A Photoluminescent Colorimetric Probe of Bovine Serum Albumin-Stabilized Gold Nanoclusters for New Psychoactive Substances: Cathinone Drugs in Seized Street Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Synthesis of BSA-Au NCs
2.3. Characterization of BSA-Au NCs
2.4. Detection of 4-CMC and Cathinone Analogues
2.5. Interference Study
2.6. Theoretical Calculation
2.7. Analysis of Actual Samples
3. Results and Discussion
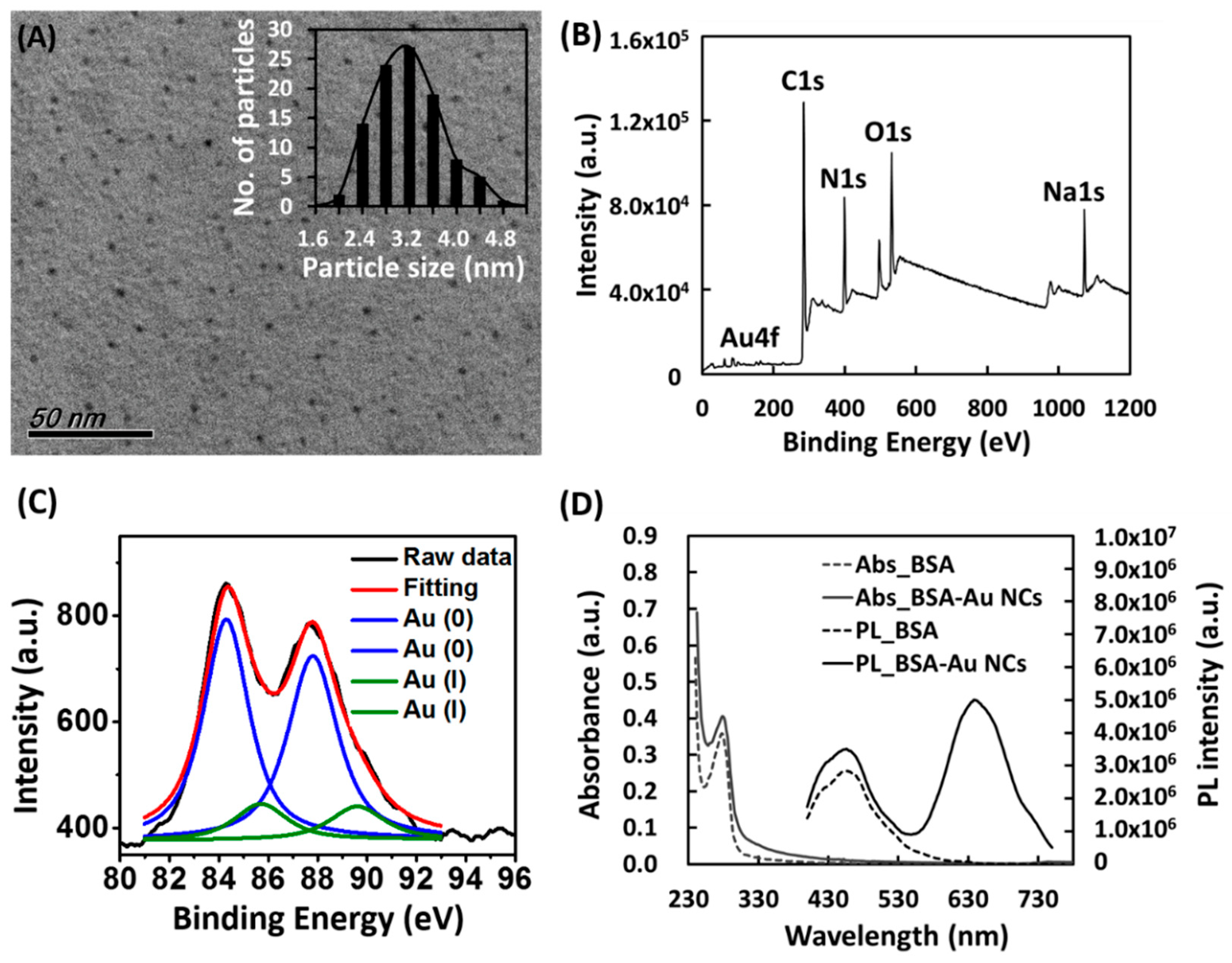
3.1. Characterization of BSA-Au NCs
3.2. Sensitivity, Selectivity, and Interference Study
3.3. Sensing Mechanism
3.4. Analysis of Actual Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). European Drug Report: Trends and Developments; Publications Office of the European Union: Luxembourg, 2018. [Google Scholar]
- Karila, L.; Megarbane, B.; Cottencin, O.; Lejoyeux, M. Synthetic cathinones: A new public health problem. Curr. Neuropharmacol. 2015, 13, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Majchrzak, M.; Celiński, R.; Kuś, P.; Kowalska, T.; Sajewicz, M. The newest cathinone derivatives as designer drugs: An analytical and toxicological review. Forensic Toxicol. 2018, 36, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, K.M.; Al-Hazmi, A.H.; Alasiri, A.M.; Ali, M.E.-S. A GC–MS method for detection and quantification of cathine, cathinone, methcathinone and ephedrine in oral fluid. J. Chromatogr. Sci. 2016, 54, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Zuba, D. Identification of cathinones and other active components of ‘legal highs’ by mass spectrometric methods. Trends Anal. Chem. 2012, 32, 15–30. [Google Scholar] [CrossRef]
- Lendoiro, E.; Jiménez-Morigosa, C.; Cruz, A.; Páramo, M.; López-Rivadulla, M.; de Castro, A. An LC-MS/MS methodological approach to the analysis of hair for amphetamine-type-stimulant (ATS) drugs, including selected synthetic cathinones and piperazines. Drug Test. Anal. 2017, 9, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Mercolini, L.; Protti, M.; Catapano, M.C.; Rudge, J.; Sberna, A.E. LC–MS/MS and volumetric absorptive microsampling for quantitative bioanalysis of cathinone analogues in dried urine, plasma and oral fluid samples. J. Pharm. Biomed. Anal. 2016, 123, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Vircks, K.E.; Mulligan, C.C. Rapid screening of synthetic cathinones as trace residues and in authentic seizures using a portable mass spectrometer equipped with desorption electrospray ionization. Rapid Commun. Mass Spectrom. 2012, 26, 2665–2672. [Google Scholar] [CrossRef]
- Guirguis, A.; Girotto, S.; Berti, B.; Stair, J.L. Identification of new psychoactive substances (NPS) using handheld Raman Spectroscopy employing both 785 and 1064 nm laser sources. Forensic Sci. Int. 2017, 273, 113–123. [Google Scholar] [CrossRef]
- Cuypers, E.; Bonneure, A.J.; Tytgat, J. The use of presumptive color tests for new psychoactive substances. Drug Test. Anal. 2016, 8, 136–140. [Google Scholar] [CrossRef]
- Smith, J.P.; Metters, J.P.; Khreit, O.I.; Sutcliffe, O.B.; Banks, C.E. Forensic electrochemistry applied to the sensing of new psychoactive substances: Electroanalytical sensing of synthetic cathinones and analytical validation in the quantification of seized street samples. Anal. Chem. 2014, 86, 9985–9992. [Google Scholar] [CrossRef]
- Tan, F.; Smith, J.P.; Sutcliffe, O.B.; Banks, C.E. Regal electrochemistry: Sensing of the synthetic cathinone class of new psychoactive substances (NPSs). Anal. Methods 2015, 7, 6470–6474. [Google Scholar] [CrossRef]
- Ellefsen, K.N.; Anizan, S.; Castaneto, M.S.; Desrosiers, N.A.; Martin, T.M.; Klette, K.L.; Huestis, M.A. Validation of the only commercially available immunoassay for synthetic cathinones in urine: Randox Drugs of Abuse V Biochip Array Technology. Drug Test. Anal. 2014, 6, 728–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.-H.; Lin, C.-A.J.; Lee, C.-H.; Lin, Y.-C.; Tseng, Y.-M.; Hsieh, C.-L.; Chen, C.-H.; Tsai, C.-H.; Hsieh, C.-T.; Shen, J.-L. Fluorescent gold nanoclusters as a biocompatible marker for in vitro and in vivo tracking of endothelial cells. ACS Nano 2011, 5, 4337–4344. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Nienhaus, G.U. Gold nanoclusters as novel optical probes for in vitro and in vivo fluorescence imaging. Biophys. Rev. 2012, 4, 313–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baral, A.; Basu, K.; Roy, S.; Banerjee, A. Blue Emitting Gold Cluster formation from Gold Nanorods: Selective and Sensitive Detection of Iron (III) ions in Aqueous Medium. ACS Sustain. Chem. Eng. 2016, 5, 1628–1637. [Google Scholar] [CrossRef]
- Tseng, Y.T.; Cherng, R.; Yuan, Z.; Wu, C.W.; Chang, H.T.; Huang, C.C. Ultrasound-mediated modulation of the emission of gold nanodots. Nanoscale 2016, 8, 5162–5169. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; He, K.; Han, Y.; Zhang, Z.; Yu, M.; Wang, H.; Huang, Y.; Nie, Z.; Yao, S. Near-infrared dual-emission quantum dots-gold nanoclusters nanohybrid via co-template synthesis for ratiometric fluorescent detection and bioimaging of ascorbic acid in vitro and in vivo. Anal. Chem. 2015, 87, 9998–10005. [Google Scholar] [CrossRef]
- Yuan, Z.; Du, Y.; Tseng, Y.T.; Peng, M.; Cai, N.; He, Y.; Chang, H.T.; Yeung, E.S. Fluorescent gold nanodots based sensor array for proteins discrimination. Anal. Chem. 2015, 87, 4253–4259. [Google Scholar] [CrossRef]
- Yuan, Z.; Chen, Y.C.; Li, H.W.; Chang, H.T. Fluorescent silver nanoclusters stabilized by DNA scaffolds. Chem. Commun. 2014, 50, 9800–9815. [Google Scholar] [CrossRef]
- Chen, P.-C.; Li, Y.-C.; Ma, J.-Y.; Huang, J.-Y.; Chen, C.-F.; Chang, H.-T. Size-tunable copper nanocluster aggregates and their application in hydrogen sulfide sensing on paper-based devices. Sci. Rep. 2016, 6, 24882. [Google Scholar] [CrossRef]
- Dutta, D.; Chattopadhyay, A.; Ghosh, S.S. Cationic BSA templated Au–Ag bimetallic nanoclusters as a theranostic gene delivery vector for HeLa cancer cells. ACS Biomater. Sci. Eng. 2016, 2, 2090–2098. [Google Scholar] [CrossRef]
- Cantelli, A.; Battistelli, G.; Guidetti, G.; Manzi, J.; Di Giosia, M.; Montalti, M. Luminescent gold nanoclusters as biocompatible probes for optical imaging and theranostics. Dyes Pigment. 2016, 135, 64–79. [Google Scholar] [CrossRef]
- Zheng, Y.; Lai, L.; Liu, W.; Jiang, H.; Wang, X. Recent advances in biomedical applications of fluorescent gold nanoclusters. Adv. Colloid Interface Sci. 2017, 242, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Gopu, C.L.; Shanti Krishna, A.; Sreenivasan, K. Fluorimetric detection of hypochlorite using albumin stabilized gold nanoclusters. Sens. Actuator B Chem. 2015, 209, 798–802. [Google Scholar] [CrossRef]
- Xie, J.; Zheng, Y.; Ying, J.Y. Protein-directed synthesis of highly fluorescent gold nanoclusters. J. Am. Chem. Soc. 2009, 131, 888–889. [Google Scholar] [CrossRef] [PubMed]
- Le Guével, X.; Hötzer, B.; Jung, G.; Hollemeyer, K.; Trouillet, V.; Schneider, M. Formation of fluorescent metal (Au, Ag) nanoclusters capped in bovine serum albumin followed by fluorescence and spectroscopy. J. Phys. Chem. C 2011, 115, 10955–10963. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Petersson, G.; Nakatsuji, H. Gaussian 16 Revision B. 01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Escudero-Francos, M.A.; Cepas, V.; Gonzalez-Menedez, P.; Badia-Laino, R.; Diaz-Garcia, M.E.; Sainz, R.M.; Mayo, J.C.; Hevia, D. Cellular uptake and tissue biodistribution of functionalized gold nanoparticles and nanoclusters. J. Biomed. Nanotechnol. 2017, 13, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Govindaraju, S.; Ankireddy, S.R.; Viswanath, B.; Kim, J.; Yun, K. Fluorescent gold nanoclusters for selective detection of dopamine in cerebrospinal fluid. Sci. Rep. 2017, 7, 40298. [Google Scholar] [CrossRef]
- Khandelwal, P.; Alam, A.; Choksi, A.; Chattopadhyay, S.; Poddar, P. Retention of anticancer activity of curcumin after conjugation with fluorescent gold quantum clusters: An in vitro and in vivo xenograft study. ACS Omega 2018, 3, 4776–4785. [Google Scholar] [CrossRef]
- Chevrier, D.M.; Chatt, A.; Zhang, P. Properties and applications of protein-stabilized fluorescent gold nanoclusters: Short review. J. Nanophotonics 2012, 6, 064504. [Google Scholar] [CrossRef]
- Hsu, N.-Y.; Lin, Y.-W. Microwave-assisted synthesis of bovine serum albumin-gold nanoclusters and their fluorescence-quenched sensing of Hg 2+ ions. New J. Chem. 2016, 40, 1155–1161. [Google Scholar] [CrossRef]
- Unnikrishnan, B.; Wei, S.-C.; Chiu, W.-J.; Cang, J.; Hsu, P.-H.; Huang, C.-C. Nitrite ion-induced fluorescence quenching of luminescent BSA-Au 25 nanoclusters: Mechanism and application. Analyst 2014, 139, 2221–2228. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Fu, C.; Ren, X.; Liu, H.; Li, L.; Meng, X. Fluorescence switching method for cascade detection of salicylaldehyde and zinc(II) ion using protein protected gold nanoclusters. Biosens. Bioelectron. 2015, 74, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Raut, S.L.; Fudala, R.; Rich, R.; Kokate, R.A.; Chib, R.; Gryczynski, Z.; Gryczynski, I. Long lived BSA Au clusters as a time gated intensity imaging probe. Nanoscale 2014, 6, 2594–2597. [Google Scholar] [CrossRef]
- Li, H.; Chen, J.; Huang, H.; Feng, J.-J.; Wang, A.-J.; Shao, L.-X. Green and facile synthesis of l-carnosine protected fluorescent gold nanoclusters for cellular imaging. Sens. Actuator B Chem. 2016, 223, 40–44. [Google Scholar] [CrossRef]
- Wu, T.H.; Hsu, Y.Y.; Lin, S.Y. A redox-switchable Au8-cluster sensor. Small 2012, 8, 2099–2105. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, C.; Mitra, P.; Chatterjee, S.; Bhattacharjee, G.; Satpati, B.; Basu, S. Photoinduced electronic interactions between acridine derivatives and small gold nanoparticles: A spectroscopic insight. J. Mol. Liq. 2018, 272, 198–208. [Google Scholar] [CrossRef]
- Halawa, M.I.; Wu, F.; Fereja, T.H.; Lou, B.; Xu, G. One-pot green synthesis of supramolecular β-cyclodextrin functionalized gold nanoclusters and their application for highly selective and sensitive fluorescent detection of dopamine. Sens. Actuator B Chem. 2018, 254, 1017–1024. [Google Scholar] [CrossRef]
- Raut, S.; Rich, R.; Fudala, R.; Butler, S.; Kokate, R.; Gryczynski, Z.; Luchowski, R.; Gryczynski, I. Resonance energy transfer between fluorescent BSA protected Au nanoclusters and organic fluorophores. Nanoscale 2014, 6, 385–391. [Google Scholar] [CrossRef]
- Mishima, M.; Huh, C.; Nakamura, H.; Fujio, M.; Tsuno, Y. Electron affinities of benzaldehydes. Substituent effects on stabilities of aromatic radical anions. Tetrahedron Lett. 1993, 34, 4223–4226. [Google Scholar] [CrossRef]





| Item | Emission at 460 nm | Emission at 650 nm |
|---|---|---|
| BSA | 11.3 ns | - |
| BSA-Au NCs | 10.5 ns | 1820.7 ns |
| BSA-Au NCs with 4-CMC | 10.4 ns | 963.4 ns |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yen, Y.-T.; Chen, T.-Y.; Chen, C.-Y.; Chang, C.-L.; Chyueh, S.-C.; Chang, H.-T. A Photoluminescent Colorimetric Probe of Bovine Serum Albumin-Stabilized Gold Nanoclusters for New Psychoactive Substances: Cathinone Drugs in Seized Street Samples. Sensors 2019, 19, 3554. https://doi.org/10.3390/s19163554
Yen Y-T, Chen T-Y, Chen C-Y, Chang C-L, Chyueh S-C, Chang H-T. A Photoluminescent Colorimetric Probe of Bovine Serum Albumin-Stabilized Gold Nanoclusters for New Psychoactive Substances: Cathinone Drugs in Seized Street Samples. Sensors. 2019; 19(16):3554. https://doi.org/10.3390/s19163554
Chicago/Turabian StyleYen, Yao-Te, Ting-Yueh Chen, Chun-Yu Chen, Chi-Lun Chang, San-Chong Chyueh, and Huan-Tsung Chang. 2019. "A Photoluminescent Colorimetric Probe of Bovine Serum Albumin-Stabilized Gold Nanoclusters for New Psychoactive Substances: Cathinone Drugs in Seized Street Samples" Sensors 19, no. 16: 3554. https://doi.org/10.3390/s19163554





