanoscale 2013, 5, 1980–1985. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, L.; Wan, Q. Nanogranular Al2O3 proton conducting films for low-voltage oxide-based homojunction thin-film transistors. J. Mater. Chem. C 2013, 1, 2781–2786. [Google Scholar] [CrossRef]
- Wu, G.; Zhang, H.; Zhou, J.; Huang, A.; Wan, Q. Proton conducting zeolite films for low-voltage oxide-based electric-double-layer thin-film transistors and logic gates. J. Mater. Chem. C 2013, 1, 5669–5674. [Google Scholar] [CrossRef]
- Jiang, J.; Cao, D.; Jiang, D.; Wu, J. Kinetic charging inversion in ionic liquid electric double layers. J. Phys. Chem. Lett. 2014, 5, 2195–2200. [Google Scholar] [CrossRef]
- Lai, Q.; Zhang, L.; Li, Z.; Stickle, W.F.; Williams, R.S.; Chen, Y. Ionic/electronic hybrid materials integrated in a synaptic transistor with signal processing and learning functions. Adv. Mater. 2010, 22, 2448–2453. [Google Scholar] [CrossRef]
- Fujimoto, T.; Matsushita, M.M.; Awaga, K. Ionic-liquid component dependence of carrier injection and mobility for electric-double-layer organic thin-film transistors. J. Phys. Chem. C 2012, 116, 5241–5246. [Google Scholar] [CrossRef]
- Schmidt, E.; Shi, S.; Ruden, P.P.; Frisbie, C.D. Characterization of the electric double layer formation dynamics of a metal/ionic liquid/metal structure. ACS Appl. Mater. Interfaces 2016, 8, 14879–14884. [Google Scholar] [CrossRef]
- Kurig, H.; Vestli, M.; Tonurist, K.; Jaenes, A.; Lust, E. Influence of room temperature ionic liquid anion chemical composition and electrical charge delocalization on the supercapacitor properties. J. Electrochem. Soc. 2012, 159, A944–A951. [Google Scholar] [CrossRef]
- Liu, N.; Zhu, L.Q.; Xiao, H.; Wan, C.J.; Liu, Y.H.; Chao, J.Y. Transient Characteristics for Proton Gating in Laterally Coupled Indium–Zinc-Oxide Transistors. ACS Appl. Mater. Interfaces 2015, 7, 6205–6210. [Google Scholar] [CrossRef]
- Bernards, D.A.; Malliaras, G.G. Steady-state and transient behavior of organic electrochemical transistors. Adv. Funct. Mater. 2007, 17, 3538–3544. [Google Scholar] [CrossRef]
- Ye, J.T.; Inoue, S.; Kobayashi, K.; Kasahara, Y.; Yuan, H.T.; Shimotani, H.; Iwasa, Y. Liquid-gated interface superconductivity on an atomically flat film. Nat. Mater. 2010, 9, 125–128. [Google Scholar] [CrossRef]
- Walter, J.; Wang, H.; Luo, B.; Frisbie, C.D.; Leighton, C. Electrostatic versus electrochemical doping and control of ferromagnetism in ion-gel-gated ultrathin La0.5Sr0.5CoO3-delta. ACS Nano 2016, 10, 7799–7810. [Google Scholar] [CrossRef]
- Fabiano, S.; Crispin, X.; Berggren, M. Ferroelectric polarization induces electric double layer bistability in electrolyte-gated field-effect transistors. ACS Appl. Mater. Interfaces 2014, 6, 438–442. [Google Scholar] [CrossRef]
- Koo, J.; Yang, J.; Cho, B.; Jo, H.; Lee, K.H.; Kang, M.S. Nonvolatile electric double-layer transistor memory devices embedded with Au nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 9563–9570. [Google Scholar] [CrossRef]
- Magliulo, M.; Mallardi, A.; Mulla, M.Y.; Cotrone, S.; Pistillo, B.R.; Favia, P.; Vikholm-Lundin, I.; Palazzo, G.; Torsi, L. Electrolyte-gated organic field-effect transistor sensors based on supported biotinylated phospholipid bilayer. Adv. Mater. 2013, 25, 2090–2094. [Google Scholar] [CrossRef]
- Kim, K.; Chen, C.L.; Truong, Q.; Shen, A.M.; Chen, Y. A carbon nanotube synapse with dynamic logic and learning. Adv. Mater. 2013, 25, 1693–1698. [Google Scholar] [CrossRef]
- Zhu, L.Q.; Wan, C.J.; Guo, L.Q.; Shi, Y.; Wan, Q. Artificial synapse network on inorganic proton conductor for neuromorphic systems. Nat. Commun. 2014, 5, 3158. [Google Scholar] [CrossRef] [Green Version]
- Barsan, N.; Weimar, U. Conduction model of metal oxide gas sensors. J. Electroceram. 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Van Hal, R.E.G.; Eijkel, J.C.T.; Bergveld, P. A general model to describe the electrostatic potential at electrolyte oxide interfaces. Adv. Colloid Interface Sci. 1996, 69, 31–62. [Google Scholar] [CrossRef] [Green Version]
- Schöning, M.J.; Poghossian, A. Bio FEDs (Field-effect devices): State-of-the-Art and new directions. Electroanalysis 2006, 18, 1893–1900. [Google Scholar] [CrossRef]
- Cherstvy, A.G. Detection of DNA hybridization by field-effect DNA-based biosensors: Mechanisms of signal generation and open questions. Biosens. Bioelectron. 2013, 46, 162–170. [Google Scholar] [CrossRef]
- Spijkman, M.J.; Brondijk, J.J.; Geuns, T.C.T.; Smits, E.C.P.; Cramer, T.; Zerbetto, F.; Stoliar, P.; Biscarini, F.; Blom, P.W.M.; de Leeuw, D.M. Dual-gate organic field-effect transistors as potentiometric sensors in aqueous solution. Adv. Funct. Mater. 2010, 20, 898–905. [Google Scholar] [CrossRef]
- Kergoat, L.; Piro, B.; Berggren, M.; Pham, M.-C.; Yassar, A.; Horowitz, G. DNA detection with a water-gated organic field-effect transistor. Org. Electron. 2012, 13, 1–6. [Google Scholar] [CrossRef]
- Gao, N.; Zhou, W.; Jiang, X.; Hong, G.; Fu, T.-M.; Lieber, C.M. General strategy for biodetection in high ionic strength solutions using transistor-based nanoelectronic sensors. Nano Lett. 2015, 15, 2143–2148. [Google Scholar] [CrossRef]
- Elnathan, R.; Kwiat, M.; Pevzner, A.; Engel, Y.; Burstein, L.; Khatchtourints, A.; Lichtenstein, A.; Kantaev, R.; Patolsky, F. Biorecognition layer engineering: Overcoming screening limitations of nanowire-based FET devices. Nano Lett. 2012, 12, 5245–5254. [Google Scholar] [CrossRef]
- Chu, C.-H.; Sarangadharan, I.; Regmi, A.; Chen, Y.-W.; Hsu, C.-P.; Chang, W.-H.; Lee, G.-Y.; Chyi, J.-I.; Chen, C.-C.; Shiesh, S.-C.; et al. Beyond the Debye length in high ionic strength solution: Direct protein detection with field-effect transistors (FETs) in human serum. Sci. Rep. 2017, 7, 5256. [Google Scholar] [CrossRef]
- Nair, P.R.; Alam, M.A. Design considerations of silicon nanowire biosensors. IEEE Trans. Electron Devices 2007, 54, 3400–3408. [Google Scholar] [CrossRef]
- Gao, X.P.; Zheng, G.; Lieber, C.M. Subthreshold regime has the optimal sensitivity for nanowire FET biosensors. Nano Lett. 2010, 10, 547–552. [Google Scholar] [CrossRef]
- Pud, S.; Jing, L.; Sibiliev, V.; Petrychuk, M.; Kovalenko, V.; Offenhausser, A.; Vitusevich, S. Liquid and back gate coupling effect: Towards biosensing with lowest detection limit. Nano Lett. 2014, 14, 578–584. [Google Scholar] [CrossRef]
- Rajan, N.K.; Routenberg, D.A.; Reed, M.A. Optimal signal-to-noise ratio for silicon nanowire biochemical sensors. Appl. Phys. Lett. 2011, 98, 264107. [Google Scholar] [CrossRef]
- Rajan, N.K.; Routenberg, D.A.; Chen, J.; Reed, M.A. 1/f noise of silicon nanowire BioFETs. IEEE Electron Device Lett. 2010, 31, 615–617. [Google Scholar] [CrossRef]
- Shirak, O.; Shtempluck, O.; Kotchtakov, V.; Bahir, G.; Yaish, Y.E. High performance horizontal gate-all-around silicon nanowire field-effect transistors. Nanotechnology 2012, 23, 395202. [Google Scholar] [CrossRef]
- Tarasov, A.; Fu, W.; Knopfmacher, O.; Brunner, J.; Calame, M.; Schonenberger, C. Signal-to-noise ratio in dual-gated silicon nanoribbon field-effect sensors. Appl. Phys. Lett. 2011, 98, 012114. [Google Scholar] [CrossRef] [Green Version]
- Buth, F.; Donner, A.; Sachsenhauser, M.; Stutzmann, M.; Garrido, J.A. Biofunctional electrolyte-gated organic field-effect transistors. Adv. Mater. 2012, 24, 4511–4517. [Google Scholar] [CrossRef]
- Casalini, S.; Leonardi, F.; Cramer, T.; Biscarini, F. Organic field-effect transistor for label-free dopamine sensing. Org. Electron. 2013, 14, 156–163. [Google Scholar] [CrossRef]
- Palazzo, G.; De Tullio, D.; Magliulo, M.; Mallardi, A.; Intranuovo, F.; Mulla, M.Y.; Favia, P.; Vikholm-Lundin, I.; Torsi, L. Detection beyond Debye’s length with an electrolyte-gated organic field-effect transistor. Adv. Mater. 2015, 27, 911–916. [Google Scholar] [CrossRef]
- Herlogsson, L.; Crispin, X.; Robinson, N.D.; Sandberg, M.; Hagel, O.J.; Gustafsson, G.; Berggren, M. Low-voltage polymer field-effect transistors gated via a proton conductor. Adv. Mater. 2007, 19, 97–101. [Google Scholar] [CrossRef]
- Lee, J.; Kaake, L.G.; Cho, J.H.; Zhu, X.Y.; Lodge, T.P.; Frisbie, C.D. Ion gel-gated polymer thin-film transistors: Operating mechanism and characterization of gate dielectric capacitance, switching speed, and stability. J. Phys. Chem. C 2009, 113, 8972–8981. [Google Scholar] [CrossRef]
- Knopfmacher, O.; Hammock, M.L.; Appleton, A.L.; Schwartz, G.; Mei, J.; Lei, T.; Pei, J.; Bao, Z. Highly stable organic polymer field-effect transistor sensor for selective detection in the marine environment. Nat. Commun. 2014, 5, 2954. [Google Scholar] [CrossRef] [Green Version]
- White, S.P.; Dorfman, K.D.; Frisbie, C.D. Label-free DNA sensing platform with low-voltage electrolyte-gated transistors. Anal. Chem. 2015, 87, 1861–1866. [Google Scholar] [CrossRef]
- White, S.P.; Dorfman, K.D.; Frisbie, C.D. Operating and sensing mechanism of electrolyte-gated transistors with floating gates: Building a platform for amplified biodetection. J. Phys. Chem. C 2016, 120, 108–117. [Google Scholar] [CrossRef]
- Schmoltner, K.; Kofler, J.; Klug, A.; List-Kratochvil, E.J. Electrolyte-gated organic field-effect transistor for selective reversible ion detection. Adv. Mater. 2013, 25, 6895–6899. [Google Scholar] [CrossRef]
- Seshadri, P.; Manoli, K.; Schneiderhan-Marra, N.; Anthes, U.; Wierzchowiec, P.; Bonrad, K.; Di Franco, C.; Torsi, L. Low-picomolar, label-free procalcitonin analytical detection with an electrolyte-gated organic field-effect transistor based electronic immunosensor. Biosens. Bioelectron. 2018, 104, 113–119. [Google Scholar] [CrossRef]
- Macchia, E.; Manoli, K.; Holzer, B.; Di Franco, C.; Picca, R.A.; Cioffi, N.; Scamarcio, G.; Palazzo, G.; Torsi, L. Selective single-molecule analytical detection of C-reactive protein in saliva with an organic transistor. Anal. Bioanal. Chem. 2019, 411, 4899–4908. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.T.K.; Tran, H.V.; Vu, T.T.; Reisberg, S.; Noel, V.; Mattana, G.; Pham, M.C.; Piro, B. Peptide-modified electrolyte-gated organic field effect transistor. Application to Cu2+ detection. Biosens. Bioelectron. 2019, 127, 118–125. [Google Scholar] [CrossRef]
- Nguyen, T.T.K.; Nguyen, T.N.; Anquetin, G.; Reisberg, S.; Noel, V.; Mattana, G.; Touzeau, J.; Barbault, F.; Pham, M.C.; Piro, B. Triggering the Electrolyte-Gated Organic Field-Effect Transistor output characteristics through gate functionalization using diazonium chemistry: Application to biodetection of 2,4-dichlorophenoxyacetic acid. Biosens. Bioelectron. 2018, 113, 32–38. [Google Scholar] [CrossRef]
- Berto, M.; Diacci, C.; D’Agata, R.; Pinti, M.; Bianchini, E.; Di Lauro, M.; Casalini, S.; Cossarizza, A.; Berggren, M.; Simon, D.; et al. EGOFET peptide aptasensor for label-free detection of inflammatory cytokines in complex fluids. Adv. Biosyst. 2018, 2, 1700072. [Google Scholar] [CrossRef]
- Berto, M.; Vecchi, E.; Baiamonte, L.; Condo, C.; Sensi, M.; Di Lauro, M.; Sola, M.; De Stradis, A.; Biscarini, F.; Minafra, A.; et al. Label free detection of plant viruses with organic transistor biosensors. Sens. Actuators B Chem. 2019, 281, 150–156. [Google Scholar] [CrossRef]
- Piro, B.; Wang, D.; Benaoudia, D.; Tibaldi, A.; Anquetin, G.; Noel, V.; Reisberg, S.; Mattana, G.; Jackson, B. Versatile transduction scheme based on electrolyte-gated organic field-effect transistor used as immunoassay readout system. Biosens. Bioelectron. 2017, 92, 215–220. [Google Scholar] [CrossRef]
- Minamiki, T.; Hashima, Y.; Sasaki, Y.; Minami, T. An electrolyte-gated polythiophene transistor for the detection of biogenic amines in water. Chem. Commun. 2018, 54, 6907–6910. [Google Scholar] [CrossRef]
- Lu, A.; Sun, J.; Jiang, J.; Wan, Q. Low-voltage transparent electric-double-layer ZnO-based thin-film transistors for portable transparent electronics. Appl. Phys. Lett. 2010, 96, 043114. [Google Scholar] [CrossRef]
- Liu, H.; Sun, J.; Jiang, J.; Tang, Q.; Wan, Q. Ultralow-voltage transparent In2O3 nanowire electric-double-layer transistors. IEEE Electron Device Lett. 2011, 32, 315–317. [Google Scholar] [CrossRef]
- Wan, C.J.; Zhou, J.M.; Shi, Y.; Wan, Q. Classical conditioning mimicked in junctionless IZO electric-double-layer thin-film transistors. IEEE Electron Device Lett. 2014, 35, 414–416. [Google Scholar] [CrossRef]
- Jiang, J.; Sun, J.; Zhu, L.; Wu, G.; Wan, Q. Dual in-plane-gate oxide-based thin-film transistors with tunable threshold voltage. Appl. Phys. Lett. 2011, 99, 113504. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, N.; Zhu, L.; Shi, Y.; Wan, Q. Energy-efficient artificial synapses based on flexible indium-gallium-zinc-oxide electric-double-layer transistors. IEEE Electron Device Lett. 2015, 36, 198–200. [Google Scholar] [CrossRef]
- Chae, M.-S.; Park, J.H.; Son, H.W.; Hwang, K.S.; Kim, T.G. IGZO-based electrolyte-gated field-effect transistor for in situ biological sensing platform. Sens. Actuators B Chem. 2018, 262, 876–883. [Google Scholar] [CrossRef]
- Liu, N.; Gan, L.; Liu, Y.; Gui, W.; Li, W.; Zhang, X. Improving pH sensitivity by field-induced charge regulation in flexible biopolymer electrolyte gated oxide transistors. Appl. Surf. Sci. 2017, 419, 206–212. [Google Scholar] [CrossRef]
- Liu, Y.; Li, B.; Zhu, L.; Feng, P.; Shi, Y.; Wan, Q. Dopamine detection based on low-voltage oxide homojunction electric-double-layer thin-film transistors. IEEE Electron Device Lett. 2016, 37, 778–781. [Google Scholar] [CrossRef]
- Guo, D.; Zhuo, M.; Zhang, X.; Xu, C.; Jiang, J.; Gao, F.; Wan, Q.; Li, Q.; Wang, T. Indium-tin-oxide thin film transistor biosensors for label-free detection of avian influenza virus H5N1. Anal. Chim. Acta 2013, 773, 83–88. [Google Scholar] [CrossRef]
- Liang, L.; Zhang, S.; Wu, W.; Zhu, L.; Xiao, H.; Liu, Y.; Zhang, H.; Javaid, K.; Cao, H. Extended-gate-type IGZO electric-double-layer TFT immunosensor with high sensitivity and low operation voltage. Appl. Phys. Lett. 2016, 109, 173501. [Google Scholar] [CrossRef]
- Liu, N.; Liu, Y.H.; Feng, P.; Zhu, L.Q.; Shi, Y.; Wan, Q. Enhancing the pH sensitivity by laterally synergic modulation in dual-gate electric-double-layer transistors. Appl. Phys. Lett. 2015, 106, 073507. [Google Scholar] [CrossRef]
- Li, B.R.; Hsieh, Y.J.; Chen, Y.X.; Chung, Y.T.; Pan, C.Y.; Chen, Y.T. An ultrasensitive nanowire-transistor biosensor for detecting dopamine release from living PC12 cells under hypoxic stimulation. J. Am. Chem. Soc. 2013, 135, 16034–16037. [Google Scholar] [CrossRef]
- Pachauri, V.; Vlandas, A.; Kern, K.; Balasubramanian, K. Site-specific self-assembled liquid-gated ZnO nanowire transistors for sensing applications. Small 2010, 6, 589–594. [Google Scholar] [CrossRef]
- Fathollahzadeh, M.; Hosseini, M.; Norouzi, M.; Ebrahimi, A.; Fathipour, M.; Kolahdouz, M.; Haghighi, B. Immobilization of glucose oxidase on ZnO nanorods decorated electrolyte-gated field effect transistor for glucose detection. J. Solid State Electrochem. 2018, 22, 61–67. [Google Scholar] [CrossRef]
- Li, W.S.; Hou, P.X.; Liu, C.; Sun, D.M.; Yuan, J.T.; Zhao, S.Y.; Yin, L.C.; Cong, H.T.; Cheng, H.M. High-quality, highly concentrated semiconducting single-wall carbon nanotubes for use in field effect transistors and biosensors. ACS Nano 2013, 7, 6831–6839. [Google Scholar] [CrossRef]
- Palaniappan, A.; Goh, W.H.; Fam, D.W.H.; Rajaseger, G.; Chan, C.E.Z.; Hanson, B.J.; Moochhala, S.M.; Mhaisalkar, S.G.; Liedberg, B. Label-free electronic detection of bio-toxins using aligned carbon nanotubes. Biosens. Bioelectron. 2013, 43, 143–147. [Google Scholar] [CrossRef]
- Bhatt, V.D.; Joshi, S.; Becherer, M.; Lugli, P. Flexible, low-cost sensor based on electrolyte gated carbon nanotube field effect transistor for organo-phosphate detection. Sensors 2017, 17, 1147. [Google Scholar] [CrossRef]
- Dong, X.; Shi, Y.; Huang, W.; Chen, P.; Li, L.-J. Electrical detection of DNA hybridization with single-base specificity using transistors based on CVD-grown graphene sheets. Adv. Mater. 2010, 22, 1649–1653. [Google Scholar] [CrossRef]
- Sohn, I.Y.; Kim, D.J.; Jung, J.H.; Yoon, O.J.; Thanh, T.N.; Quang, T.T.; Lee, N.E. pH sensing characteristics and biosensing application of solution-gated reduced graphene oxide field-effect transistors. Biosens. Bioelectron. 2013, 45, 70–76. [Google Scholar] [CrossRef]
- Sudibya, H.G.; He, Q.Y.; Zhang, H.; Chen, P. Electrical detection of metal ions using field-effect transistors based on micropatterned reduced graphene oxide films. ACS Nano 2011, 5, 1990–1994. [Google Scholar] [CrossRef]
- Campos, R.; Borme, J.; Guerreiro, J.R.; Machado, G.; Cerqueira, M.F.G.; Petrovykh, D.Y.; Alpuim, P. Attomolar label-free detection of DNA hybridization with electrolyte-gated graphene field-effect transistors. ACS Sens. 2019, 4, 286–293. [Google Scholar] [CrossRef]
- Sarkar, D.; Liu, W.; Xie, X.; Anselmo, A.C.; Mitragotri, S.; Banerjee, K. MoS2 field-effect transistor for next-generation label-free biosensors. ACS Nano 2014, 8, 3992–4003. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Park, S.; Vosguerichian, M.; Bao, Z. A review of fabrication and applications of carbon nanotube film-based flexible electronics. Nanoscale 2013, 5, 1727–1752. [Google Scholar] [CrossRef]
- Ramnani, P.; Saucedo, N.M.; Mulchandani, A. Carbon nanomaterial-based electrochemical biosensors for label-free sensing of environmental pollutants. Chemosphere 2016, 143, 85–98. [Google Scholar] [CrossRef] [Green Version]
- Rosenblatt, S.; Yaish, Y.; Park, J.; Gore, J.; Sazonova, V.; McEuen, P.L. High performance electrolyte gated carbon nanotube transistors. Nano Lett. 2002, 2, 869–872. [Google Scholar] [CrossRef]
- Melzer, K.; Bhatt, V.P.; Jaworska, E.; Mittermeier, R.; Maksymiuk, K.; Michalska, A.; Lugli, P. Enzyme assays using sensor arrays based on ion-selective carbon nanotube field-effect transistors. Biosens. Bioelectron. 2016, 84, 7–14. [Google Scholar] [CrossRef]
- Jacobs, C.B.; Peairs, M.J.; Venton, B.J. Review: Carbon nanotube based electrochemical sensors for biomolecules. Anal. Chim. Acta 2010, 662, 105–127. [Google Scholar] [CrossRef]
- Gruner, G. Carbon nanotube transistors for biosensing applications. Anal. Bioanal. Chem. 2006, 384, 322–335. [Google Scholar] [CrossRef]
- Sorgenfrei, S.; Chiu, C.-Y.; Nuckolls, C.; Shepard, K.L.; Gonzalez Jr, R.L.; Yu, Y.-J.; Kim, P.; Rossnagel, S.M. Label-free single-molecule detection of DNA-hybridization kinetics with a carbon nanotube field-effect transistor. Nat. Nanotechnol. 2011, 6, 126–132. [Google Scholar] [CrossRef] [Green Version]
- Weiss, N.O.; Zhou, H.; Liao, L.; Liu, Y.; Jiang, S.; Huang, Y.; Duan, X. Graphene: An emerging electronic material. Adv. Mater. 2012, 24, 5782–5825. [Google Scholar] [CrossRef]
- Feng, L.; Liu, Z. Graphene in biomedicine: Opportunities and challenges. Nanomedicine 2011, 6, 317–324. [Google Scholar] [CrossRef]
- Sun, C.-L.; Lee, H.-H.; Yang, J.-M.; Wu, C.-C. The simultaneous electrochemical detection of ascorbic acid, dopamine, and uric acid using graphene/size-selected Pt nanocomposites. Biosens. Bioelectron. 2011, 26, 3450–3455. [Google Scholar] [CrossRef]
- Moldovan, O.; Iniguez, B.; Deen, M.J.; Marsal, L.F. Graphene electronic sensors—Review of recent developments and future challenges. IET Circuits Devices Syst. 2015, 9, 446–453. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, H.L.; Aldalbahi, A.; Zuo, X.L.; Fan, C.H.; Mi, X.Q. Fluorescent biosensors enabled by graphene and graphene oxide. Biosens. Bioelectron. 2017, 89, 96–106. [Google Scholar] [CrossRef]
- Fu, W.; El Abbassi, M.; Hasler, T.; Jung, M.; Steinacher, M.; Calame, M.; Schoenenberger, C.; Puebla-Hellmann, G.; Hellmueller, S.; Ihn, T.; et al. Electrolyte gate dependent high-frequency measurement of graphene field-effect transistor for sensing applications. Appl. Phys. Lett. 2014, 104, 013102. [Google Scholar] [CrossRef] [Green Version]
- Ang, P.K.; Chen, W.; Wee, A.T.S.; Loh, K.P. Solution-gated epitaxial graphene as pH sensor. J. Am. Chem. Soc. 2008, 130, 14392–14393. [Google Scholar] [CrossRef]
- Lin, P.; Luo, X.; Hsing, I.M.; Yan, F. Organic electrochemical transistors integrated in flexible microfluidic systems and used for label-free DNA sensing. Adv. Mater. 2011, 23, 4035–4040. [Google Scholar] [CrossRef]
- An, J.H.; Park, S.J.; Kwon, O.S.; Bae, J.; Jang, J. High-performance flexible graphene aptasensor for mercury detection in mussels. ACS Nano 2013, 7, 10563–10571. [Google Scholar] [CrossRef]
- Chen, T.Y.; Loan, P.T.; Hsu, C.L.; Lee, Y.H.; Tse-Wei Wang, J.; Wei, K.H.; Lin, C.T.; Li, L.J. Label-free detection of DNA hybridization using transistors based on CVD grown graphene. Biosens. Bioelectron. 2013, 41, 103–109. [Google Scholar] [CrossRef]
- Fu, W.Y.; Feng, L.Y.; Mayer, D.; Panaitov, G.; Kireev, D.; Offenhausser, A.; Krause, H.J. Electrolyte-gated graphene ambipolar frequency multipliers for biochemical sensing. Nano Lett. 2016, 16, 2295–2300. [Google Scholar] [CrossRef]
- John, R.A.; Ko, J.; Kulkarni, M.R.; Tiwari, N.; Chien, N.A.; Ing, N.G.; Leong, W.L.; Mathews, N. Flexible ionic-electronic hybrid oxide synaptic TFTs with programmable dynamic plasticity for brain-inspired neuromorphic computing. Small 2017, 13, 1701193. [Google Scholar] [CrossRef]
- Kim, Y.; Chortos, A.; Xu, W.; Liu, Y.; Oh, J.Y.; Son, D.; Kang, J.; Foudeh, A.M.; Zhu, C.; Lee, Y.; et al. A bioinspired flexible organic artificial afferent nerve. Science 2018, 360, 998–1003. [Google Scholar] [CrossRef] [Green Version]
- Wan, C.; Chen, G.; Fu, Y.; Wang, M.; Matsuhisa, N.; Pan, S.; Pan, L.; Yang, H.; Wan, Q.; Zhu, L.; et al. An artificial sensory neuron with tactile perceptual learning. Adv. Mater. 2018, 30, 1801291. [Google Scholar] [CrossRef]
- Zang, Y.; Shen, H.; Huang, D.; Di, C.A.; Zhu, D. A dual-organic-transistor-based tactile-perception system with signal-processing functionality. Adv Mater 2017, 29, 1606088. [Google Scholar] [CrossRef]
- Brunel, N.; Hakim, V.; Richardson, M.J.E. Single neuron dynamics and computation. Curr. Opin. Neurobiol. 2014, 25, 149–155. [Google Scholar] [CrossRef]
- Zhu, L.Q.; Wan, C.J.; Gao, P.Q.; Liu, Y.H.; Xiao, H.; Ye, J.C.; Wan, Q. Flexible proton-gated oxide synaptic transistors on Si membrane. ACS Appl. Mater. Interfaces 2016, 8, 21770–21775. [Google Scholar] [CrossRef]
- Shao, F.; Yang, Y.; Zhu, L.Q.; Feng, P.; Wan, Q. Oxide-based synaptic transistors gated by sol-gel silica electrolytes. ACS Appl. Mater. Interfaces 2016, 8, 3050–3055. [Google Scholar] [CrossRef]
- Wan, C.J.; Liu, Y.H.; Feng, P.; Wang, W.; Zhu, L.Q.; Liu, Z.P.; Shi, Y.; Wan, Q. Flexible metal oxide/graphene oxide hybrid neuromorphic transistors on flexible conducting graphene substrates. Adv. Mater. 2016, 28, 5878–5886. [Google Scholar] [CrossRef]
- Liu, N.; Zhu, L.Q.; Feng, P.; Wan, C.J.; Liu, Y.H.; Shi, Y.; Wan, Q. Flexible sensory platform based on oxide-based neuromorphic transistors. Sci. Rep. 2015, 5, 18082. [Google Scholar] [CrossRef]
- Liu, N.; Liu, Y.; Hu, J.; He, Y.; Zhang, X.; Wan, Q. PH-dependent plasticity regulation in proton/electron hybrid oxide-based synaptic transistors. Appl. Surf. Sci. 2019, 481, 1412–1417. [Google Scholar] [CrossRef]







| Channel | Electrolyte | Target Species | Receptor/Immobilization Site | Carrier Mobility (cm2·V−1·s−1) | Sensitivity/LOD | Selective Detection | Refs. |
|---|---|---|---|---|---|---|---|
| P3HT | 10−2 M NaCl solution | Na+ | Polymeric ion selective membrane/electrolyte | 0.02 | 62 mV·dec−1 | Less sensitive to K+ | [94] |
| P3HT | PBS solution | Procalcitonin (PCT) | BSA + anti-PCT antibody/channel | 0.001–0.01 | LOD is 2.2 pM | No response to milk powder | [95] |
| P3HT | High pure water | C-reactive protein (CRP) | BSA + anti-CRP/gate electrode | nr | LOD as low as 13 ± 4 proteins | No response with sole BSA | [96] |
| DPP-DTT | PBS solution | Cu2+ | Gly-Gly-His peptide/ gate electrode | nr | 1 mA·dec−1; LOD is 1 pM | Less sensitive to Fe2+, Mn2+ | [97] |
| DPP-DTT | Water | 2,4-dichlorophenoxyacetic acid (2,4-D) | 2,4-D-C1-alkyne + antibody/ gate electrode | ~1 | LOD is 2.5 fM | No response to 2,4,5- trichlorophenoxyacetic acid | [98] |
| Pentacene | PBS solution | TNFα | Peptide aptamers/gate electrode | nr | LOD is 1 pM | No response to interleukin-6 | [99] |
| Pentacene | PBS solution | Plum Pox Virus (PPV) | Protein G + anti-PPV antibody/gate electrode | nr | LOD reaches sub ng·mL−1 | No response with anti-TNFα antibody | [100] |
| pBTTT | PBS solution | Bisphenol A (BPA) | Alkyl-BPA + antibody/channel | nr | LOD is 2 pg·mL−1 | Less sensitive to dibutyl phthalate | [101] |
| P3CPT | 10−1 M NaCl + MES solution | Histamine | H2 histamine receptor/channel | nr | LOD is 1.6 nM | Less sensitive to putrescine, tyramine and histidine | [102] |
| Nano Channel | Gate Electrolyte | Target Species | Receptor | Carrier Mobility (cm2·V−1·s−1) | Sensitivity/LOD | Selective Detection | Refs. |
|---|---|---|---|---|---|---|---|
| Silicon nanowire | PBS solution | Prostate specific antigen (PSA) | APTES + PSA antibody | nr | LOD is 1.5 fM | nr | [80] |
| Silicon nanowire | PBS solution | Dopamine released from Living Cells | DNA oligonucleotide of 57-mer | nr | LOD is 1 fM | No response without cells seeding | [114] |
| ZnO nanowire | pH buffer solutions | H+ | APTES | 1.85 | ~90 mV·pH−1 | nr | [115] |
| ZnO nanorods | PBS solution | glucose | Glucose oxidase | nr | LOD is 3.8 μM | nr | [116] |
| SWNTs | PBS solution | Dopamine | nr | 21.1 | LOD is 10−18 M | nr | [117] |
| SWNTs | PBS solution | Epsilon toxin (ETX) | PBSE + ETX antibody | nr | LOD is 2 nM | nr | [118] |
| CNTs | PBS solution | Acetylcholine | Acetylcholinesterase | nr | 5.7 µA·dec−1 | No response to serine and glycine | [119] |
| CVD-grown graphene sheet | PBS solution | Complementary DNA | Probe DNA | nr | LOD is 0.01 nM | Less sensitive to mismatched DNA | [120] |
| Reduced graphene oxide | PBS solution | Acetylcholine | Acetylcholinesterase | 0.5 | ΔI/Imin = 1.06 dec−1 in the range of 0.1–10 mM | nr | [121] |
| Reduced graphene oxide | PBS solution | Ca2+, Hg2+ | Calmodulin; metallothionein type II protein | nr | LOD is 1 nM | No response to lake water | [122] |
| Single-layer graphene | PBS solution | Complementary DNA | Probe DNA | nr | 24 mV·dec−1; LOD is 25 aM | Less sensitive to SNP | [123] |
| MoS2 nanosheet | PBS solution | Streptavidin | Biotin | nr | I/I0 = 196 at 100 fM | nr | [124] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, N.; Chen, R.; Wan, Q. Recent Advances in Electric-Double-Layer Transistors for Bio-Chemical Sensing Applications. Sensors 2019, 19, 3425. https://doi.org/10.3390/s19153425
Liu N, Chen R, Wan Q. Recent Advances in Electric-Double-Layer Transistors for Bio-Chemical Sensing Applications. Sensors. 2019; 19(15):3425. https://doi.org/10.3390/s19153425
Chicago/Turabian StyleLiu, Ning, Ru Chen, and Qing Wan. 2019. "Recent Advances in Electric-Double-Layer Transistors for Bio-Chemical Sensing Applications" Sensors 19, no. 15: 3425. https://doi.org/10.3390/s19153425
APA StyleLiu, N., Chen, R., & Wan, Q. (2019). Recent Advances in Electric-Double-Layer Transistors for Bio-Chemical Sensing Applications. Sensors, 19(15), 3425. https://doi.org/10.3390/s19153425




