Freeze-Damage Detection in Lemons Using Electrochemical Impedance Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
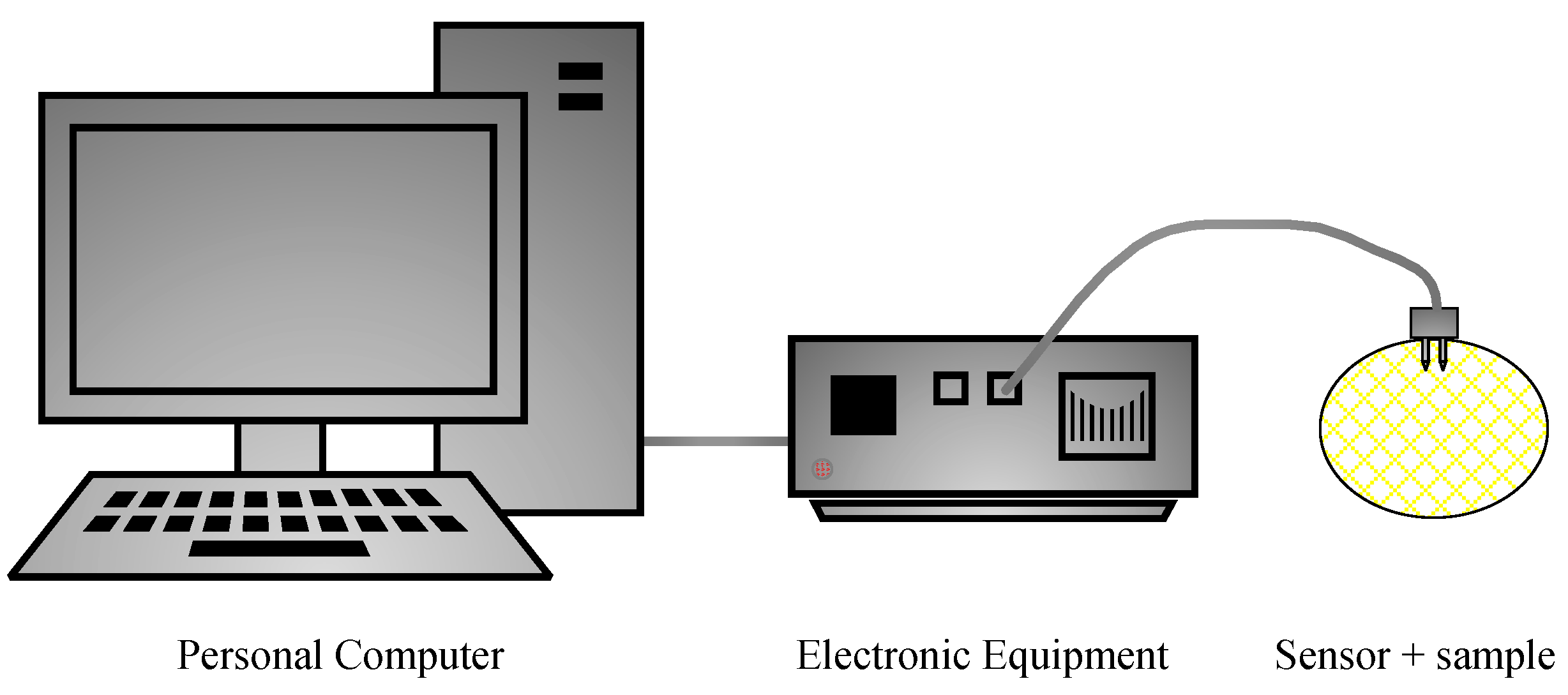
2.2. Electrochemical Impedance Spectroscopy System
2.2.1. Software Application
2.2.2. Electronic Equipment
2.3. Electrochemical Impedance Spectroscopy Analyses
2.4. Data Treatment
2.4.1. Multivariate Analysis
2.4.2. ANNs
3. Results
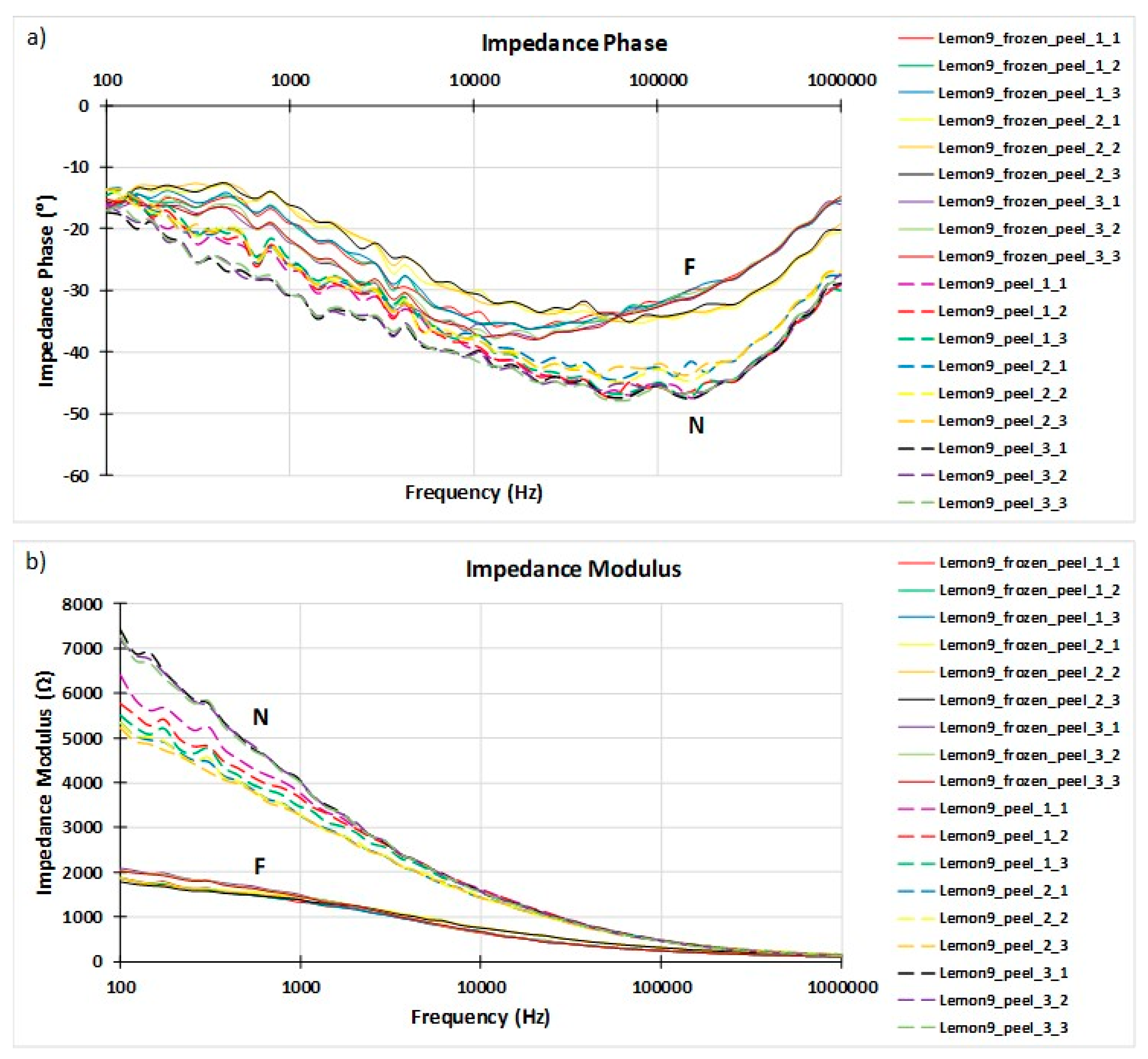
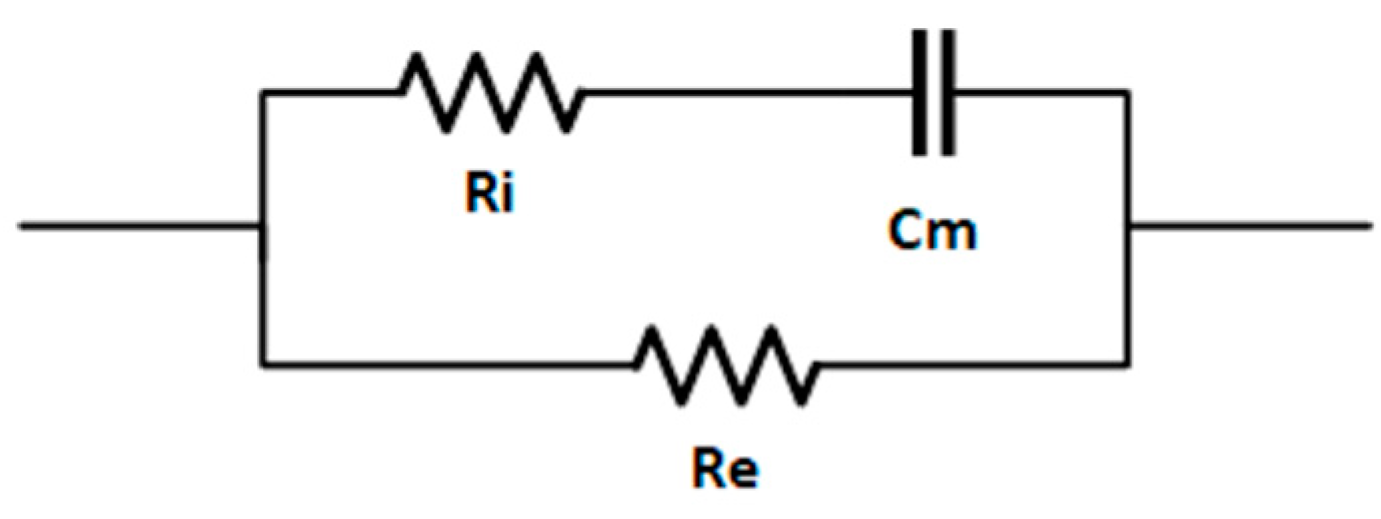
3.1. Impedance Spectroscopy Results
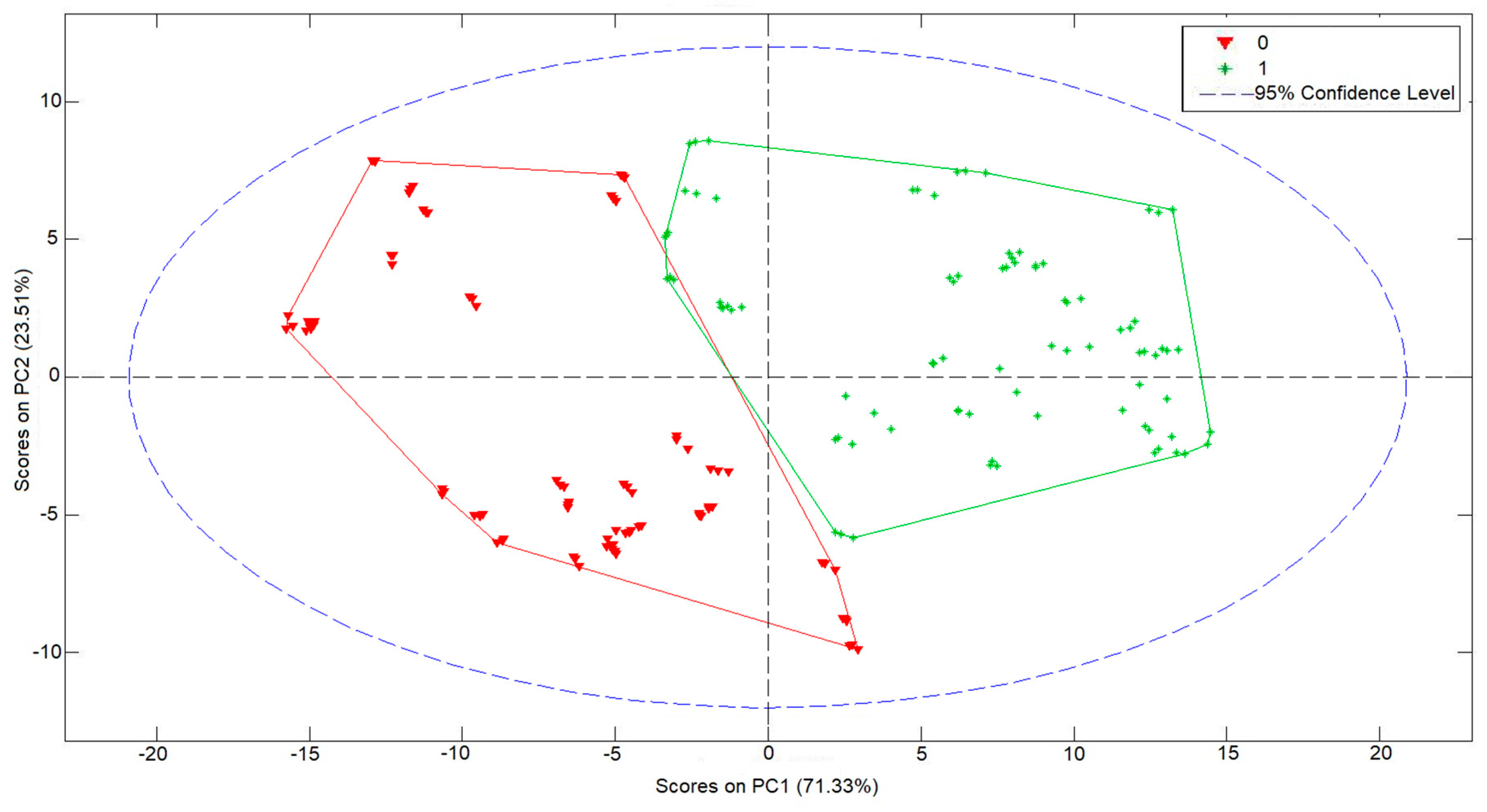
3.2. Multivariate Analysis Results
3.3. ANNs Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- FAO. Citrus Fruit Fresh and Processed Statistical Bulletin 2016; Food and Agriculture Organization: Rome, Italy, 2017; p. 66. [Google Scholar]
- El-Otmani, M.; Ait-Oubahou, A.; Zacarías, L. Citrus spp.: Orange, mandarin, tangerine, clementine, grapefruit, pomelo, lemon and lime. In Woodhead Publishing Series in Food Science, Technology and Nutrition, Postharvest Biology and Technology of Tropical and Subtropical Fruits; Yahia, E.M., Ed.; Woodhead Publishing: Sawston, UK, 2011; pp. 437–516. [Google Scholar]
- Zabihi, H.; Vogeler, I.; Amin, Z.M.Y.; Gourabi, B.R. Mapping the sensitivity of citrus crops to freeze stress using a geographical information system in Ramsar, Iran. Weather Clim. Extrem. 2016, 14, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Artes, F.; Artes-Hernandez, F. Daños por frío en la postrecolección de frutas y hortalizas. In Avances en Ciencias y Técnicas del Frío 1; Gómez, A.L., Calero, F.A., Esnoz, A., Nicuesa, A.E., Eds.; Universidad Politécnica de Cartagena: Cartagena, Spain, 2003; pp. 299–310. [Google Scholar]
- Tan, E.S.; Slaughter, D.C.; Thompson, J.F. Freeze damage detection in oranges using gas sensors. Postharvest Biol. Technol. 2005, 35, 177–182. [Google Scholar] [CrossRef]
- Slaughter, D.C.; Obenland, D.M.; Thompson, J.F.; Arpaia, M.L.; Margosan, D.A. Non-destructive freeze damage detection in oranges using machine vision and ultraviolet fluorescence. Postharvest Biol. Technol. 2008, 48, 341–346. [Google Scholar] [CrossRef]
- Martínez, L.; Ibacache, A.; Rojas, L. Daños por heladas en frutales. Tierra Adentro 2008, 80, 32–35. [Google Scholar]
- Wang, C.Y. Chilling and freezing injury. In The Commercial Storage of Fruits, Vegetables, and Florist and Nursery Stocks. Agriculture Handbook 66; Gross, K.C., Wang, C.Y., Saltveir, M., Eds.; Agriculture Handbook: Washington, DC, USA, 2016. [Google Scholar]
- Snyder, R.L.; Melo-Abreu, J.P.; Villar-Mir, J.M. Protección Contra Las Heladas: Fundamentos, Práctica y Economía; Food and Agriculture Organization: Rome, Italy, 2010; p. 68. [Google Scholar]
- Urbina Vallejo, V. Daños por heladas en frutales. Sintomatología y evaluación. In Curs de Valoració de Danys Climatològics i Incendis, Reus-Tarragona, Spain, May 28th–29th 2007 Centre de Formació i Estudis Agrorurals; Generalitat de Catalunya—Departament d’Agricultura, Alimentació i Acció Rural: Barcelona, Spain, 2007; p. 19. [Google Scholar]
- Sala, J.M.; Sanchez-Ballesta, M.T.; Alférez, F.; Mulas, M.; Zacarias, L.; Lafuente, M.T. A comparative study of the postharvest performance of an ABA deficient mutant of oranges II. Antioxidant enzymatic system and phenylalanine ammonia-lyase in non-chilling and chilling peel disorders of citrus fruit. Postharvest Biol. Technol. 2005, 37, 232–240. [Google Scholar] [CrossRef]
- Siboza, X.I.; Bertling, I.; Odindo, A.O. Salicylic acid and methyl jasmonate improve chilling tolerance in cold-stored lemon fruit (Citrus limon). J. Plant Physiol. 2014, 171, 1722–1731. [Google Scholar] [CrossRef] [PubMed]
- Jha, P.K.; Xanthakis, E.; Chevallier, S.; Jury, V.; Le-Bail, A. Assessment of freeze damage in fruits and vegetables. Food Res. Int. 2018, 121, 479–496. [Google Scholar] [CrossRef] [PubMed]
- Sala, J.M.; Lafuente, M.T. Catalase in the heat-induced chilling tolerance of cold-stored hybrid Fortune mandarin fruits. J. Agric. Food Chem. 1999, 47, 2410–2414. [Google Scholar] [CrossRef] [PubMed]
- USDA. Arizona California Citrus Loss Adjustment Standards Handbook; USDA: Washington, DC, USA, 1999; p. 35.
- Hatton, T.T.; Cubbedge, R.H. Separation of frozen grapefruit by using emulsions of differing specific gravities. Proc. Fla. State Hortic. Soc. 1978, 91, 126–128. [Google Scholar]
- Miller, W.M.; Wardowski, W.F.; Grierson, W. Separation and grading of freeze- damaged fruit. In Fresh Citrus Fruits; Wardowski, W.F., Miller, W.M., Hall, D.J., Grierson, W., Eds.; Florida Science Source, Inc.: Longboat Key, FL, USA, 2006; pp. 299–306. [Google Scholar]
- Moomkesh, S.; Ahmad Mireei, S.; Sadeghi, M.; Mazeri, M. Early detection of freezing damage in sweet lemons using Vis/SWNIR spectroscopy. Biosyst. Eng. 2017, 164, 157–170. [Google Scholar] [CrossRef]
- Obenland, D.M.; Aung, L.H.; Bridges, D.L.; Mackey, B.E. Volatile emissions of navel oranges as predictors of freeze damage. J. Agric. Food Chem. 2003, 51, 3367–3371. [Google Scholar] [CrossRef] [PubMed]
- Gambhir, P.N.; Choi, Y.J.; Slaughter, D.C.; Thompson, J.F.; McCarthy, M.J. Proton spin–spin relaxation time of peel and flesh of navel orange varieties exposed to freezing temperature. J. Sci. Food Agric. 2005, 85, 2482–2486. [Google Scholar] [CrossRef]
- Fuentes, A.; Masot, R.; Fernández-Segovia, I.; Ruiz-Rico, M.; Alcañiz, M.; Barat, J.M. Differentiation between fresh and frozen-thawed sea bream (Sparus aurata) using impedance spectroscopy techniques. Innov. Food Sci. Emerg. Technol. 2013, 19, 210–217. [Google Scholar] [CrossRef]
- Conesa, C.; Gracía-Breijo, E.; Loeff, E.; Seguí, L.; Fito, P.; Laguarda-Miró, N. An Electrochemical Impedance Spectroscopy-Based Technique to Identify and Quantify Fermentable Sugars in Pineapple Waste Valorization for Bioethanol Production. Sensors 2015, 15, 22941–22955. [Google Scholar] [CrossRef] [Green Version]
- Macdonald, J.R.; Barsoukov, E. Impedance Spectroscopy. Theory, Experiment and Applications, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; p. 595. [Google Scholar]
- Wu, L.; Ogawa, Y.; Tagawa, A. Electrical impedance spectroscopy analysis of eggplant pulp and effects of drying and freezing-thawing treatments on its impedance characteristics. J. Food Eng. 2008, 87, 274–280. [Google Scholar] [CrossRef]
- Serrano-Pallicer, E.; Muñoz-Albero, M.; Pérez-Fuster, C.; Masot Peris, R.; Laguarda-Miró, N. Early Detection of Freeze Damage in Navelate Oranges with Electrochemical Impedance Spectroscopy. Sensors 2018, 18, 4503. [Google Scholar] [CrossRef]
- Grossi, M.; Riccò, B. Electrical impedance spectroscopy (EIS) for biological analysis and food characterization: A review. J. Sens. Sens. Syst. 2017, 6, 303–325. [Google Scholar] [CrossRef]
- Chowdhury, A.; Bera, T.K.; Ghoshal, D.; Chakraborty, B. Electrical Impedance Variations in Banana Ripening: An Analytical Study with Electrical Impedance Spectroscopy. J. Food Process Eng. 2017, 40, 1–14. [Google Scholar] [CrossRef]
- Bauchot, A.D.; Harker, F.R.; Arnold, W.M. The use of electrical impedance spectroscopy to assess the physiological condition of kiwifruit. Postharvest Biol. Technol. 2000, 18, 9–18. [Google Scholar] [CrossRef]
- Figuereido, A.; Cárdenas, N.; Rabelo, E.; Pequeño de Oliveira, H. Determination of mango ripening degree by electrical impedance spectroscopy. Comput. Electron. Agric. 2017, 143, 222–226. [Google Scholar] [CrossRef]
- Benavente, J.; Ramos-Barrado, J.R.; Heredia, A. A study of the electrical behaviour of isolated tomato cuticular membranes and cutin by impedance spectroscopy measurements. Colloids Surf. A 1998, 140, 333–338. [Google Scholar] [CrossRef]
- Ando, Y.; Maeda, Y.; Mizutani, K.; Wakatsuki, N.; Hagiwara, S.; Nabetani, H. Impact of blanching and freeze-thaw pretreatment on drying rate of carrot roots in relation to changes in cell membrane function and cell structure. LWT Food Sci. Technol. 2016, 71, 40–46. [Google Scholar] [CrossRef]
- Ando, Y.; Maeda, Y.; Mizutani, K.; Wakatsuki, N.; Hagiwara, S.; Nabetani, H. Effect of air-dehydration pretreatment before freezing on the electrical impedance characteristics and texture of carrots. J. Food Eng. 2016, 169, 114–121. [Google Scholar] [CrossRef]
- Fuentes, A.; Vázquez-Gutiérrez, J.L.; Pérez-Gago, M.B.; Vonasek, E.; Nitin, N.; Barret, D.M. Application of nondestructive impedance spectroscopy to determination of the effect of temperature on potato microstructure and texture. J. Food Eng. 2014, 133, 16–22. [Google Scholar] [CrossRef]
- M’hiri, N.; Veys-Renaux, D.; Rocca, E.; Ioannou, I.; Mihoubi Bourdinova, N.; Ghoul, M. Corrosion inhibition of carbon steel in acidic medium by orange peel extract and its main antioxidant compounds. Corros. Sci. 2016, 102, 55–62. [Google Scholar] [CrossRef]
- Conesa, C.; Ibáñez, J.; Seguí, L.; Fito, P.; Laguarda-Miro, N. An Electrochemical Impedance Spectroscopy System for Monitoring Pineapple Waste Saccharification. Sensors 2016, 16, 188. [Google Scholar] [CrossRef]
- Conesa, C.; Gil, L.; Seguí, L.; Fito, P.; Laguarda-Miro, N. Ethanol quantification in pineapple waste by an electrochemical impedance spectroscopy-based system and artificial neural networks. Chemom. Intell. Lab. Syst. 2017, 161, 1–7. [Google Scholar] [CrossRef]
- Ulrich, C.; Petersson, H.; Sundgren, H.; Björefors, F.; Krantz-Rülcker, C. Simultaneous estimation of soot and diesel contamination in engine oil using electrochemical impedance spectroscopy. Sens. Actuators B Chem. 2007, 127, 613–618. [Google Scholar] [CrossRef]
- Olivati, C.A.; Riul, A.; Balogh, D.T.; Oliveira, O.N.; Ferreira, M. Detection of phenolic compounds using impedance spectroscopy measurements. Bioprocess Biosyst. Eng. 2009, 32, 41–46. [Google Scholar] [CrossRef]
- Martinez Gil, P.; Laguarda-Miró, N.; Soto Camino, J.; Masot Peris, R. Glyphosate detection with ammonium nitrate and humic acids as potential interfering substances by pulsed voltammetry technique. Talanta 2013, 115, 702–705. [Google Scholar] [CrossRef]
- Górski, Ł.; Sordoń, W.; Ciepiela, F.; Kubiak, W.W.; Jakubowska, M. Voltammetric classifcation of ciders with PLS-DA. Talanta 2016, 146, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Buchheit, R.G. Use of Artificial Neural Network Models to Predict Coated Component Life from Short-Term Electrochemical Impedance Spectroscopy Measurements. Corrosion 2008, 64, 241–254. [Google Scholar] [CrossRef]
- Eddahech, A.; Briat, O.; Bertrand, N.; Delétage, J.Y.; Vinassa, J.M. Behavior and state-of-health monitoring of Li-ion batteries using impedance spectroscopy and recurrent neural networks. Int. J. Electron. Power Energy Syst. 2012, 42, 487–494. [Google Scholar] [CrossRef]
- Conesa, C.; Seguí, L.; Laguarda-Miró, N.; Fito, P. Microwaves as a pretreatment for enhancing enzymatic hydrolysis of pineapple industrial waste for bioethanol production. Food. Bioprod. Process. 2016, 100, 203–213. [Google Scholar] [CrossRef]
- Council of Europe. Technical Document. Guidelines on Metals and Alloys Used as Food Contact Materials. In Partial Agreement Department in the Social and Public Health Field; Council of Europe: Strasburg, France, 2002; p. 88. Available online: http://www.mast.is/Uploads/document/guidelines_metals_alloys_used_as_food_contact_materials.pdf (accessed on 11 September 2019).
- Masot, R.; Alcañiz, M.; Fuentes, A.; Schmidt, F.C.; Barat, J.M.; Gil, L.; Baigts, D.; Martínez-Máñez, R.; Soto, J. Design of a low-cost non-destructive system for punctual measurements of salt levels in food products using impedance spectroscopy. Sens. Actuators A Phys. 2010, 158, 217–223. [Google Scholar] [CrossRef]
- Wold, S.; Sjostrom, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Legin, E.; Zadorozhnaya, O.; Khaydukova, M.; Kirsanov, D.; Rybakin, V.; Zagrebin, A.; Ignatyeva, N.; Ashina, J.; Sarkar, S.; Mukherjee, S.; et al. Rapid Evaluation of Integral Quality and Safety of Surface and Waste Waters by a Multisensor System (Electronic Tongue). Sensors 2019, 19, 2019. [Google Scholar] [CrossRef] [PubMed]
- Kasuba, T. Simplified fuzzy ARTMAP. AI Expert 1993, 8, 18–25. [Google Scholar]
- Garcia-Breijo, E.; Atkinson, J.; Gil-Sanchez, L.; Masot, R.; Ibañez, J.; Garrigues, J.; Glanc, M.; Laguarda-Miro, N.; Olguin, C. A comparison study of pattern recognition algorithms implemented on a microcontroller for use in an electronic tongue for monitoring drinking waters. Sens. Actuators A Phys. 2011, 2, 570–582. [Google Scholar] [CrossRef]
- Rajasekaran, S.; Vijayalakshmi Pai, G.A. Neural Networks, Fuzzy Logic and Genetic Algorithms: Synthesis and Applications; Prentice Hall: New Delhi, India, 2004; p. 456. [Google Scholar]
- Garcia-Breijo, E.; Garrigues, J.; Gil Sanchez, L.; Laguarda-Miró, N. An Embedded Simplified Fuzzzy ARTMAP Implemented on a Microcontroller for Food Classification. Sensors 2013, 13, 10418–10429. [Google Scholar] [CrossRef]
- Brezmes, J.; Cabre, P.; Rojo, S.; Llobet, E.; Xilanova, X.; Correig, X. Discrimination between different samples of olive oil using variable selection techniques and modified fuzzy artmap neural networks. IEEE Sens. J. 2005, 5, 463–470. [Google Scholar] [CrossRef]
- Ibáñez Civera, J.; Garcia Breijo, E.; Laguarda Miró, N.; Gil Sánchez, L.; Garrigues Baixauli, J.; Romero Gil, I.; Masot Peris, R.; Alcañiz Fillol, M. Artificial neural network onto Eight Bit microcontroller for Secchi depth calculation. Sens. Actuators B Chem. 2011, 156, 132–139. [Google Scholar] [CrossRef]
- Del Brío, B.M.; Molina, A.S. Redes Neuronales y Sistemas Borrosos, 2nd ed.; Ra-Ma: Madrid, Spain, 2001; p. 399. [Google Scholar]
- Fricke, H.; Morse, S. The electric resistance and capacity of blood for frequencies between 800 and 4(1/2) million cycles. J. Gen. Physiol. 1925, 9, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Damez, J.-L.; Clerjon, S.; Abouelkaram, S.; Lepetit, J. Dielectric behavior of beef meat in the 1–1500 kHz range: Simulation with the Fricke/Cole-Cole model. Meat Sci. 2007, 77, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shen, H.; Lun, Y. Study on the electric conduction properties of fresh and frozen-thawed grass carp (Ctenopharyngodon idellus) and tilapia (Oreochromis niloticus). Int. J. Food Sci. Technol. 2010, 45, 2560–2564. [Google Scholar] [CrossRef]


| Parameter | Specifications |
|---|---|
| Frequency range | 1 Hz–1 MHz |
| Signal amplitude | Up to 500 mV |
| Type of signal | Sinusoidal |
| Impedance calculation | Discrete Fourier Transform |
| Measured parameters | Current and Voltage |
| Output data | Modulus and phase of the impedance |
| Data set per assay | Up to 100 data (50 for modulus and 50 for phase) |
| ANN Architecture: 20-13-1 | |||
|---|---|---|---|
| Training | Validation | Test | Overall |
| CCR = 100% | CCR = 100% | CCR = 100% | CCR = 100% |
 |  |  |  |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ochandio Fernández, A.; Olguín Pinatti, C.A.; Masot Peris, R.; Laguarda-Miró, N. Freeze-Damage Detection in Lemons Using Electrochemical Impedance Spectroscopy. Sensors 2019, 19, 4051. https://doi.org/10.3390/s19184051
Ochandio Fernández A, Olguín Pinatti CA, Masot Peris R, Laguarda-Miró N. Freeze-Damage Detection in Lemons Using Electrochemical Impedance Spectroscopy. Sensors. 2019; 19(18):4051. https://doi.org/10.3390/s19184051
Chicago/Turabian StyleOchandio Fernández, Adrián, Cristian Ariel Olguín Pinatti, Rafael Masot Peris, and Nicolás Laguarda-Miró. 2019. "Freeze-Damage Detection in Lemons Using Electrochemical Impedance Spectroscopy" Sensors 19, no. 18: 4051. https://doi.org/10.3390/s19184051
APA StyleOchandio Fernández, A., Olguín Pinatti, C. A., Masot Peris, R., & Laguarda-Miró, N. (2019). Freeze-Damage Detection in Lemons Using Electrochemical Impedance Spectroscopy. Sensors, 19(18), 4051. https://doi.org/10.3390/s19184051






