Peptide Nanotube Encapsulated Enzyme Biosensor for Vapor Phase Detection of Malathion, an Organophosphorus Compound
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Investigation of Electrode Configuration
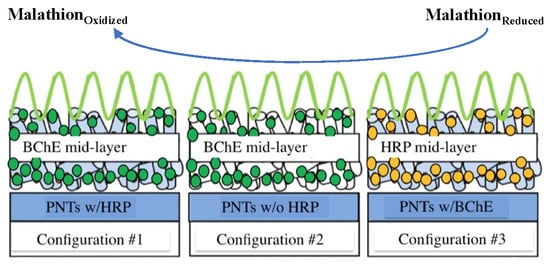
2.1.1. Different Layering Configurations of Biosensors
2.1.2. The Effect of Nafion Layer on the Electrode Efficiency
2.2. Biosensor Detection
2.2.1. Detection of Malathion
2.2.2. Biosensor Test Data Results
2.3. Longevity Experiment
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Synthesis of PNTs and the Encapsulation of BChE Inside the PNTs
3.2.2. Biosensor Fabrication and Reconstitution
3.2.3. Cyclic Voltammogram
3.2.4. Malathion Detection
3.2.5. Longevity Experiment
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mirzaei, M.; Azami, S.; Latifi, A.M.; Sariri, R.; Rastegar, S.; Panahi, M. Polymer Based Formulation and Immobilization Approaches in Enzymatic Detoxification of Organophosphorus Compounds: A review. J. Appl. Biotechnol. Rep. 2016, 3, 387–393. [Google Scholar]
- Boss, M.J.; Day, D.W. Building Vulnerability Assessments: Industrial Hygiene and Engineering Concepts; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Lu, X.; Tao, L.; Song, D.; Li, Y.; Gao, F. Bimetallic Pd@Au nanorods based ultrasensitive acetylcholinesterase biosensor for determination of organophosphate pesticides. Sens. Actuators B Chem. 2018, 255, 2575–2581. [Google Scholar] [CrossRef]
- Cui, H.F.; Wu, W.W.; Li, M.M.; Song, X.; Lv, Y.; Zhang, T.T. A highly stable acetylcholinesterase biosensor based on chitosan-TiO2-graphene nanocomposites for detection of organophosphate pesticides. Biosens. Bioelectron. 2018, 99, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Arduini, F.; Forchielli, M.; Amine, A.; Neagu, D.; Cacciotti, I.; Nanni, F.; Moscone, D.; Palleschi, G. Screen-printed biosensor modified with carbon black nanoparticles for the determination of paraoxon based on the inhibition of butyrylcholinesterase. Microchim. Acta 2015, 182, 643–651. [Google Scholar] [CrossRef]
- Baker, P.A.; Goltz, M.N.; Schrand, A.M.; Kim, D.S. Organophosphate vapor detection on gold electrodes using peptide nanotubes. Biosens. Bioelectron. 2014, 61, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Arduini, F.; Palleschi, G. Disposable electrochemical biosensor based on cholinesterase inhibition with improved shelf-life and working stability for nerve agent detection. In Portable Chemical Sensors; Springer: Dordrecht, Netherlands, 2012; pp. 261–278. [Google Scholar]
- Pohanka, M. Biosensors containing acetylcholinesterase and butyrylcholinesterase as recognition tools for detection of various compounds. Chem. Pap. 2015, 69, 4–16. [Google Scholar] [CrossRef]
- Sigolaeva, L.V.; Gladyr, S.Y.; Mergel, O.; Gelissen, A.P.H.; Noyong, M.; Simon, U.; Pergushov, D.V.; Kurochkin, I.N.; Plamper, F.A.; Richtering, W. Easy-preparable butyrylcholinesterase/microgel construct for facilitated organophosphate biosensing. Anal. Chem. 2017, 89, 6091–6098. [Google Scholar] [CrossRef] [PubMed]
- Arduini, F.; Amine, A.; Moscone, D.; Ricci, F.; Palleschi, G. Fast, sensitive and cost-effective detection of nerve agents in the gas phase using a portable instrument and an electrochemical biosensor. Anal. Bioanal. Chem. 2007, 388, 1049–1057. [Google Scholar] [CrossRef]
- Campanella, L.; Lelo, D.; Martini, E.; Tomassetti, M. Organophosphorus and carbamate pesticide analysis using an inhibition tyrosinase organic phase enzyme sensor; comparison by butyrylcholinesterase+ choline oxidase opee and application to natural waters. Anal. Chim. Acta 2007, 587, 22–32. [Google Scholar] [CrossRef]
- Skladal, P.; Mascini, M. Sensitive detection of pesticides using amperometric sensors based on cobalt phthalocyanine-modified composite electrodes and immobilized cholinesterases. Biosens. Bioelectron. 1992, 7, 335–343. [Google Scholar] [CrossRef]
- Volotovskky, V.; Kim, N.S. Ion-sensitive field effect transistor-based multienzyme sensor for alternative detection of mercury ions, cyanide, and pesticide. J. Microbiol. Biotechnol. 2003, 13, 373–377. [Google Scholar]
- Andreescu, S.; Avramescu, A.; Bala, C.; Magearu, V.; Marty, J.L. Detection of organophosphorus insecticides with immobilized acetylcholinesterase–comparative study of two enzyme sensors. Anal. Bioanal. Chem. 2002, 374, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Andreescu, S.; Marty, J.L. Twenty years research in cholinesterase biosensors: From basic research to practical applications. Biomol. Eng. 2006, 23, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sahin, A.; Dooley, K.; Cropek, D.M.; West, A.C.; Banta, S. A dual enzyme electrochemical assay for the detection of organophosphorus compounds using organophosphorus hydrolase and horseradish peroxidase. Sens. Actuators B Chem. 2011, 158, 353–360. [Google Scholar] [CrossRef]
- Park, B.W.; Yoon, D.Y.; Kim, D.S. Recent progress in bio-sensing techniques with encapsulated enzymes. Biosens. Bioelectron. 2010, 26, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Park, B.W.; Ko, K.A.; Yoon, D.Y.; Kim, D.S. Enzyme activity assay for horseradish peroxidase encapsulated in peptide nanotubes. Enzyme Microb. Technol. 2012, 51, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, P.; Faridbod, F.; Larijani, B.; Ganjali, M.R. Glucose biosensor based on MWCNTs-gold nanoparticles in a nafion film on the glassy carbon electrode using flow injection FFT continuous cyclic voltammetry. Int. J. Electrochem. Sci. 2010, 5, 1213–1224. [Google Scholar]





| Configuration # | Potential (V) | Inhibition (%) | Average |
|---|---|---|---|
| 1 | −0.30 through −0.32 | 39.41%, 41.11% | 40.18% |
| 2 | −0.30 through −0.34 | 39.44%, 51.51% | 45.28% |
| 3 | −0.34 | 39.41%, 57.18% | 50.95% |
| Electrode # | Inhibition (%) | Electrode # | Inhibition (%) |
|---|---|---|---|
| 1 (With Nafion) | 68.16 | 4 (Without Nafion) | 53.61 |
| 2 (With Nafion) | 65.74 | 5 (Without Nafion) | 32.39 |
| 3 (With Nafion) | 63.13 | 6 (Without Nafion) | 38.04 |
| Peak Average | 65.68 | Peak Average | 34.03 |
| (Std Dev) | (2.52) | (Std Dev) | (12.3) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edwards, C.; Duanghathaipornsuk, S.; Goltz, M.; Kanel, S.; Kim, D.-S. Peptide Nanotube Encapsulated Enzyme Biosensor for Vapor Phase Detection of Malathion, an Organophosphorus Compound. Sensors 2019, 19, 3856. https://doi.org/10.3390/s19183856
Edwards C, Duanghathaipornsuk S, Goltz M, Kanel S, Kim D-S. Peptide Nanotube Encapsulated Enzyme Biosensor for Vapor Phase Detection of Malathion, an Organophosphorus Compound. Sensors. 2019; 19(18):3856. https://doi.org/10.3390/s19183856
Chicago/Turabian StyleEdwards, Christopher, Surachet Duanghathaipornsuk, Mark Goltz, Sushil Kanel, and Dong-Shik Kim. 2019. "Peptide Nanotube Encapsulated Enzyme Biosensor for Vapor Phase Detection of Malathion, an Organophosphorus Compound" Sensors 19, no. 18: 3856. https://doi.org/10.3390/s19183856