Real-Time Cardiac Beat Detection and Heart Rate Monitoring from Combined Seismocardiography and Gyrocardiography
Abstract
:1. Introduction
2. Methods
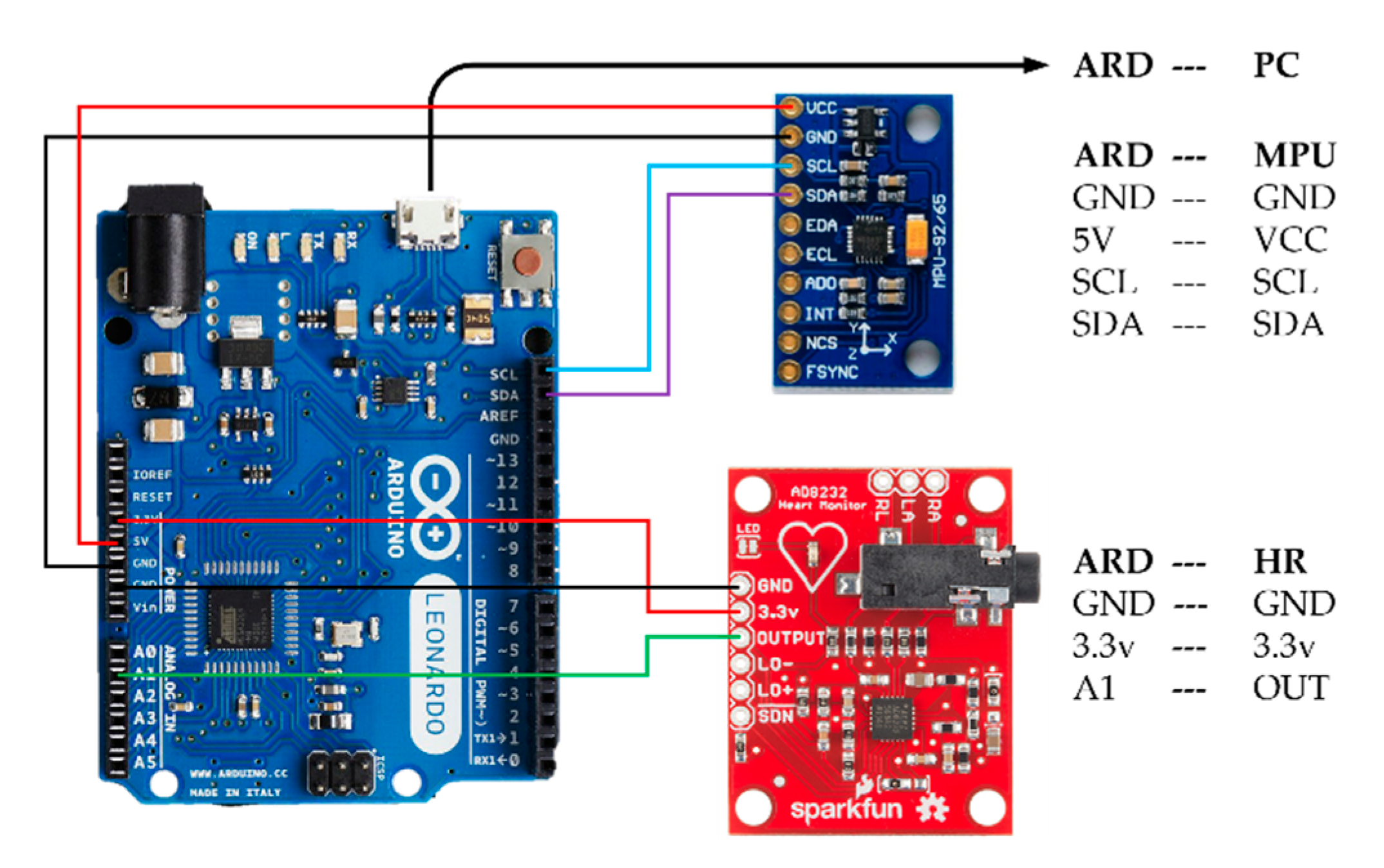
2.1. System Configuration
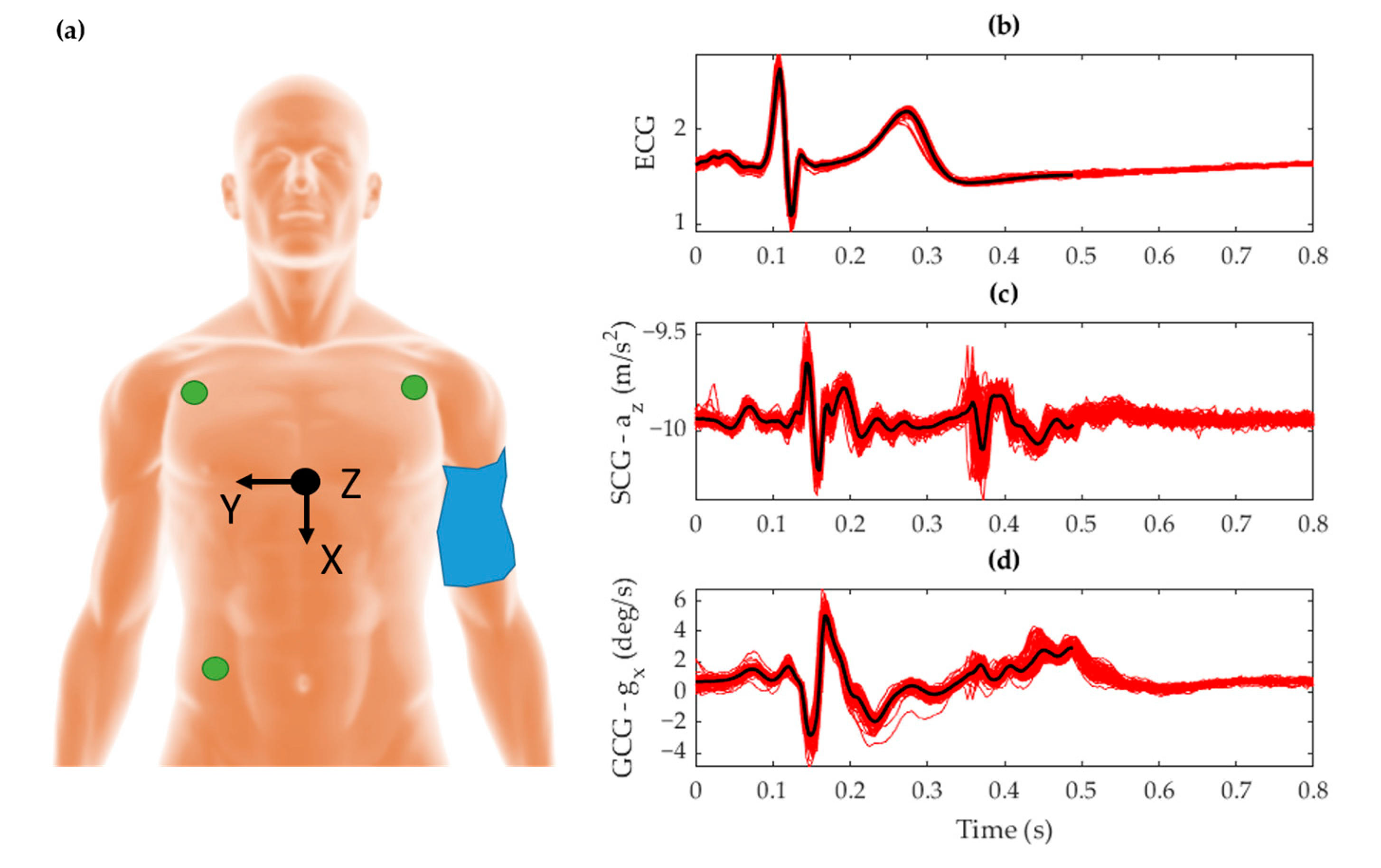
2.2. Measurement Protocol
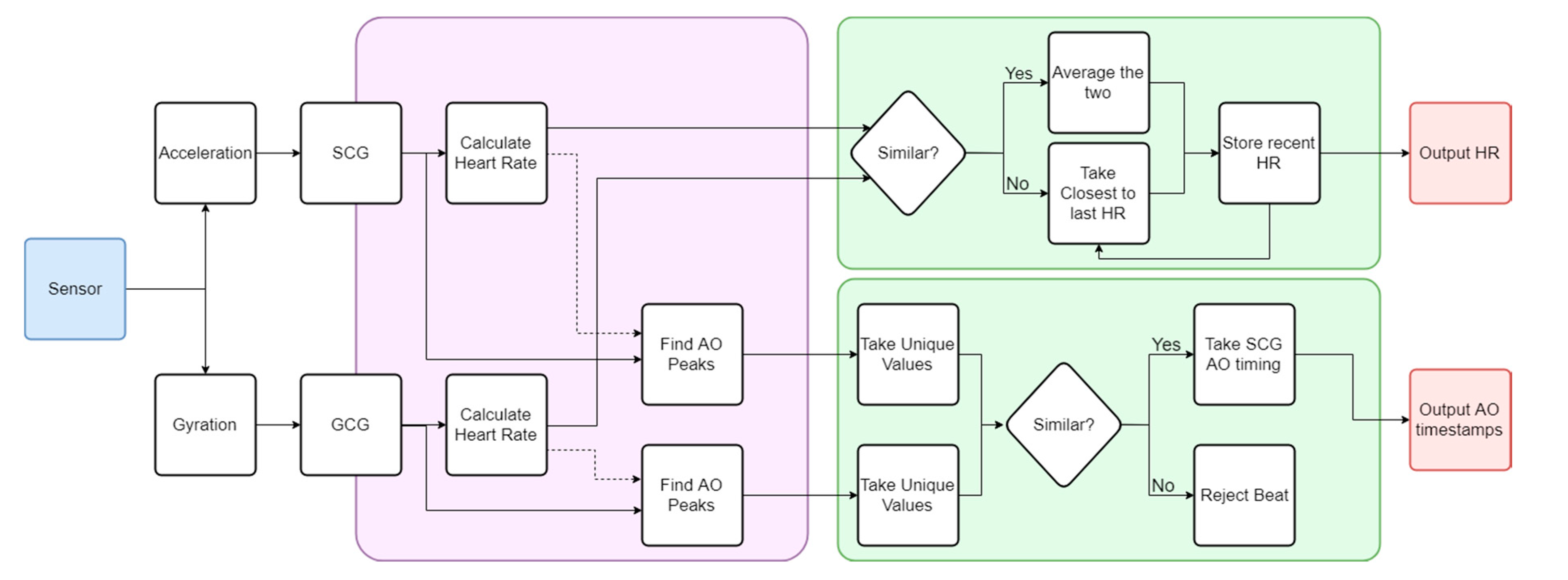
2.3. Algorithm Workflow
2.4. Performance Characterization
3. Algorithm Architecture
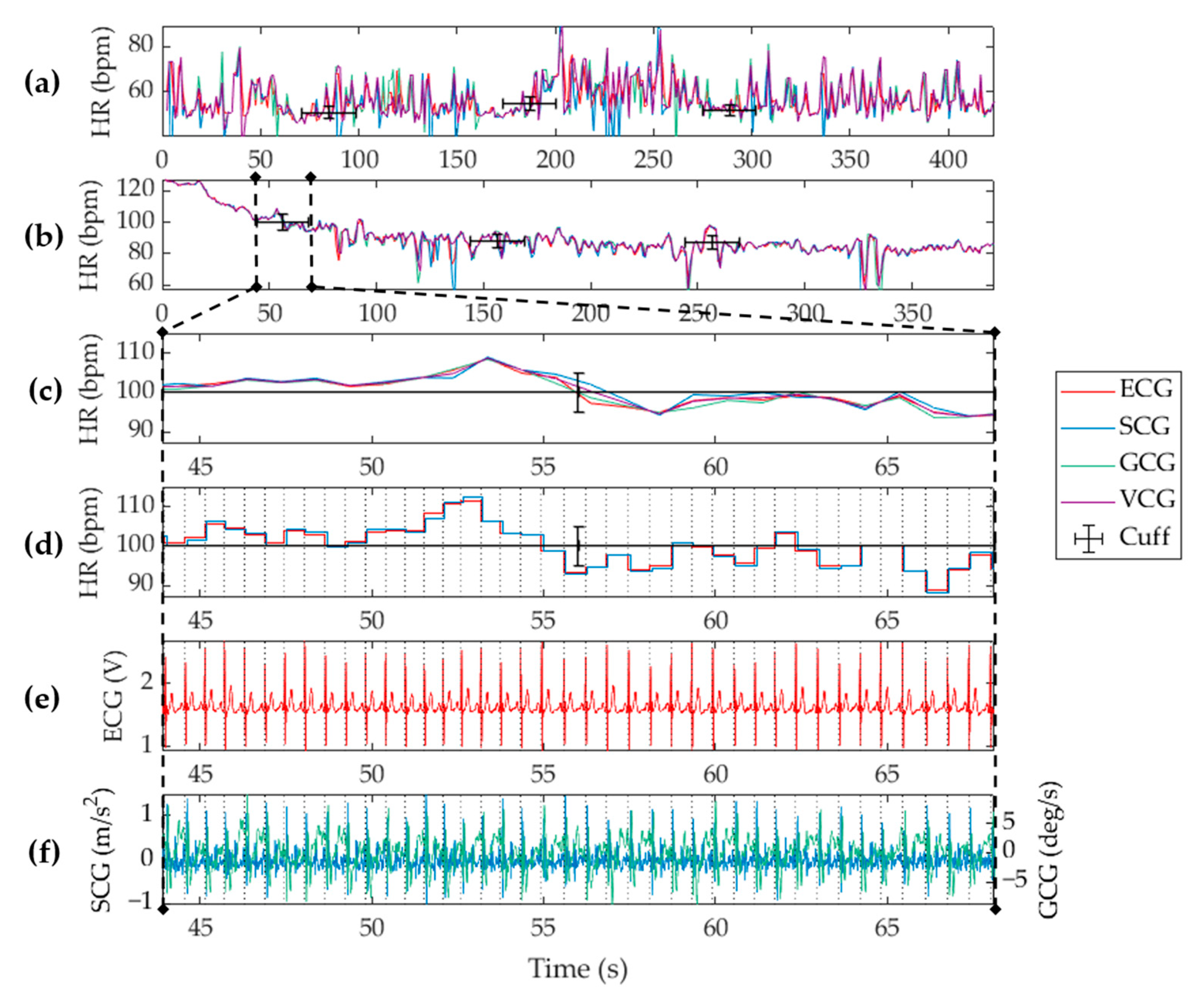
3.1. Heart Rate Calculation
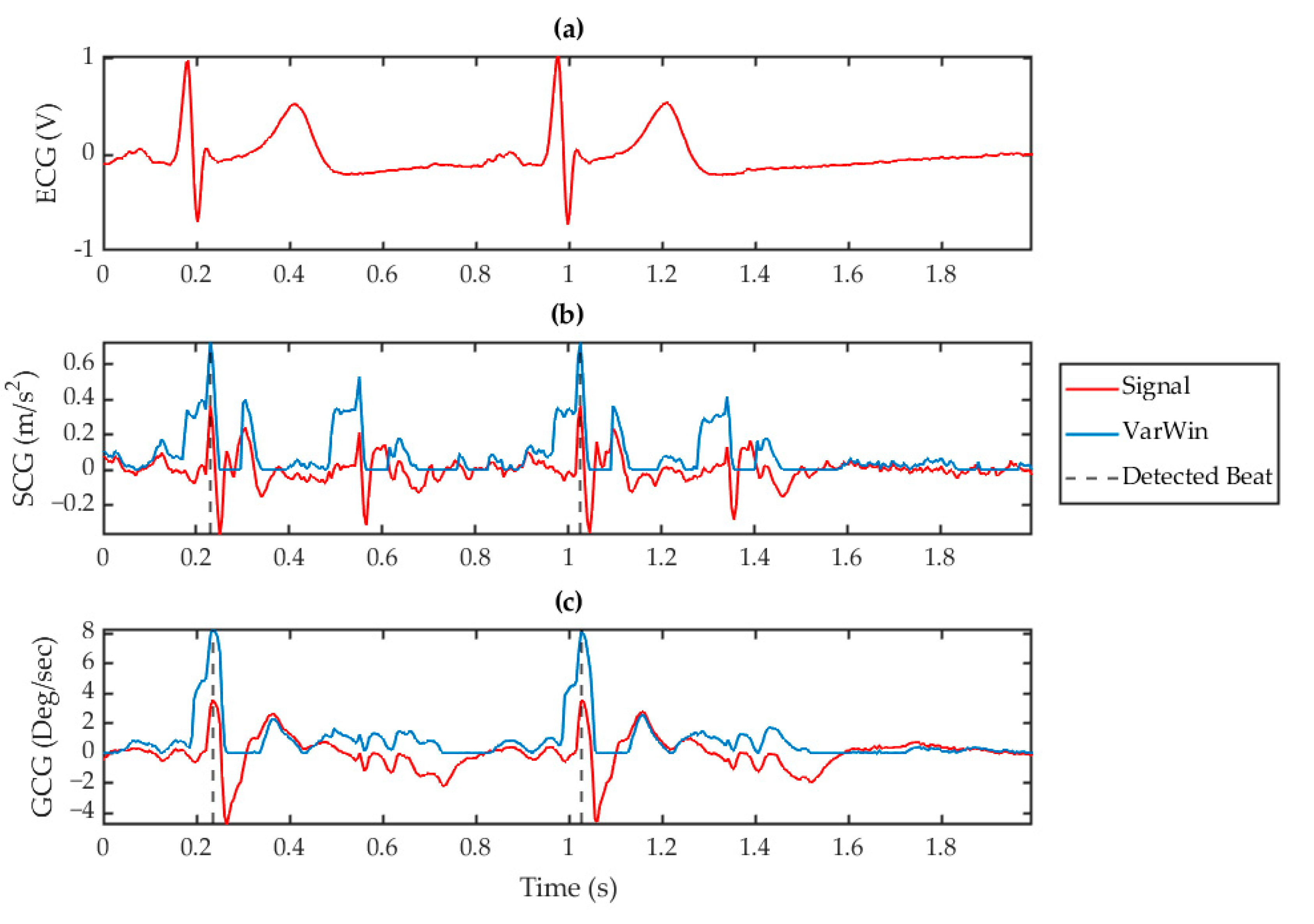
3.2. Beat Identification
3.3. Consolidation of SCG and GCG
4. Results
4.1. Heart Rate Measurement
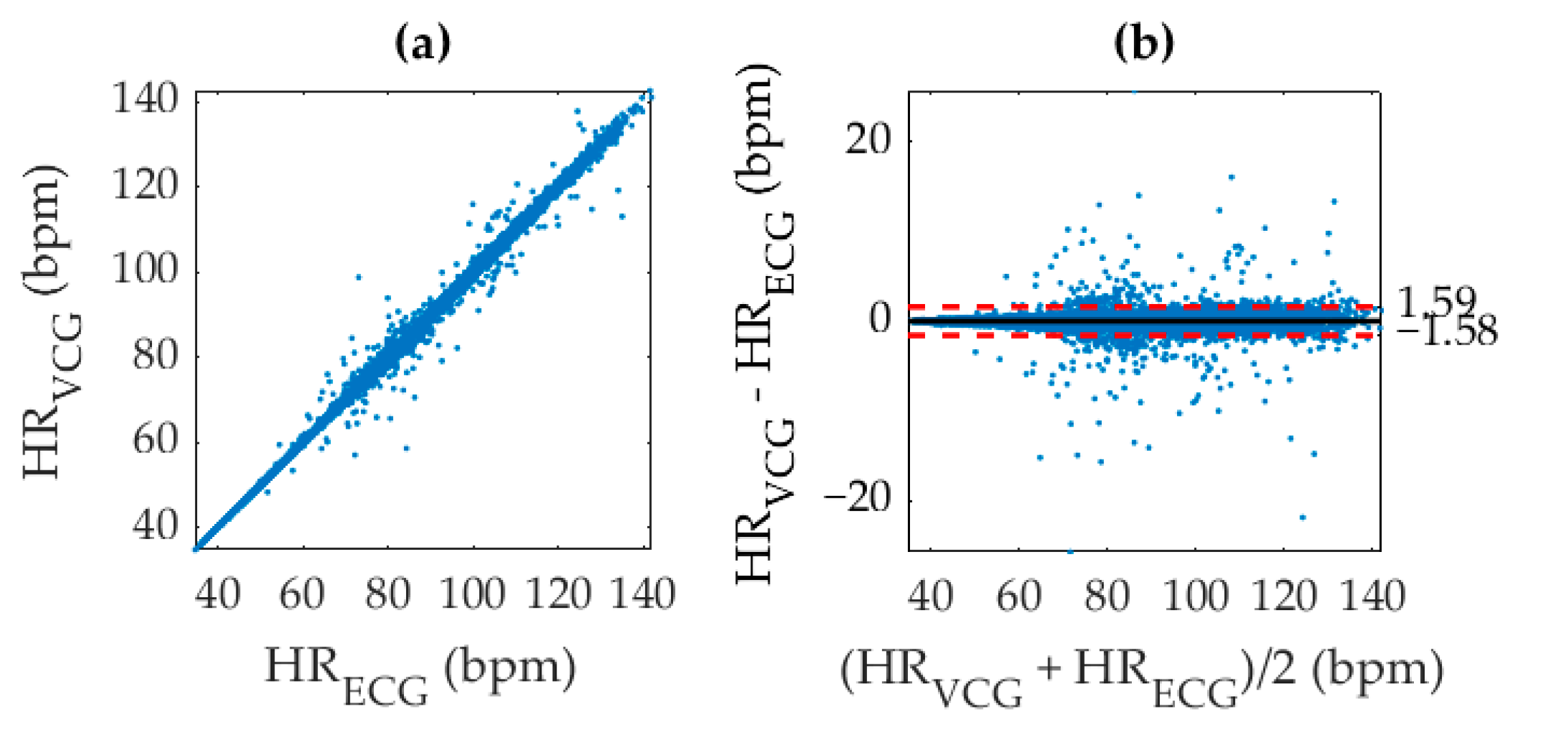
4.2. Statistical Analysis
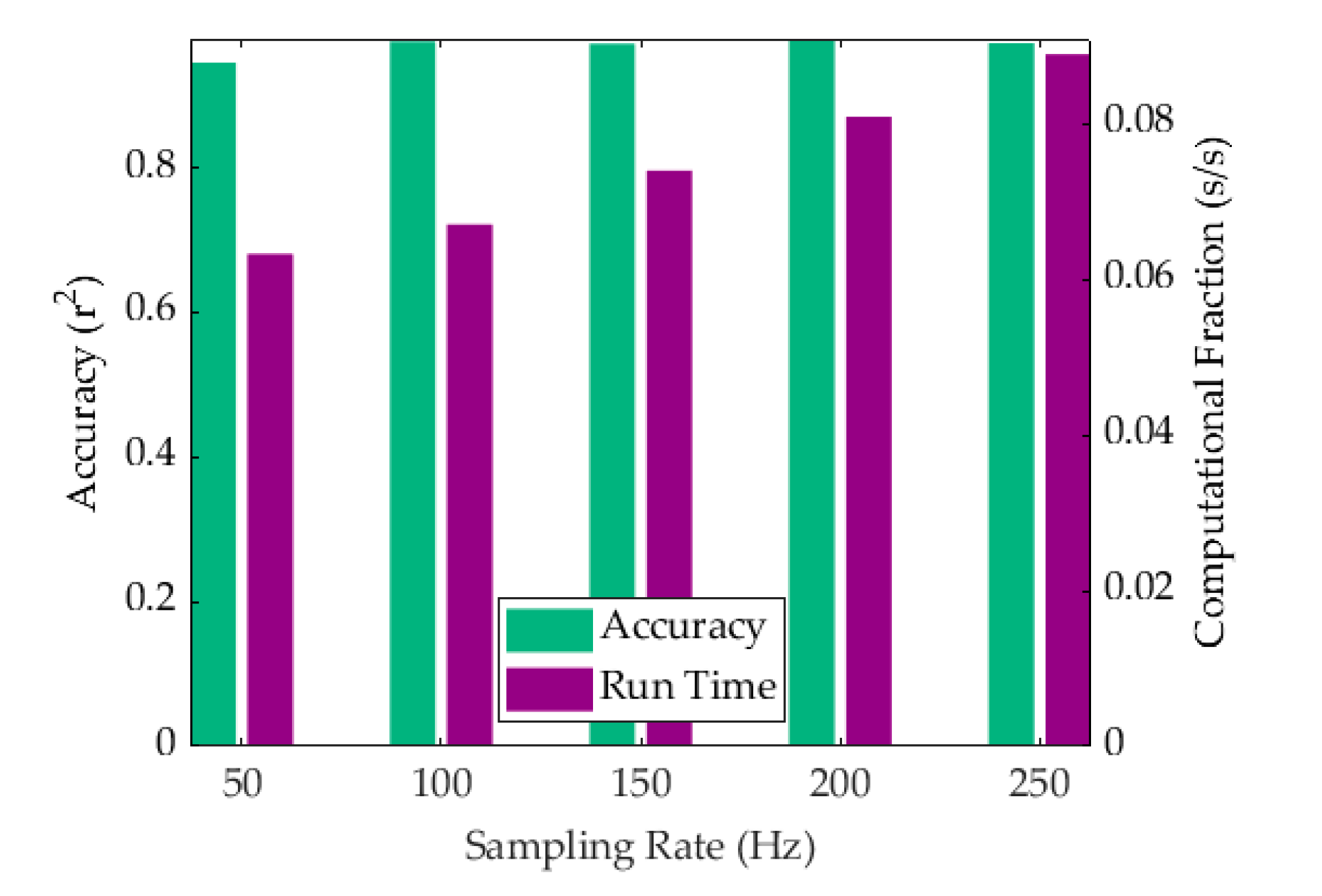
4.3. Computational Efficiency
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R. Heart disease and stroke statistics—2018 update: A report from the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef] [PubMed]
- Gersh, B.J.; Sliwa, K.; Mayosi, B.M.; Yusuf, S. Novel therapeutic concepts the epidemic of cardiovascular disease in the developing world: Global implications. Eur. Heart J. 2010, 31, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; Tiwari, A.K. Heart monitoring systems—A review. Comput. Biol. Med. 2014, 54, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bloom, D.E.; Cafiero, E.; Jané-Llopis, E.; Abrahams-Gessel, S.; Bloom, L.R.; Fathima, S.; Feigl, A.B.; Gaziano, T.; Mowafi, M.; Pandya, A. The Global Economic Burden of Noncommunicable Diseases. Available online: http://www3.weforum.org/docs/WEF_Harvard_HE_GlobalEconomicBurdenNonCommunicableDiseases_2011.pdf (accessed on 6 August 2019).
- Franklin, C.; Mathew, J. Developing strategies to prevent inhospital cardiac arrest: Analyzing responses of physicians and nurses in the hours before the event. Resuscitation 1994, 28, 167–168. [Google Scholar] [CrossRef]
- Krantz, D.S.; Manuck, S.B. Acute psychophysiologic reactivity and risk of cardiovascular disease: A review and methodologic critique. Psychol. Bull. 1984, 96, 435–464. [Google Scholar] [CrossRef] [PubMed]
- MacIntosh, E.; Rajakulendran, N.; Khayat, Z.; Wise, A. Transforming Health: Shifting from Reactive to Proactive and Predictive Care; MaRS Discovery District: Toronto, ON, Canada, 2014. [Google Scholar]
- Paré, G.; Jaana, M.; Sicotte, C. Systematic review of home telemonitoring for chronic diseases: The evidence base. J. Am. Med Inform. Assoc. 2007, 14, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Acharya, U.R.; Fujita, H.; Lih, O.S.; Adam, M.; Tan, J.H.; Chua, C.K. Automated detection of coronary artery disease using different durations of ECG segments with convolutional neural network. Knowl. Based Syst. 2017, 132, 62–71. [Google Scholar] [CrossRef]
- Fullman, N.; Yearwood, J.; Abay, S.M.; Abbafati, C.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; Abebe, Z.; Abebo, T.A.; Aboyans, V. Measuring performance on the Healthcare Access and Quality Index for 195 countries and territories and selected subnational locations: A systematic analysis from the Global Burden of Disease Study 2016. Lancet 2018, 391, 2236–2271. [Google Scholar] [CrossRef]
- Subbe, C.; Kruger, M.; Rutherford, P.; Gemmel, L. Validation of a modified Early Warning Score in medical admissions. QJM 2001, 94, 521–526. [Google Scholar] [CrossRef] [Green Version]
- Weng, S.F.; Reps, J.; Kai, J.; Garibaldi, J.M.; Qureshi, N. Can machine-learning improve cardiovascular risk prediction using routine clinical data? PLoS ONE 2017, 12, e0174944. [Google Scholar] [CrossRef]
- Pomeranz, B.; Macaulay, R.J.; Caudill, M.A.; Kutz, I.; Adam, D.; Gordon, D.; Kilborn, K.M.; Barger, A.C.; Shannon, D.C.; Cohen, R.J.; et al. Assessment of autonomic function in humans by heart rate spectral analysis. Am. J. Physiol. Heart Circ. Physiol. 1985, 248, H151–H153. [Google Scholar] [CrossRef] [PubMed]
- Foo, J.Y.A.; Wilson, S.J. A computational system to optimise noise rejection in photoplethysmography signals during motion or poor perfusion states. Med. Biol. Eng. Comput. 2006, 44, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Thakor, N.V.; Webster, J.G. Electrode studies for the long-term ambulatory ECG. Med. Biol. Eng. Comput. 1985, 23, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Patterson, R. Fundamentals of impedance cardiography. IEEE Eng. Med. Biol. Mag. 1989, 8, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Wick, C.A.; Su, J.-J.; McClellan, J.H.; Brand, O.; Bhatti, P.T.; Buice, A.L.; Stillman, A.E.; Tang, X.; Tridandapani, S. A system for seismocardiography-based identification of quiescent heart phases: Implications for cardiac imaging. IEEE Trans. Inf. Technol. Biomed. 2012, 16, 869–877. [Google Scholar] [CrossRef]
- Inan, O.T.; Migeotte, P.-F.; Park, K.-S.; Etemadi, M.; Tavakolian, K.; Casanella, R.; Zanetti, J.M.; Tank, J.; Funtova, I.; Prisk, G.K. Ballistocardiography and seismocardiography: A review of recent advances. IEEE J. Biomed. Health Inform. 2015, 19, 1414–1427. [Google Scholar] [CrossRef]
- McGee, S. Evidence-Based Physical Diagnosis E-Book; Elsevier Health Sciences: Philadelphia, PA, USA, 2012. [Google Scholar]
- Casanella, R.; Khosrow-khavar, F.; Schmidt, S.; Zanetti, J.; Tavakolian, K. Cardiac Mechanical Signals. In Cardiovascular Computing—Methodologies and Clinical Applications; Golemati, S., Nikita, K.S., Eds.; Springer: Singapore, 2019. [Google Scholar]
- Bozhenko, B.S. Seismocardiography—A new method in the study of functional conditions of the heart. Ter. Arkhiv 1961, 33, 55–64. [Google Scholar]
- Tadi, M.J.; Lehtonen, E.; Pankäälä, M.; Saraste, A.; Vasankari, T.; Terás, M.; Koivisto, T. Gyrocardiography: A new non-invasive approach in the study of mechanical motions of the heart. Concept, method and initial observations. In Proceedings of the 2016 IEEE 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 17–20 August 2016. [Google Scholar]
- Salerno, D.M.; Zanetti, J. Seismocardiography for monitoring changes in left ventricular function during ischemia. Chest 1991, 100, 991–993. [Google Scholar] [CrossRef]
- Tadi, M.J.; Lehtonen, E.; Saraste, A.; Tuominen, J.; Koskinen, J.; Teräs, M.; Airaksinen, J.; Pänkäälä, M.; Koivisto, T. Gyrocardiography: A new non-invasive monitoring method for the assessment of cardiac mechanics and the estimation of hemodynamic variables. Sci. Rep. 2017, 7, 6823. [Google Scholar] [CrossRef]
- Crow, R.S.; Hannan, P.; Jacobs, D.; Hedquist, L.; Salerno, D.M. Relationship between seismocardiogram and echocardiogram for events in the cardiac cycle. Am. J. Noninvasive Cardiol. 1994, 8, 39–46. [Google Scholar] [CrossRef]
- Taebi, A.; Solar, B.E.; Bomar, A.J.; Sandler, R.H.; Mansy, H.A. Recent Advances in Seismocardiography. Vibration 2019, 2, 64–86. [Google Scholar] [CrossRef] [Green Version]
- Migeotte, P.-F.; Mucci, V.; Delière, Q.; Lejeune, L.; van de Borne, P. Multi-dimensional kineticardiography a new approach for wearable cardiac monitoring through body acceleration recordings. In Proceedings of the XIV Mediterranean Conference on Medical and Biological Engineering and Computing, Paphos, Cyprus, 31 March–2 April 2016. [Google Scholar]
- Yang, C.; Tavassolian, N. Combined Seismo-and Gyro-Cardiography: A More Comprehensive Evaluation of Heart-Induced Chest Vibrations. IEEE J. Biomed. Health Inform. 2018, 22, 1466–1475. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Tavassolian, N. An Independent Component Analysis Approach to Motion Noise Cancellation of Cardio-Mechanical Signals. IEEE Trans. Biomed. Eng. 2018, 66, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, H.; Whang, M. An Enhanced Method to Estimate Heart Rate from Seismocardiography via Ensemble Averaging of Body Movements at Six Degrees of Freedom. Sensors 2018, 18, 238. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Whang, M. Heart Rate Estimated from Body Movements at Six Degrees of Freedom by Convolutional Neural Networks. Sensors 2018, 18, 1392. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Tang, S.; Tavassolian, N. Utilizing gyroscopes towards the automatic annotation of seismocardiograms. IEEE Sens. J. 2017, 17, 2129–2136. [Google Scholar] [CrossRef]
- D’Mello, Y.; Skoric, J.; Xu, S.; Akhras, M.; Roche, P.J.; Lortie, M.; Gagnon, S.; Plant, D.V. Autocorrelated Differential Algorithm for Real-time Seismocardiography Analysis. IEEE Sens. J. 2019, 19, 5127–5140. [Google Scholar] [CrossRef]
- Landreani, F.; Morri, M.; Martin-Yebra, A.; Casellato, C.; Pavan, E.; Frigo, C.; Caiani, E.G. Ultra-short-term heart rate variability analysis on accelerometric signals from mobile phone. In Proceedings of the 6th IEEE International Conference on E-Health and Bioengineering (EHB), Sinaia, Romania, 22–24 June 2017. [Google Scholar]
- Iftikhar, Z.; Lahdenoja, O.; Tadi, M.J.; Hurnanen, T.; Vasankari, T.; Kiviniemi, T.; Airaksinen, J.; Koivisto, T.; Pänkäälä, M. Multiclass Classifier based Cardiovascular Condition Detection Using Smartphone Mechanocardiography. Sci. Rep. 2018, 8, 9344. [Google Scholar] [CrossRef] [PubMed]
- Lahdenoja, O.; Hurnanen, T.; Iftikhar, Z.; Nieminen, S.; Knuutila, T.; Saraste, A.; Kiviniemi, T.; Vasankari, T.; Airaksinen, J.; Pänkäälä, M. Atrial fibrillation detection via accelerometer and gyroscope of a smartphone. IEEE J. Biomed. Health Inform. 2018, 22, 108–118. [Google Scholar] [CrossRef]
- Tadi, M.J.; Lehtonen, E.; Hurnanen, T.; Koskinen, J.; Eriksson, J.; Pänkäälä, M.; Teräs, M.; Koivisto, T. A real-time approach for heart rate monitoring using a Hilbert transform in seismocardiograms. Physiol. Meas. 2016, 37, 1885–1909. [Google Scholar] [CrossRef]
- Wahlström, J.; Skog, I.; Händel, P.; Khosrow-Khavar, F.; Tavakolian, K.; Stein, P.K.; Nehorai, A. A hidden markov model for seismocardiography. IEEE Trans. Biomed. Eng. 2017, 64, 2361–2372. [Google Scholar] [CrossRef] [PubMed]
- Khosrow-Khavar, F.; Tavakolian, K.; Blaber, A.; Menon, C. Automatic and Robust Delineation of the Fiducial Points of the Seismocardiogram Signal for Noninvasive Estimation of Cardiac Time Intervals. IEEE Trans. Biomed. Eng. 2017, 64, 1701–1710. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, T.; Sharma, L.N.; Bhuyan, M.K. Automatic Detection of Aortic Valve Opening using Seismocardiography in Healthy Individuals. IEEE J. Biomed. Health Inform. 2018, 23, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Laurin, A.; Khosrow-Khavar, F.; Blaber, A.; Tavakolian, K. Accurate and consistent automatic seismocardiogram annotation without concurrent ECG. Physiol. Meas. 2016, 37, 1588. [Google Scholar] [CrossRef]
- Malcangi, M.; Quan, H.; Vaini, E.; Lombardi, P.; Di Rienzo, M. Applying the EFuNN Evolving Paradigm to the Recognition of Artefactual Beats in Continuous Seismocardiogram Recordings. In Proceedings of the International Conference on Engineering Applications of Neural Networks, Athens, Greece, 25–27 August 2017. [Google Scholar]
- Khosrow-Khavar, F.; Tavakolian, K.; Blaber, A.P.; Zanetti, J.M.; Fazel-Rezai, R.; Menon, C. Automatic annotation of seismocardiogram with high-frequency precordial accelerations. IEEE J. Biomed. Health Inform. 2015, 19, 1428–1434. [Google Scholar] [CrossRef]
- Etemadi, M.; Inan, O.T.; Heller, J.A.; Hersek, S.; Klein, L.; Roy, S. A wearable patch to enable long-term monitoring of environmental, activity and hemodynamics variables. IEEE Trans. Biomed. Circuits Syst. 2016, 10, 280–288. [Google Scholar] [CrossRef]
- Di Rienzo, M.; Vaini, E.; Lombardi, P. An algorithm for the beat-to-beat assessment of cardiac mechanics during sleep on Earth and in microgravity from the seismocardiogram. Sci. Rep. 2017, 7, 15634. [Google Scholar] [CrossRef] [Green Version]
- Sahoo, P.K.; Thakkar, H.K.; Lee, M.-Y. A cardiac early warning system with multi channel SCG and ECG monitoring for mobile health. Sensors 2017, 17, 711. [Google Scholar] [CrossRef]
- Tadi, M.J.; Koivisto, T.; Pänkäälä, M.; Paasio, A.; Knuutila, T.; Teräs, M.; Hänninen, P. A new algorithm for segmentation of cardiac quiescent phases and cardiac time intervals using seismocardiography. In Proceedings of the Sixth International Conference on Graphic and Image Processing (ICGIP 2014), Beijing, China, 24–26 October 2014. [Google Scholar]
- Javaid, A.Q.; Ashouri, H.; Dorier, A.; Etemadi, M.; Heller, J.A.; Roy, S.; Inan, O.T. Quantifying and Reducing Motion Artifacts in Wearable Seismocardiogram Measurements During Walking to Assess Left Ventricular Health. IEEE Trans. Biomed. Eng. 2017, 64, 1277–1286. [Google Scholar] [CrossRef]
- MPU-9250|TDK. Available online: https://www.invensense.com/products/motion-tracking/9-axis/mpu-9250/ (accessed on 25 June 2019).
- SparkFun Single Lead Heart Rate Monitor-AD8232. Available online: https://www.sparkfun.com/products/12650 (accessed on 25 June 2019).
- Arduino Leonardo. Available online: https://www.arduino.cc/en/Main/Arduino_BoardLeonardo (accessed on 25 June 2019).
- Zanetti, J.M.; Salerno, D.M. Seismocardiography: A technique for recording precordial acceleration. In Proceedings of the Computer-Based Medical Systems-Proceedings of the Fourth Annual IEEE Symposium, Baltimore, MD, USA, 12–14 May 1991. [Google Scholar]
- Wilson, F.N.; Johnston, F.D.; Rosenbaum, F.F.; Barker, P.S. On Einthoven’s triangle, the theory of unipolar electrocardiographic leads, and the interpretation of the precordial electrocardiogram. Am Heart J. 1946, 32, 277–310. [Google Scholar] [CrossRef]
- Ozemek, C.; Cochran, H.L.; Strath, S.J.; Byun, W.; Kaminsky, L.A. Estimating relative intensity using individualized accelerometer cutpoints: The importance of fitness level. BMC Med. Res. Methodol. 2013, 13, 53. [Google Scholar] [CrossRef] [PubMed]
- Tadi, M.J.; Lehtonen, E.; Koivisto, T.; Pänkäälä, M.; Paasio, A.; Teräs, M. Seismocardiography: Toward heart rate variability (HRV) estimation. In Proceedings of the 2015 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Torino, Italy, 7–9 May 2015. [Google Scholar]
- AAMI. Cardiac monitors, heart rate meters, and alarms. In American National Standard (ANSI/AAMI EC13: 2002); AAMI: Arlington, VA, USA, 2002; pp. 1–87. [Google Scholar]
- Pan, J.; Tompkins, W.J. A real-time QRS detection algorithm. IEEE Trans. Biomed. Eng. 1985, 32, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Ashouri, H.; Inan, O.T. Automatic detection of seismocardiogram sensor misplacement for robust pre-ejection period estimation in unsupervised settings. IEEE Sens. J. 2017, 17, 3805–3813. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-Y.; Chou, W.-C.; Chang, P.-C.; Chou, C.-C.; Wen, M.-S.; Ho, M.-Y.; Lee, W.-C.; Hsieh, M.-J.; Tsai, T.-H.; Lee, M.-Y. Identification of location specific feature points in a cardiac cycle using a novel seismocardiogram spectrum system. IEEE J. Biomed. Health Inform. 2016, 22, 442–449. [Google Scholar] [CrossRef] [PubMed]

| Type | File | # Beats | TP | FP | FN | TPR | PPV | r2 |
|---|---|---|---|---|---|---|---|---|
| Rest | 1 | 478 | 478 | 0 | 0 | 1 | 1 | 0.9983 |
| 2 | 505 | 505 | 0 | 0 | 1 | 1 | 0.9959 | |
| 3 | 565 | 537 | 1 | 28 | 0.9504 | 0.9981 | 0.9773 | |
| 4 | 584 | 577 | 0 | 7 | 0.988 | 1 | 0.9945 | |
| 5 | 569 | 568 | 4 | 1 | 0.9982 | 0.993 | 0.9949 | |
| 6 | 437 | 437 | 6 | 0 | 1 | 0.9865 | 0.9997 | |
| 7 | 449 | 438 | 0 | 11 | 0.9755 | 1 | 0.9952 | |
| 8 | 539 | 501 | 11 | 38 | 0.9295 | 0.9785 | 0.9924 | |
| 9 | 353 | 353 | 0 | 0 | 1 | 1 | 0.9978 | |
| 10 | 527 | 518 | 0 | 9 | 0.9829 | 1 | 0.9757 | |
| 11 | 466 | 466 | 0 | 0 | 1 | 1 | 0.9962 | |
| 12 | 450 | 450 | 0 | 0 | 1 | 1 | 0.9931 | |
| 13 | 577 | 574 | 0 | 3 | 0.9948 | 1 | 0.8487 | |
| 14 | 395 | 395 | 0 | 0 | 1 | 1 | 0.9995 | |
| 15 | 505 | 498 | 1 | 7 | 0.9861 | 0.998 | 0.9223 | |
| 16 | 438 | 400 | 12 | 38 | 0.9132 | 0.9709 | 0.9724 | |
| 17 | 316 | 316 | 0 | 0 | 1 | 1 | 0.9975 | |
| 18 | 461 | 457 | 0 | 4 | 0.9913 | 1 | 0.9982 | |
| 19 | 323 | 302 | 0 | 21 | 0.935 | 1 | 0.9987 | |
| 20 | 531 | 523 | 0 | 8 | 0.9849 | 1 | 0.9958 | |
| 21 | 511 | 511 | 1 | 0 | 1 | 0.998 | 0.9972 | |
| 22 | 508 | 419 | 0 | 89 | 0.8248 | 1 | 0.9293 | |
| 23 | 502 | 460 | 2 | 42 | 0.9163 | 0.9957 | 0.9996 | |
| 24 | 423 | 423 | 0 | 0 | 1 | 1 | 0.9983 | |
| 25 | 496 | 324 | 0 | 172 | 0.6532 | 1 | 0.9966 | |
| Recovery | 1 | 656 | 655 | 1 | 1 | 0.9985 | 0.9985 | 0.9707 |
| 2 | 655 | 651 | 0 | 4 | 0.9939 | 1 | 0.9991 | |
| 3 | 577 | 520 | 0 | 57 | 0.9012 | 1 | 0.8099 | |
| 4 | 699 | 699 | 1 | 0 | 1 | 0.9986 | 0.9975 | |
| 5 | 752 | 717 | 1 | 35 | 0.9535 | 0.9986 | 0.9837 | |
| 7 | 483 | 473 | 1 | 7 | 0.9854 | 0.9979 | 0.9986 | |
| 9 | 583 | 579 | 0 | 4 | 0.9931 | 1 | 0.9994 | |
| 10 | 620 | 607 | 0 | 13 | 0.979 | 1 | 0.994 | |
| 11 | 682 | 678 | 0 | 4 | 0.9941 | 1 | 0.9949 | |
| 12 | 464 | 463 | 1 | 1 | 0.9978 | 0.9978 | 0.9986 | |
| 13 | 655 | 648 | 0 | 7 | 0.9893 | 1 | 0.9964 | |
| 14 | 578 | 577 | 0 | 1 | 0.9983 | 1 | 0.9988 | |
| 16 | 502 | 420 | 5 | 82 | 0.8367 | 0.9882 | 0.9979 | |
| 17 | 382 | 381 | 0 | 1 | 0.9974 | 1 | 0.9996 | |
| 18 | 482 | 468 | 11 | 14 | 0.971 | 0.977 | 0.9986 | |
| 19 | 430 | 421 | 0 | 9 | 0.9791 | 1 | 0.8693 | |
| 20 | 664 | 601 | 4 | 63 | 0.9051 | 0.9934 | 0.9603 | |
| 21 | 634 | 621 | 11 | 13 | 0.9795 | 0.9826 | 0.986 | |
| 22 | 364 | 364 | 1 | 0 | 1 | 0.9973 | 0.9955 | |
| 24 | 471 | 455 | 0 | 16 | 0.966 | 1 | 0.9994 | |
| 25 | 743 | 734 | 0 | 9 | 0.9879 | 1 | 0.9942 | |
| Total | 46 | 23,984 | 23,162 | 75 | 819 | 0.9657 | 0.9968 | 0.9982 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Mello, Y.; Skoric, J.; Xu, S.; Roche, P.J.R.; Lortie, M.; Gagnon, S.; Plant, D.V. Real-Time Cardiac Beat Detection and Heart Rate Monitoring from Combined Seismocardiography and Gyrocardiography. Sensors 2019, 19, 3472. https://doi.org/10.3390/s19163472
D’Mello Y, Skoric J, Xu S, Roche PJR, Lortie M, Gagnon S, Plant DV. Real-Time Cardiac Beat Detection and Heart Rate Monitoring from Combined Seismocardiography and Gyrocardiography. Sensors. 2019; 19(16):3472. https://doi.org/10.3390/s19163472
Chicago/Turabian StyleD’Mello, Yannick, James Skoric, Shicheng Xu, Philip J. R. Roche, Michel Lortie, Stephane Gagnon, and David V. Plant. 2019. "Real-Time Cardiac Beat Detection and Heart Rate Monitoring from Combined Seismocardiography and Gyrocardiography" Sensors 19, no. 16: 3472. https://doi.org/10.3390/s19163472