Monitoring of Chemical Risk Factors for Sudden Infant Death Syndrome (SIDS) by Hydroxyapatite-Graphene-MWCNT Composite-Based Sensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Synthesis of Graphene Oxide
2.3. Synthesis of HA-GN-MWCNT Composite
2.4. Characterization
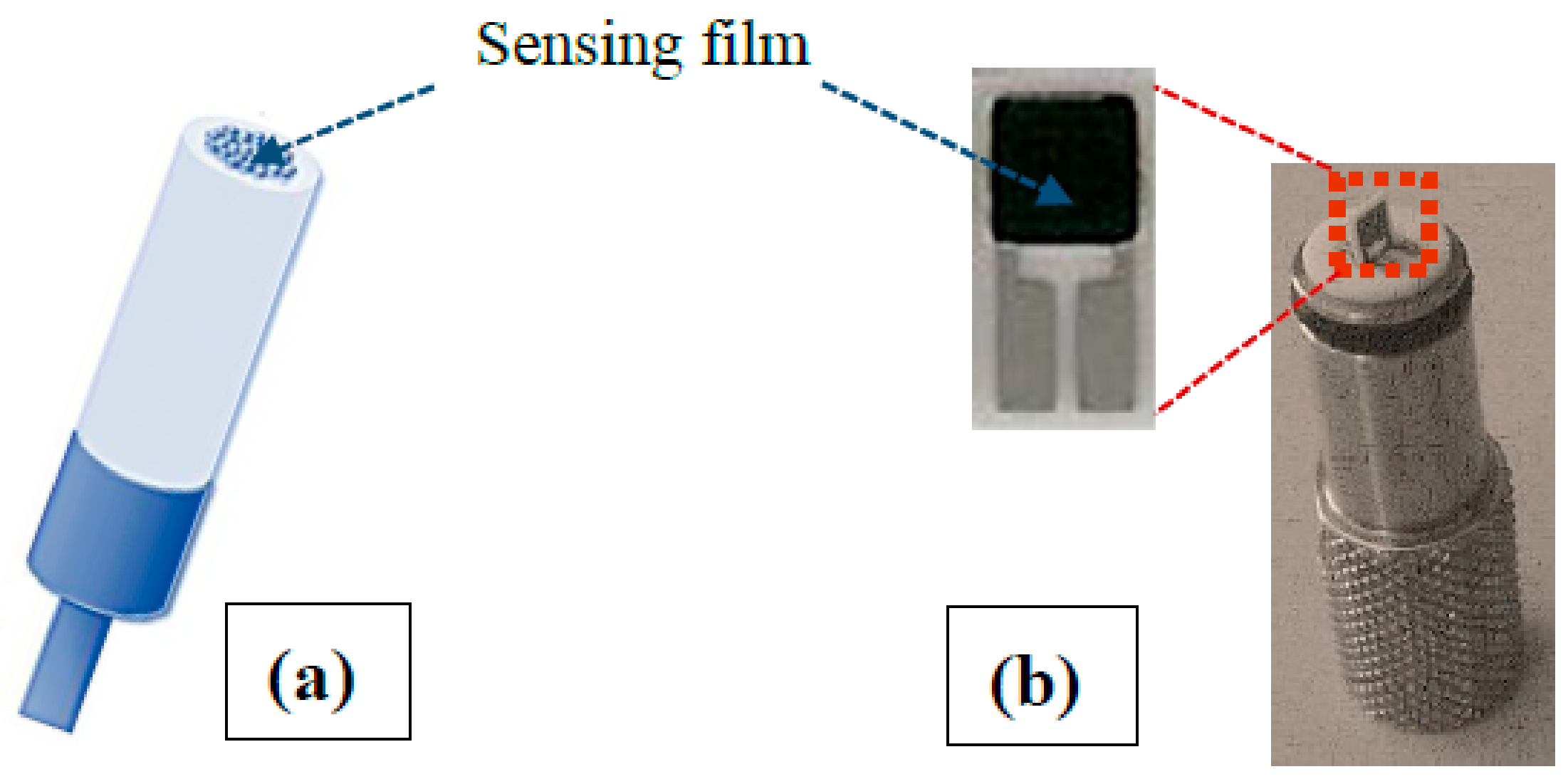
2.5. Electrochemical and Gas Sensing Tests
3. Results and Discussion
3.1. Synthesis and Characterization of HA-GN-MWCNT Composite
3.2. Electrochemical Behavior of HA-GN-MWCNT/GCE
3.2.1. Electrochemical Determination of Nicotine and Caffeine
3.2.2. Selective Determination of Nicotine and Caffeine
3.2.3. Simultaneous Determination of Nicotine and Caffeine
3.2.4. Interference and Reproducibility Studies
3.2.5. Real Sample Analysis
3.3. HA-GN-MWCNT Conductometric Sensor for Environmental CO2
Conductometric Tests with HA-GN-MWCNT for CO2 Monitoring
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dales, R.; Burnett, R.T.; Smith-Doiron, M.; Stieb, D.M.; Brook, J.R. Air Pollution and Sudden Infant Death Syndrome. Pediatrics 2004, 113, 628–631. [Google Scholar] [CrossRef] [PubMed]
- Corbyn, J.A. Sudden Infant Death due to carbon dioxide and other pollutant accumulation at the face of a sleeping baby. Med. Hypotheses 1993, 41, 483–494. [Google Scholar] [CrossRef]
- Ferng, S.-F.; Lee, L.-W. Indoor air quality assessment of daycare facilities with carbon dioxide, temperature, and humidity as indicators. J. Environ. Health 2002, 65, 14–18. [Google Scholar] [PubMed]
- Indoor Environmental Quality: Building Ventilation; National Institute for Occupational Safety and Health: Washington, DC, USA, 2011.
- Adgent, M.A. Environmental tobacco smoke and sudden infant death syndrome: A review. Birth Defects Res. B 2006, 77, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, E.A.; Ford, R.P.; Stewart, A.W.; Taylor, B.J.; Becroft, D.M.; Thompson, J.M.; Scragg, R.; Hassall, I.B.; Barry, D.M.; Allen, E.M. Smoking and the sudden infant death syndrome. Pediatrics 1993, 91, 893–896. [Google Scholar] [CrossRef] [PubMed]
- Calvaresi, V.; Escuder, D.; Minutillo, A.; Bastons-Compta, A.; García-Algar, O.; Alonso, C.R.P.; Pacifici, R.; Pichini, S. Transfer of nicotine, cotinine and caffeine intobreast milk in a smoker mother consuming caffeinated drinks. J. Anal. Toxicol. 2016, 40, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Ford, R.P.K.; Schluter, P.J.; Mitchell, E.A.; Taylor, B.J.; Scragg, R.; Stewart, A.W. Heavy Caffeine Intake in Pregnancy and Sudden Infant Death Syndrome. Obstet. Gynecol. Surv. 1998, 53, 535–536. [Google Scholar] [CrossRef] [Green Version]
- Rusted, J.; Graupner, L.; O’Connell, N.; Nicholls, C. Does nicotine improve cognitive function? Psychopharmacology 1994, 115, 547–549. [Google Scholar] [CrossRef]
- Bishop, D. Dietary supplements and team-sport performance. Sports Med. 2010, 40, 995. [Google Scholar] [CrossRef]
- Conger, S.A.; Warren, G.L.; Hardy, M.A.; Millard-Stafford, M.L. Does Caffeine Added to Carbohydrate Provide Additional Ergogenic Benefit for Endurance? Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 71–84. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.-J.; Kim, G.; Kwon, Y.-K. Molecular adsorption study of nicotine and caffeine on single-walled carbon nanotubes from first principles. Chem. Phys. Lett. 2013, 580, 57–61. [Google Scholar] [CrossRef]
- Van Hieu, N.; Khoang, N.D.; Trung, D.D.; Toan, L.D.; Van Duy, N.; Hoa, N.D. Comparative study on CO2 and CO sensing performance of LaOCl-coated ZnO nanowires. J. Hazard. Mater. 2013, 244, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Neri, G. First Fifty Years of Chemoresistive Gas Sensors. Chemosensors 2015, 3, 1–20. [Google Scholar] [CrossRef]
- D’Orazio, P. Electrochemical sensors: A review of techniques and applications in point of care testing point of care. J. Near-Patient Test. Technol. 2004, 3, 49–59. [Google Scholar] [CrossRef]
- Huixia, L.; Yong, L.; Lanlan, L.; Yanni, T.; Qing, Z.; Kun, L. Development of ammonia sensors by using conductive polymer/hydroxyapatite composite materials. Mater. Sci. Eng. C 2016, 59, 438–444. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.; Zhang, Q.; Li, K.; Liu, W.; Liu, Y.; Banks, C.E. Green electrochemical sensing platforms: Utilizing hydroxyapatite derived from natural fish scales as a novel electrochemical material for the sensitive detection of kidney injury molecule 1 (KIM-1). Analyst 2014, 139, 536. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zhang, L.; Zhang, X.; Huan, S.; Shen, G.; Yu, R. A novel tyrosinase biosensor based on hydroxyapatite–chitosan nanocomposite for the detection of phenolic compounds. Anal. Chim. Acta 2010, 665, 146–151. [Google Scholar] [CrossRef]
- Chidembo, A.T.; Ozoemena, K.I.; Agboola, B.O.; Gupta, V.; Wildgoose, G.G.; Compton, R.G. Nickel(ii) tetra-aminophthalocyanine modified MWCNTs as potential nanocomposite materials for the development of supercapacitors. Energy Environ. Sci. 2010, 3, 228–236. [Google Scholar] [CrossRef]
- Canobre, S.C.; Almeida, D.A.; Fonseca, C.P.; Neves, S. Synthesis and characterization of hybrid composites based on carbon nanotubes. Electrochim. Acta 2009, 54, 6383–6388. [Google Scholar] [CrossRef]
- Khomenko, V.; Raymundo-Pinero, E.; Beguin, F. Optimization of an asymmetric manganese oxide/activated carbon capacitor working at 2 V in aqueous medium. J. Power Sources 2016, 153, 183–190. [Google Scholar] [CrossRef]
- Wu, M.-S.; Lin, K.-H. One-step Electrophoretic Deposition of Ni-Decorated Activated-Carbon Film as an Electrode Material for Supercapacitors. J. Phys. Chem. C 2010, 114, 6190–6196. [Google Scholar] [CrossRef]
- Dikin, D.A.; Stankovich, S.; Zimney, E.J.; Piner, R.D.; Dommett, G.H.B.; Evmenenko, G.; Nguyen, S.T.; Ruoff, R.S. Preparation and characterization of graphene oxide paper. Nature 2007, 448, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Petit, C.; Seredych, M.; Bandosz, T.J. Revisiting the chemistry of graphite oxides and its effect on ammonia adsorption. J. Mater. Chem. 2009, 19, 9176. [Google Scholar] [CrossRef]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Aboutalebi, S.H.; Chidembo, A.T.; Salari, M.; Wexler, D.; Liu, H.K.; Dou, S.X.; Konstantinov, K. Comparison of GO, GO/MWCNTs composite and MWCNTs as potential electrode materials for supercapacitors. Energy Environ. Sci. 2011, 4, 1855. [Google Scholar] [CrossRef]
- Shao, G.; Lu, Y.; Wu, F.; Yang, C.; Zeng, F.; Wu, Q. Graphene oxide: The mechanisms of oxidation and exfoliation. J. Mater. Sci. 2012, 47, 4400–4409. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, J.; Wang, Z.; Ran, H.; Li, Y.; Niu, L.; Gong, P.; Liu, B.; Yang, S. One-pot synthesis of graphene/hydroxyapatite nanorod composite for tissue engineering. Carbon 2014, 66, 407–416. [Google Scholar] [CrossRef]
- Núñez, J.D.; Benito, A.M.; González, R.; Aragón, J.; Arenal, R.; Maser, W.K. Integration and bioactivity of hydroxyapatite grown on carbon nanotubes and graphene oxide. Carbon 2014, 79, 590–604. [Google Scholar] [CrossRef] [Green Version]
- Wan, W.-B.; Zhao, Z.-B.; Hu, H.; Zhou, Q.; Fan, Y.-R.; Qiu, J.-S. “Green” reduction of graphene oxide to graphene by sodium citrate. Carbon 2011, 49, 2878. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, J.; Li, H. Synthesis of hydroxyapatite–reduced graphite oxide nanocomposites for biomedical applications: Oriented nucleation and epitaxial growth of hydroxyapatite. J. Mater. Chem. B 2013, 1, 1826. [Google Scholar] [CrossRef]
- Lavanya, N.; Fazio, E.; Neri, F.; Bonavita, A.; Leonardi, S.G.; Neri, G.; Sekar, C. Electrochemical sensor for simultaneous determination of ascorbic acid, uric acid and folic acid based on Mn-SnO2 nanoparticles modified glassy carbon electrode. J. Electroanal. Chem. 2016, 770, 23–32. [Google Scholar] [CrossRef]
- Mirzaei, A.; Janghorban, K.; Hashemi, B.; Bonyani, M.; Leonardi, S.G.; Neri, G. Highly stable and selective ethanol sensor based on α-Fe2O3 nanoparticles prepared by Pechini sol–gel method. Ceram. Int. 2016, 42, 6136–6144. [Google Scholar] [CrossRef]
- Dhahri, R.; Hjiri, M.; El Mir, L.; Fazio, E.; Neri, F.; Barreca, F.; Donato, N.; Bonavita, A.; Leonardi, S.G.; Neri, G. ZnO:Ca nanopowders with enhanced CO2 sensing properties. J. Phys. D Appl. Phys. 2015, 48, 255503. [Google Scholar] [CrossRef]
- Dhahri, R.; Leonardi, S.G.; Hjiri, M.; Mir, L.E.; Bonavita, A.; Donato, N.; Iannazzo, D.; Neri, G. Enhanced performance of novel calcium/aluminum co-doped zinc oxide for CO2 sensors. Sens. Actuators B 2017, 239, 36–44. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Neri, G.; Pinna, N. Nanostructured materials for room temperature gas sensors. Adv. Mater. 2016, 28, 795–831. [Google Scholar] [CrossRef] [PubMed]
- Mahabole, P.M.; Mene, R.U.; Khairnar, R.S. Gas sensing and dielectric studies on cobalt doped hydroxyapatite thick films. Adv. Mater. Lett. 2013, 4, 46–52. [Google Scholar] [CrossRef]
- Prim, A.; Pellicer, E.; Rossinyol, E.; Peiró, F.; Cornet, A.; Morante, J.R. A novel mesoporous CaO-loaded In2O3 material for CO2 sensing. Adv. Funct. Mater. 2007, 17, 2957–2963. [Google Scholar] [CrossRef]
- De La Iglesia, D.H.; De Paz, J.F.; Gonzalez, G.V.; Barriuso, A.L.; Bajo, J. A Context-Aware Indoor Air Quality System for Sudden Infant Death Syndrome Prevention. Sensors 2018, 18, 757. [Google Scholar] [CrossRef]











© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sudhan, N.; Lavanya, N.; Leonardi, S.G.; Neri, G.; Sekar, C. Monitoring of Chemical Risk Factors for Sudden Infant Death Syndrome (SIDS) by Hydroxyapatite-Graphene-MWCNT Composite-Based Sensors. Sensors 2019, 19, 3437. https://doi.org/10.3390/s19153437
Sudhan N, Lavanya N, Leonardi SG, Neri G, Sekar C. Monitoring of Chemical Risk Factors for Sudden Infant Death Syndrome (SIDS) by Hydroxyapatite-Graphene-MWCNT Composite-Based Sensors. Sensors. 2019; 19(15):3437. https://doi.org/10.3390/s19153437
Chicago/Turabian StyleSudhan, Narayanan, Nehru Lavanya, Salvatore Gianluca Leonardi, Giovanni Neri, and Chinnathambi Sekar. 2019. "Monitoring of Chemical Risk Factors for Sudden Infant Death Syndrome (SIDS) by Hydroxyapatite-Graphene-MWCNT Composite-Based Sensors" Sensors 19, no. 15: 3437. https://doi.org/10.3390/s19153437
APA StyleSudhan, N., Lavanya, N., Leonardi, S. G., Neri, G., & Sekar, C. (2019). Monitoring of Chemical Risk Factors for Sudden Infant Death Syndrome (SIDS) by Hydroxyapatite-Graphene-MWCNT Composite-Based Sensors. Sensors, 19(15), 3437. https://doi.org/10.3390/s19153437