Non-Covalent Functionalization of Carbon Nanotubes for Electrochemical Biosensor Development
Abstract
1. Introduction
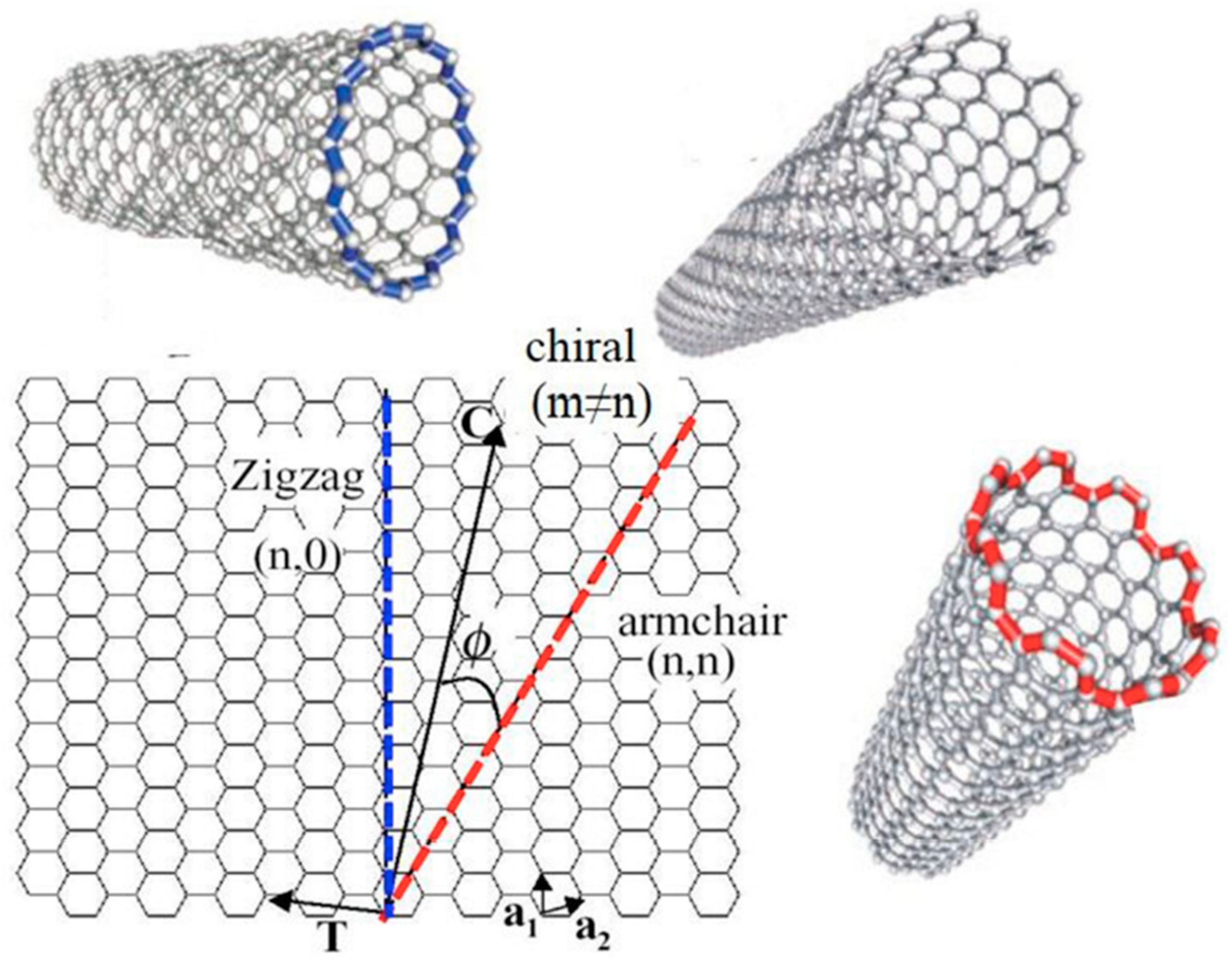
2. Functionalization of Carbon Nanotubes
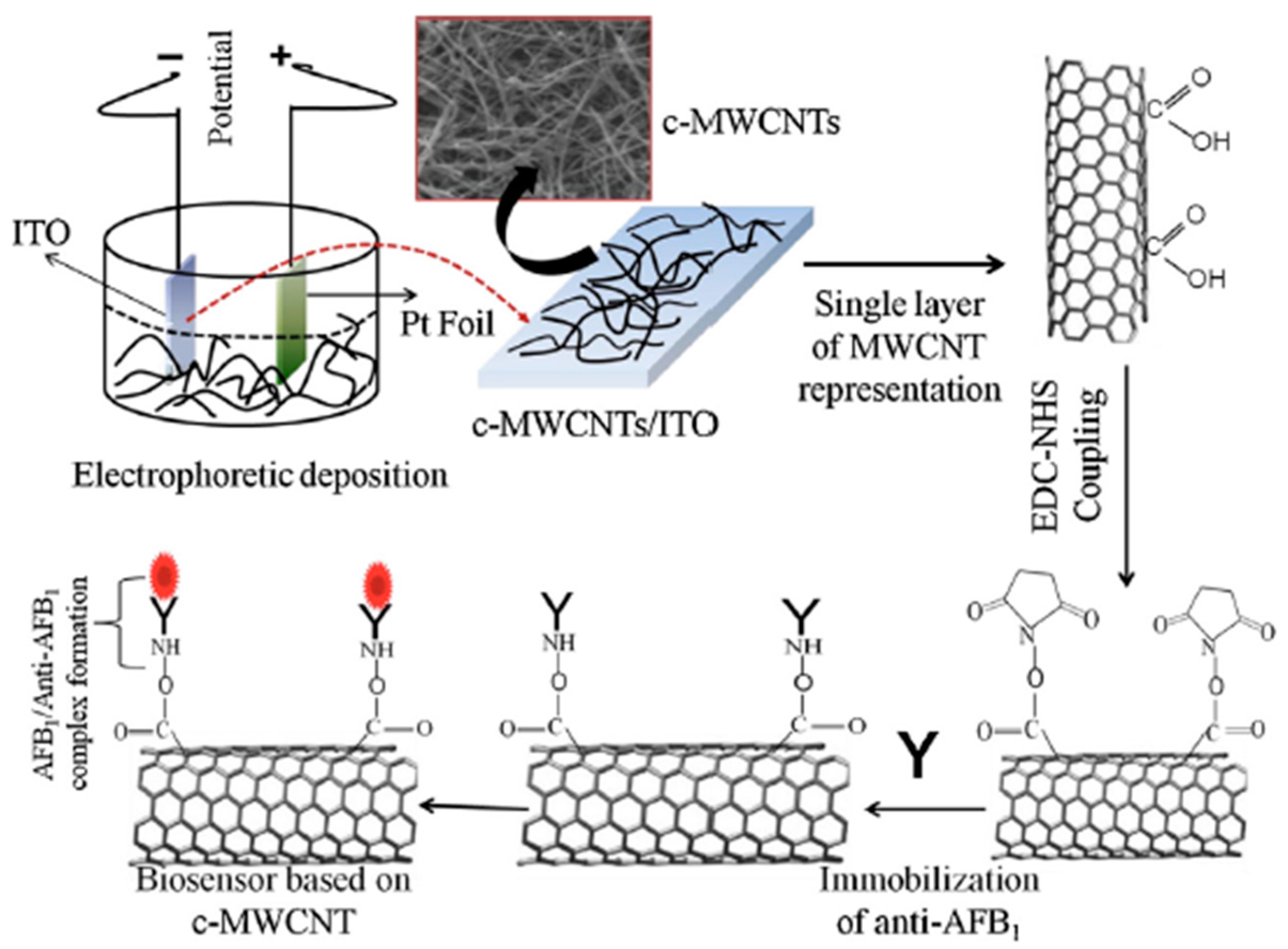
2.1. Covalent Functionalization
2.2. Non-Covalent Functionalization
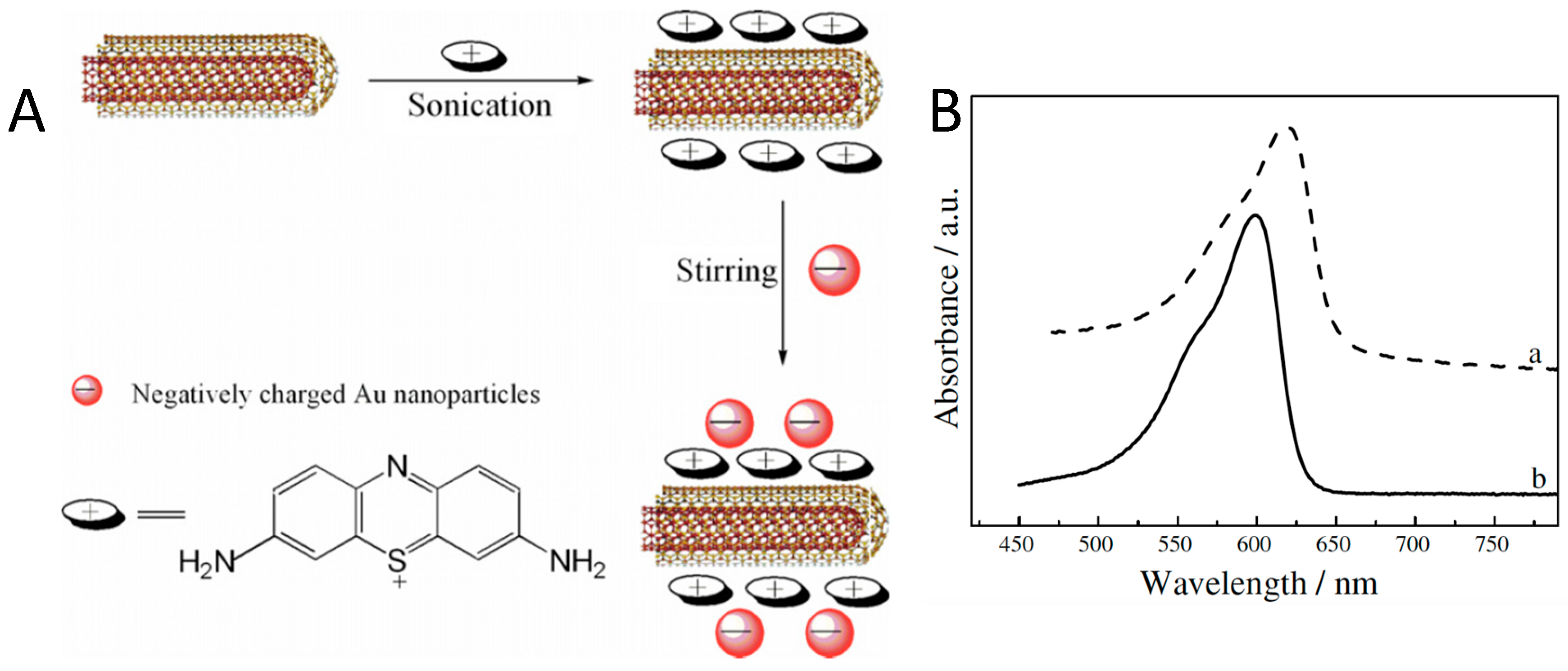
2.2.1. Π-Π Interactions with Aromatic Molecules
2.2.2. Π-Π Interaction with Polymers
2.2.3. Electrostatic Interaction with Polymers
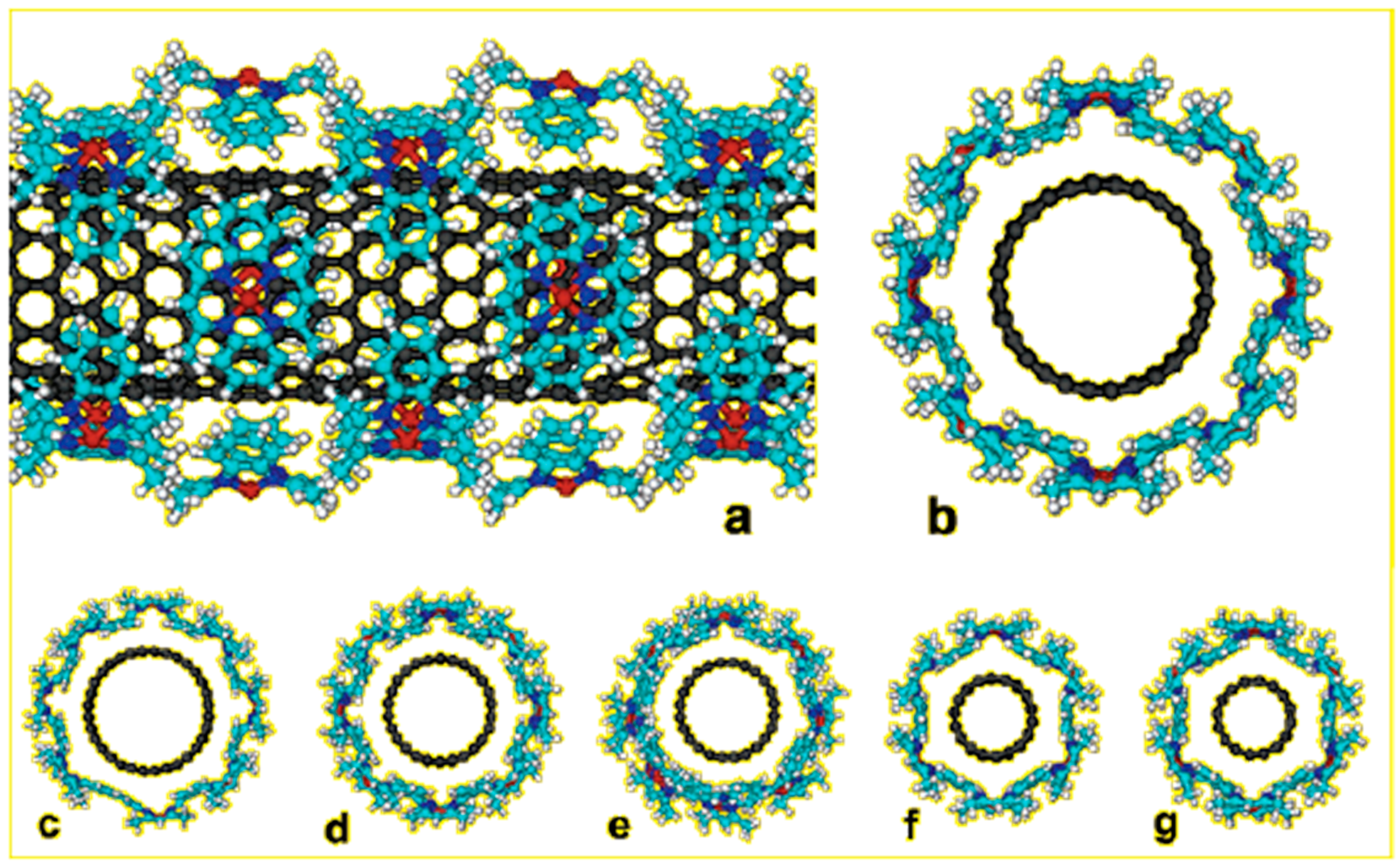
2.2.4. CH-Π Interactions
2.2.5. Non-Covalent Functionalization without Coupling Agent
3. Applications of Non-Covalent Functionalization of CNT Using Different Bio-Recognition Elements for Electrochemical Biosensors
3.1. Proteins
3.2. Enzymes
3.3. Antibodies
3.4. Viruses
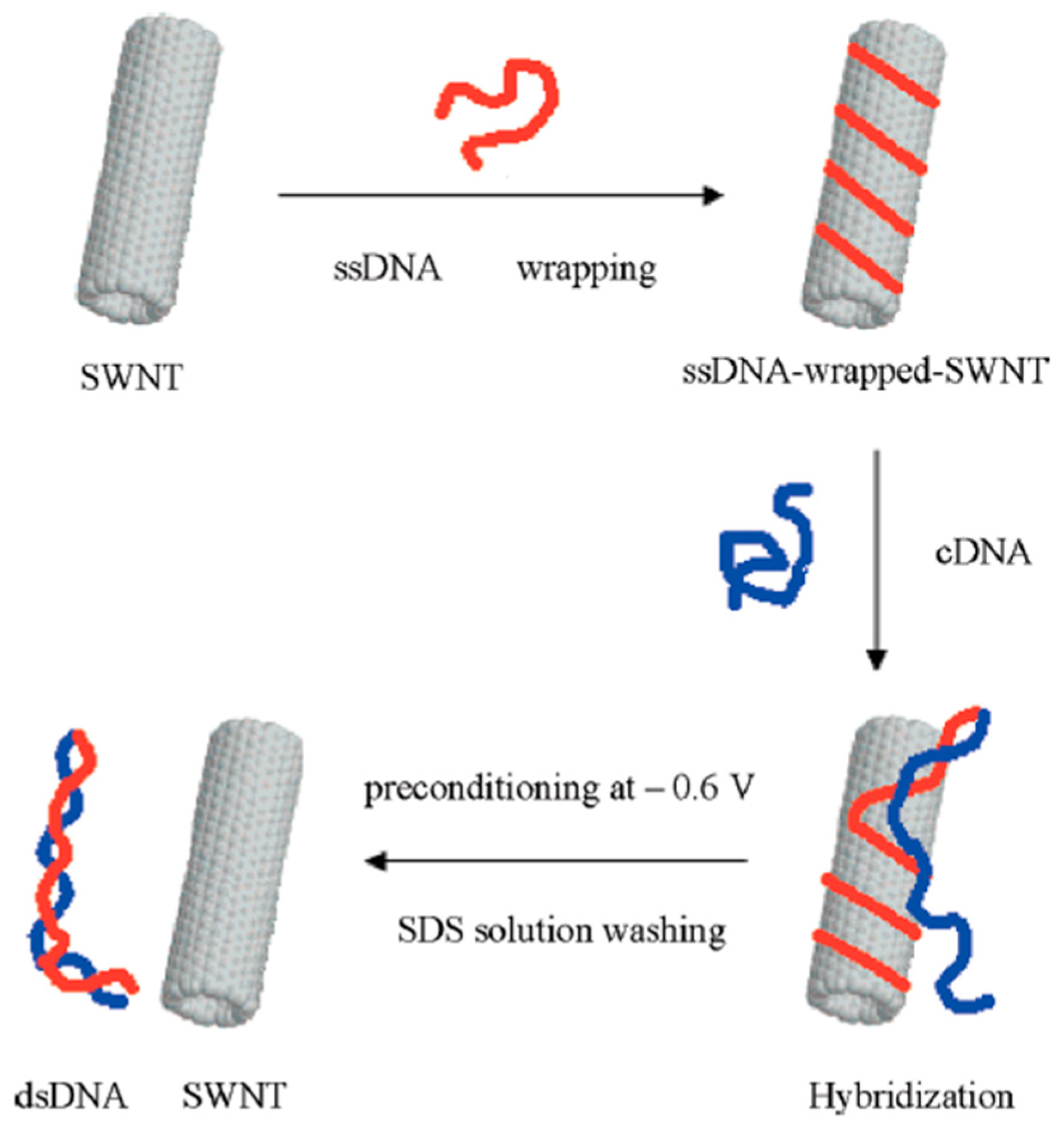
3.5. DNA
4. Conclusions and Outlook
Funding
Conflicts of Interest
References
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical Biosensors Sensor Principles and Architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef] [PubMed]
- Pangule, R.C.; Brooks, S.J.; Dinu, C.Z.; Bale, S.S.; Salmon, S.L.; Zhu, G.; Metzger, D.W.; Kane, R.S.; Dordick, J.S. Antistaphylococcal Nanocomposite Films Based on Enzyme−Nanotube Conjugates. ACS Nano 2010, 7, 3993–4000. [Google Scholar] [CrossRef] [PubMed]
- Senthil Kumar, A.; Gayathri, P.; Barathi, P.; Vijayaraghavan, R. Improved Electric Wiring of Hemoglobin with Impure-Multiwalled Carbon Nanotube/Nafion Modified Glassy Carbon Electrode and Its Highly Selective Hydrogen Peroxide Biosensing. J. Phys. Chem. C 2012, 116, 23692–23703. [Google Scholar] [CrossRef]
- Turner, A.P. Biosensors: Sense and sensibility. Chem. Soc. Rev. 2013, 42, 3184–3196. [Google Scholar] [CrossRef] [PubMed]
- Holzinger, M.; Le Goff, A.; Cosnier, S. Nanomaterials for biosensing applications: A review. Front. Chem. 2014, 2, 63. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Umasankar, Y.; Ramasamy, R.P. Laccase-TiO2 nanoconjugates as catalysts for oxygen reduction reaction in biocathodes. J. Electrochem. Soc. 2015, 162, H911–H917. [Google Scholar] [CrossRef]
- Guo, X. Surface plasmon resonance based biosensor technique: A review. J. Biophotonics 2012, 5, 483–501. [Google Scholar] [CrossRef]
- Solanki, P.R.; Kaushik, A.; Agrawal, V.V.; Malhotra, B.D. Nanostructured metal oxide-based biosensors. NPG Asia Mater. 2011, 3, 17–24. [Google Scholar] [CrossRef]
- Fang, Y.; Umasankar, Y.; Ramasamy, R.P. Electrochemical detection of p-ethylguaiacol, a fungi infected fruit volatile using metal oxide nanoparticles. Analyst 2014, 139, 3804–3810. [Google Scholar] [CrossRef]
- Mundra, R.V.; Wu, X.; Sauer, J.; Dordick, J.S.; Kane, R.S. Nanotubes in biological applications. Curr. Opin. Biotechnol. 2014, 28, 25–32. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Balasubramanian, K.; Burghard, M. Chemically functionalized carbon nanotubes. Small 2005, 1, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Gan, Z.; Zhuang, Q. Electrochemical sensors based on carbon nanotubes. Electroanalysis 2002, 14, 1609–1613. [Google Scholar] [CrossRef]
- Tilmaciu, C.M.; Morris, M.C. Carbon nanotube biosensors. Front. Chem. 2015, 3, 59. [Google Scholar] [CrossRef]
- Lawal, A.T. Synthesis and utilization of carbon nanotubes for fabrication of electrochemical biosensors. Mater. Res. Bull. 2016, 73, 308–350. [Google Scholar] [CrossRef]
- Ahn, J.H.; Kim, J.H.; Reuel, N.F.; Barone, P.W.; Boghossian, A.A.; Zhang, J.; Yoon, H.; Chang, A.C.; Hilmer, A.J.; Strano, M.S. Label-free, single protein detection on a near-infrared fluorescent single-walled carbon nanotube/protein microarray fabricated by cell-free synthesis. Nano Lett. 2011, 11, 2743–2752. [Google Scholar] [CrossRef] [PubMed]
- Crescenzo, A.D.; Ettorre, V.; Fontana, A. Non-covalent and reversible functionalization of carbon nanotubes. Beilstein J. Nanotechnol. 2014, 5, 1675–1690. [Google Scholar] [CrossRef]
- Britz, D.A.; Khlobystov, A.N. Noncovalent interactions of molecules with single walled carbon nanotubes. Chem. Soc. Rev. 2006, 35, 637–659. [Google Scholar] [CrossRef]
- Kanoun, O.; Muller, C.; Benchirouf, A.; Sanli, A.; Dinh, T.N.; Al-Hamry, A.; Bu, L.; Gerlach, C.; Bouhamed, A. Flexible carbon nanotube films for high performance strain sensors. Sensors 2014, 14, 10042–10071. [Google Scholar] [CrossRef]
- Trojanowicz, M. Analytical applications of carbon nanotubes: A review. TrAC Trends Anal. Chem. 2006, 25, 480–489. [Google Scholar] [CrossRef]
- Yang, W.; Ratinac, K.R.; Ringer, S.P.; Thordarson, P.; Gooding, J.J.; Braet, F. Carbon nanomaterials in biosensors: Should you use nanotubes or graphene? Angew. Chem. Int. Ed. Engl. 2010, 49, 2114–2138. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.-G.; Delaney, P.; Louie, S.G. Quantum conductance of multiwall carbon nanotubes. Phys. Rev. B 2002, 66, 073407. [Google Scholar] [CrossRef]
- Bachtold, A.; Strunk, C.; Salvetat, J.P.; Bonard, J.M.; Forró, L.; Nussbaumer, T.; Schönenberger, C. Aharonov–Bohm oscillations in carbon nanotubes. Nature 1999, 397, 673–675. [Google Scholar] [CrossRef]
- Eatemadi, A.; Daraee, H.; Karimkhanloo, H.; Kouhi, M.; Zarghami, N.; Akbarzadeh, A.; Abasi, M.; Hanifehpour, Y.; Joo, S.W. Carbon nanotubes: Properties, synthesis, purification, and medical applications. Nanoscale Res. Lett. 2014, 9, 393. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Dai, Z. Carbon nanomaterial-based electrochemical biosensors: An overview. Nanoscale 2015, 7, 6420–6431. [Google Scholar] [CrossRef] [PubMed]
- Tasis, D.; Tagmatarchis, N.; Bianco, A.; Prato, M. Chemistry of Carbon Nanotubes. Chem. Rev. 2006, 106, 1105–1136. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Chen, X.; Hu, N.; Tam, U.C.; Blixt, O.; Zettl, A.; Bertozzi, C.R. Biocompatible Carbon Nanotubes Generated by Functionalization with Glycodendrimers. Angew. Chem. 2008, 120, 5100–5103. [Google Scholar] [CrossRef]
- Tournus, F.; Charlier, J.C. Ab initiostudy of benzene adsorption on carbon nanotubes. Phys. Rev. B 2005, 71, 165421. [Google Scholar] [CrossRef]
- Blanford, C.F.; Foster, C.E.; Heath, R.S.; Armstrong, F.A. Efficient electrocatalytic oxygen reduction by the ‘blue’ copper oxidase, laccase, directly attached to chemically modified carbons. Faraday Discuss. 2009, 140, 319–335. [Google Scholar] [CrossRef]
- Bellino, M.G.; Soler-Illia, G.J.A.A. Nano-Designed Enzyme–Functionalized Hierarchical Metal–Oxide Mesoporous Thin Films: En Route to Versatile Biofuel Cells. Small 2014, 10, 2834–2839. [Google Scholar] [CrossRef]
- Besteman, K.; Lee, J.-O.; Wiertz, F.G.M.; Heering, H.A.; Dekker, C. Enzyme-Coated Carbon Nanotubes as Single-Molecule Biosensors. Nano Lett. 2003, 3, 727–730. [Google Scholar] [CrossRef]
- Jain, S. Development of an Antibody Functionalized Carbon Nanotube Biosensor for Foodborne Bacterial Pathogens. J. Biosens. Bioelectron. 2012, 11. [Google Scholar] [CrossRef]
- Aravind, S.S.J.; Ramaprabhu, S. Noble metal dispersed multiwalled carbon nanotubes immobilized ss-DNA for selective detection of dopamine. Sens. Actuators B Chem. 2011, 155, 679–686. [Google Scholar] [CrossRef]
- Bianco, A.; Kostarelos, K.; Partidos, C.D.; Prato, M. Biomedical applications of functionalised carbon nanotubes. Chem. Commun. 2005, 5, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, J.; Shen, Y.; Wang, M.; Li, J. Poly-l-lysine Functionalization of Single-Walled Carbon Nanotubes. J. Phys. Chem. B 2004, 108, 15343–15346. [Google Scholar] [CrossRef]
- Zeng, Y.-L.; Huang, Y.-F.; Jiang, J.-H.; Zhang, X.-B.; Tang, C.-R.; Shen, G.-L.; Yu, R.-Q. Functionalization of multi-walled carbon nanotubes with poly(amidoamine) dendrimer for mediator-free glucose biosensor. Electrochem. Commun. 2007, 9, 185–190. [Google Scholar] [CrossRef]
- Singh, C.; Srivastava, S.; Ali, M.A.; Gupta, T.K.; Sumana, G.; Srivastava, A.; Mathur, R.B.; Malhotra, B.D. Carboxylated MWCNT based biosensor for aflatoxin detection. Sens. Actuators B 2013, 185, 258–264. [Google Scholar]
- Viswanathan, S.; Rani, C.; Vijay Anand, A.; Ho, J.A. Disposable electrochemical immunosensor for carcinoembryonic antigen using ferrocene liposomes and MWCNT screen-printed electrode. Biosens. Bioelectron. 2009, 24, 1984–1989. [Google Scholar] [CrossRef]
- Periasamy, A.P.; Chang, Y.J.; Chen, S.M. Amperometric glucose sensor based on glucose oxidase immobilized on gelatin-multiwalled carbon nanotube modified glassy carbon electrode. Bioelectrochemistry 2011, 80, 114–120. [Google Scholar] [CrossRef]
- Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. BioTechniques 2004, 37, 790–802. [Google Scholar] [CrossRef]
- Calcio Gaudino, E.; Tagliapietra, S.; Martina, K.; Barge, A.; Lolli, M.; Terreno, E.; Lembo, D.; Cravotto, G. A novel SWCNT platform bearing DOTA and beta-cyclodextrin units. “One shot” multidecoration under microwave irradiation. Org. Biomol. Chem. 2014, 12, 4708–4715. [Google Scholar] [CrossRef] [PubMed]
- Dinesh, B.; Bianco, A.; Menard-Moyon, C. Designing multimodal carbon nanotubes by covalent multi-functionalization. Nanoscale 2016, 8, 18596–18611. [Google Scholar] [CrossRef] [PubMed]
- Lamanna, G.; Battigelli, A.; Ménard-Moyon, C.; Bianco, A. Multifunctionalized carbon nanotubes as advanced multimodal nanomaterials for biomedical applications. Nanotechnol. Rev. 2012, 1, 17–29. [Google Scholar] [CrossRef]
- Tuncel, D. Non-covalent interactions between carbon nanotubes and conjugated polymers. Nanoscale 2011, 3, 3545–3554. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-L.; Stoddart, J.F. Noncovalent Functionalization of Single-Walled Carbon Nanotubes. Acc. Chem. Res. 2009, 42, 1161–1171. [Google Scholar] [CrossRef]
- Gao, C.; Guo, Z.; Liu, J.H.; Huang, X.J. The new age of carbon nanotubes: An updated review of functionalized carbon nanotubes in electrochemical sensors. Nanoscale 2012, 4, 1948–1963. [Google Scholar] [CrossRef]
- Chen, R.J.; Bangsaruntip, S.; Drouvalakis, K.A.; Kam, N.W.S.; Shim, M.; Li, Y.; Kim, W.; Utz, P.J.; Dai, H. Noncovalent functionalization of carbon nanotubes for highly specific electronic biosensors. Proc. Natl. Acad. Sci. USA 2003, 100, 4984–4989. [Google Scholar] [CrossRef]
- Star, A.; Han, T.-R.; Gabriel, J.-C.P.; Bradley, K.; Gruner, G. Interaction of Aromatic Compounds with CN Correlation to the Hammett Parameter of the Substituent and Measured Carbon Nanotube FET Response. Nano Lett. 2003, 3, 1421–1423. [Google Scholar] [CrossRef]
- Woods, L.M.; Bădescu, Ş.C.; Reinecke, T.L. Adsorption of simple benzene derivatives on carbon nanotubes. Phys. Rev. B 2007, 75, 155415. [Google Scholar] [CrossRef]
- Chen, R.J.; Zhang, Y.; Wang, D.; Dai, H. Noncovalent Sidewall Functionalization of Single-Walled Carbon Nanotubes for Protein Immobilization. J. Am. Chem. Soc. 2001, 123, 3838–3839. [Google Scholar] [CrossRef]
- Shim, M.; Kam, N.W.S.; Chen, R.J.; Li, Y.; Dai, H. Functionalization of CNT for biocomatibility and biiomelecurlar recognition. Nano Lett. 2002, 2, 285–288. [Google Scholar] [CrossRef]
- Assali, M.; Leal, M.P.; Fernández, I.; Baati, R.; Mioskowski, C.; Khiar, N. Non-covalent functionalization of carbon nanotubes with glycolipids: Glyconanomaterials with specific lectin-affinity. Soft Matter 2009, 5, 948–950. [Google Scholar] [CrossRef]
- Chen, Y.; Vedala, H.; Kotchey, G.P.; Audfray, A.; Cecioni, S.; Imberty, A.; Vidal, S.; Star†, A. Electronic Detection of Lectins using carbohydrate functionalized Graphene versus CNT. ACS Nano 2012, 6, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Bourourou, M.; Elouarzaki, K.; Lalaoui, N.M.; Agns, C.; Goff, A.L.; Holzinger, M.; Maaref, A.; Cosnier, S. Supramolecular Immobilization of Laccase on Carbon Nanotube Electrodes Functionalized with (Methylpyrenylaminomethyl)anthraquinone for Direct Electron Reduction of Oxygen. Chem. Eur. J. 2013, 2013, 9371–9375. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, R.P.; Luckarift, H.R.; Ivnitski, D.M.; Atanassov, P.B.; Johnson, G.R. High electrocatalytic activity of tethered multicopper oxidase- carbon nanotube conjugates. Chem. Commun. 2010, 46, 5977–6188. [Google Scholar] [CrossRef] [PubMed]
- Parimi, N.S.; Umasankar, Y.; Atanassov, P.; Ramasamy, R.P. Kinetic and Mechanistic Parameters of Laccase Catalyzed Direct Electrochemical Oxygen Reduction Reaction. ACS Catal. 2012, 2, 38–44. [Google Scholar] [CrossRef]
- Lau, C.; Adkins, E.R.; Ramasamy, R.P.; Luckarift, H.R.; Johnson, G.R.; Atanassov, P. Design of carbon nanotube-based gas-diffusion cathode for O2 reduction by multicopper oxidases. Adva. Energy Mater. 2012, 2, 162–168. [Google Scholar] [CrossRef]
- Sekar, N.; Umasankar, Y.; Ramasamy, R.P. Photocurrent generation by immobilized cyanobacteria via direct electron transport in photo-bioelectrochemical cells. Phys. Chem. Chem. Phys. 2014, 16, 7862–7871. [Google Scholar] [CrossRef]
- Goff, A.L.; Moggia, F.; Debou, N.; Jegou, P.; Artero, V.; Fontecave, M.; Jousselme, B.; Palacin, S. Facile and tunable functionalization of carbon nanotube electrodes with ferrocene by covalent coupling and π-stacking interactions and their relevance to glucose bio-sensing. J. Electroanal. Chem. 2010, 641, 57–63. [Google Scholar] [CrossRef]
- Basiuk, E.V.; Rybak-Akimova, E.V.; Basiuk, V.A.; Acosta-Najarro, D.; Saniger, J.M. Adsorption Modification of Single-Walled Carbon Nanotubes with Tetraazaannulene Macrocyclic Complexes. Nano Lett. 2002, 2, 1249–1252. [Google Scholar]
- Holzinger, M.; Baur, J.; Haddad, R.; Wang, X.; Cosnier, S. Multiple functionalization of single-walled carbon nanotubes by dip coating. Chem. Commun. 2011, 47, 2450–2452. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lu, Y.; Ma, Y.; Li, Y.; Du, F.; Chen, Y. Noncovalent nanohybrid of ferrocene with single-walled carbon nanotubes and its enhanced electrochemical property. Chem. Phys. Lett. 2006, 420, 416–420. [Google Scholar] [CrossRef]
- Huang, X.-J.; Im, H.-S.; Lee, D.-H.; Kim, H.-S.; Choi, Y.-K. Ferrocene functionalized single-walled carbon nanotube bundles hybrid interdigitated construction film for L-glutamate detection. J. Phys. Chem. C 2007, 111, 1200–1206. [Google Scholar] [CrossRef]
- Giroud, F.; Minteer, S.D. Anthracene-modified pyrenes immobilized on carbon nanotubes for direct electroreduction of O2 by laccase. Electrochem. Commun. 2013, 34, 157–160. [Google Scholar] [CrossRef]
- Zhang, J.; Lee, J.-K.; Wu, Y.; Murray, R.W. Photoluminescence and Electronic Interaction of Anthracene Derivatives Adsorbed on Sidewalls of SWCNT. Nano Lett. 2003, 3, 403–407. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, J.; Yan, H.; He, M.; Liu, Z. Thionine-mediated chemistry of carbon nanotubes. Carbon 2004, 42, 287–291. [Google Scholar] [CrossRef]
- Wang, Z.; Li, M.; Zhang, Y.; Yuan, J.; Shen, Y.; Niu, L.; Ivaska, A. Thionine-interlinked multi-walled carbon nanotube/gold nanoparticle composites. Carbon 2007, 45, 2111–2115. [Google Scholar] [CrossRef]
- Ghica, M.E.; Brett, C.M. Poly(brilliant green) and poly(thionine) modified carbon nanotube coated carbon film electrodes for glucose and uric acid biosensors. Talanta 2014, 130, 198–206. [Google Scholar] [CrossRef]
- Li, S.; Zhu, X.; Zhang, W.; Xie, G.; Feng, W. Hydrogen peroxide biosensor based on gold nanoparticles/thionine/gold nanoparticles/multi-walled carbon nanotubes–chitosans composite film-modified electrode. Appl. Surf. Sci. 2012, 258, 2802–2807. [Google Scholar] [CrossRef]
- Deng, L.; Wang, Y.; Shang, L.; Wen, D.; Wang, F.; Dong, S. A sensitive NADH and glucose biosensor tuned by visible light based on thionine bridged carbon nanotubes and gold nanoparticles multilayer. Biosens. Bioelectron. 2008, 24, 957–963. [Google Scholar] [CrossRef]
- Hashemnia, S.; Eskanari, M. Preparation and Electrochemical Characterization of an Enzyme Electrode Based on Catalase Immobilized onto a Multiwall Carbon Nanotube-Thionine Film. J. Chin. Chem. Soc. 2014, 61, 903–909. [Google Scholar] [CrossRef]
- Panczyk, T.; Wolski, P.; Jagusiak, A.; Drach, M. Molecular dynamics study of Congo red interaction with carbon nanotubes. RSC Adv. 2014, 4, 47304–47312. [Google Scholar] [CrossRef]
- Zhang, Y.; Wen, Y.; Liu, Y.; Li, D.; Li, J. Functionalization of single-walled carbon nanotubes with Prussian blue. Electrochem. Commun. 2004, 6, 1180–1184. [Google Scholar] [CrossRef]
- Li, P.; Liu, H.; Ding, Y.; Wang, Y.; Chen, Y.; Zhou, Y.; Tang, Y.; Wei, H.; Cai, C.; Lu, T. Synthesis of water-soluble phosphonate functionalized single-walled carbon nanotubes and their applications in biosensing. J. Mater. Chem. 2012, 22, 15370–15378. [Google Scholar] [CrossRef]
- Mao, X.; Wu, Y.; Xu, L.; Cao, X.; Cui, X.; Zhu, L. Electrochemical biosensors based on redox carbon nanotubes prepared by noncovalent functionalization with 1,10-phenanthroline-5,6-dione. Analyst 2011, 136, 293–298. [Google Scholar] [CrossRef]
- Zhang, H.X.; Hu, J.S.; Yan, C.J.; Jiang, L.; Wan, L.J. Functionalized carbon nanotubes as sensitive materials for electrochemical detection of ultra-trace 2,4,6-trinitrotoluene. Phys. Chem. Chem. Phys. 2006, 8, 3567–3572. [Google Scholar] [CrossRef] [PubMed]
- Murakami, H.; Nomura, T.; Nakashima, N. Noncovalent porphyrin-functionalized single-walled carbon nanotubes in solution and the formation of porphyrin–nanotube nanocomposites. Chem. Phys. Lett. 2003, 378, 481–485. [Google Scholar] [CrossRef]
- Tu, W.; Lei, J.; Ju, H. Functionalization of carbon nanotubes with water-insoluble porphyrin in ionic liquid: Direct electrochemistry and highly sensitive amperometric biosensing for trichloroacetic acid. Chemistry 2009, 15, 779–784. [Google Scholar] [CrossRef]
- Wei, Y.; Kong, L.T.; Yang, R.; Wang, L.; Liu, J.H.; Huang, X.J. Electrochemical impedance determination of polychlorinated biphenyl using a pyrenecyclodextrin-decorated single-walled carbon nanotube hybrid. Chem. Commun. 2011, 47, 5340–5342. [Google Scholar] [CrossRef]
- Zheng, D.; Li, H.; Lu, B.; Xu, Z.; Chen, H. Electrochemical properties of ferrocene adsorbed on multi-walled carbon nanotubes electrode. Thin Solid Films 2008, 516, 2151–2157. [Google Scholar] [CrossRef]
- Wu, J.; Fu, Q.; Lu, B.; Li, H.; Xu, Z. Surfactant-associated electrochemical properties of ferrocene adsorbed on a glassy carbon electrode modified with multi-walled carbon nanotubes. Thin Solid Films 2010, 518, 3240–3245. [Google Scholar] [CrossRef]
- Fabre, B.; Samorì, C.; Bianco, A. Immobilization of double functionalized carbon nanotubes on glassy carbon electrodes for the electrochemical sensing of the biotin–avidin affinity. J. Electroanal. Chem. 2012, 665, 90–94. [Google Scholar] [CrossRef]
- Cluff, K.J.; Blümel, J. Adsorption of Ferrocene on Carbon Nanotubes, Graphene, and Activated Carbon. Organometallics 2016, 35, 3939–3948. [Google Scholar] [CrossRef]
- Meredith, M.T.; Minson, M.; Hickey, D.; Artyushkova, K.; Glatzhofer, D.T.; Minteer, S.D. Anthracene-modified multi-walled carbon nanotubes as direct electron transfer scaffolds for enzymatic oxygen reduction. ACS Catal. 2011, 1, 1683–1690. [Google Scholar] [CrossRef]
- Song, M.; Wang, X.; Liu, W.; Zuo, J. New anthracene-tetrathiafulvalene derivative-encapsulated SWNT nanocomposite and its application for biosensing. J. Colloid Interface Sci. 2010, 343, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhang, M.; Gong, K.; Su, L.; Guo, Z.; Mao, L. Adsorption of methylene blue dye onto carbon nanotubes a route to an electrochemically functional nanostructure and Its LbL assembled nanocomposite. Chem. Mater. 2005, 17, 3457–3463. [Google Scholar] [CrossRef]
- Hu, C.; Hu, S. Carbon nanotube-based electrochemical sensors: Principles and applications in biomedical systems. J. Sens. 2009, 2009, 1–40. [Google Scholar] [CrossRef]
- Hu, C.; Chen, Z.; Shen, A.; Shen, X.; Li, J.; Hu, S. Water-soluble single-walled carbon nanotubes via noncovalent functionalization by a rigid, planar and conjugated diazo dye. Carbon 2006, 44, 428–434. [Google Scholar] [CrossRef]
- Yang, C.; Xu, Y.; Hu, C.; Hu, S. Voltammetric Detection of Ofloxacin in Human Urine at a Congo Red Functionalized Water-Soluble Carbon Nanotube Film Electrode. Electroanalysis 2008, 20, 144–149. [Google Scholar] [CrossRef]
- Shahrokhian, S.; Zare-Mehrjardi, H.R.; Khajehsharifi, H. Modification of carbon paste with congo red supported on multi-walled carbon nanotube for voltammetric determination of uric acid in the presence of ascorbic acid. J. Solid State Electrochem. 2008, 13, 1567–1575. [Google Scholar] [CrossRef]
- Zheng, W.; Li, Q.; Su, L.; Yan, Y.; Zhang, J.; Mao, L. Direct Electrochemistry of Multi-Copper Oxidases at Carbon Nanotubes Noncovalently Functionalized with Cellulose Derivatives. Electroanalysis 2006, 18, 587–594. [Google Scholar] [CrossRef]
- Chen, J.; Liu, H.; Weimer, W.A.; Halls, M.D.; Waldeck, D.H.; Walker, G.C. Noncovalent Engineering of Carbon Nanotube Surfaces by Rigid, Functional Conjugated Polymers. J. Am. Chem. Soc. 2002, 124, 9034–9035. [Google Scholar] [CrossRef]
- Cesarino, I.; Moraes, F.C.; Lanza, M.R.; Machado, S.A. Electrochemical detection of carbamate pesticides in fruit and vegetables with a biosensor based on acetylcholinesterase immobilised on a composite of polyaniline-carbon nanotubes. Food Chem. 2012, 135, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.-M.; Chang, H.-L.; Lin, Y.-W. Synthesis and characterization of conductive polypyrrole/multi-walled carbon nanotubes composites with improved solubility and conductivity. Compos. Sci. Technol. 2009, 69, 639–644. [Google Scholar] [CrossRef]
- Silveira, C.M.; Baur, J.; Holzinger, M.; Moura, J.J.G.; Cosnier, S.; Almeida, M.G. Enhanced Direct Electron Transfer of a Multihemic Nitrite Reductase on Single-walled Carbon Nanotube Modified Electrodes. Electroanalysis 2010, 22, 2973–2978. [Google Scholar] [CrossRef]
- Zhu, L.; Yang, R.; Zhai, J.; Tian, C. Bienzymatic glucose biosensor based on co-immobilization of peroxidase and glucose oxidase on a carbon nanotubes electrode. Biosens. Bioelectron. 2007, 23, 528–535. [Google Scholar] [CrossRef]
- Tam, P.D.; Hieu, N.V. Conducting polymer film-based immunosensors using carbon nanotube/antibodies doped polypyrrole. Appl. Surf. Sci. 2011, 257, 9817–9824. [Google Scholar] [CrossRef]
- Holzinger, M.; Bouffier, L.; Villalonga, R.; Cosnier, S. Adamantane/beta-cyclodextrin affinity biosensors based on single-walled carbon nanotubes. Biosens. Bioelectron. 2009, 24, 1128–1134. [Google Scholar] [CrossRef]
- Pang, X.; Imin, P.; Zhitomirsky, I.; Adronov, A. Amperometric Detection of Glucose Using a Conjugated Polyelectrolyte Complex with Single-Walled Carbon Nanotubes. Macromolecules 2010, 43, 10376–10381. [Google Scholar] [CrossRef]
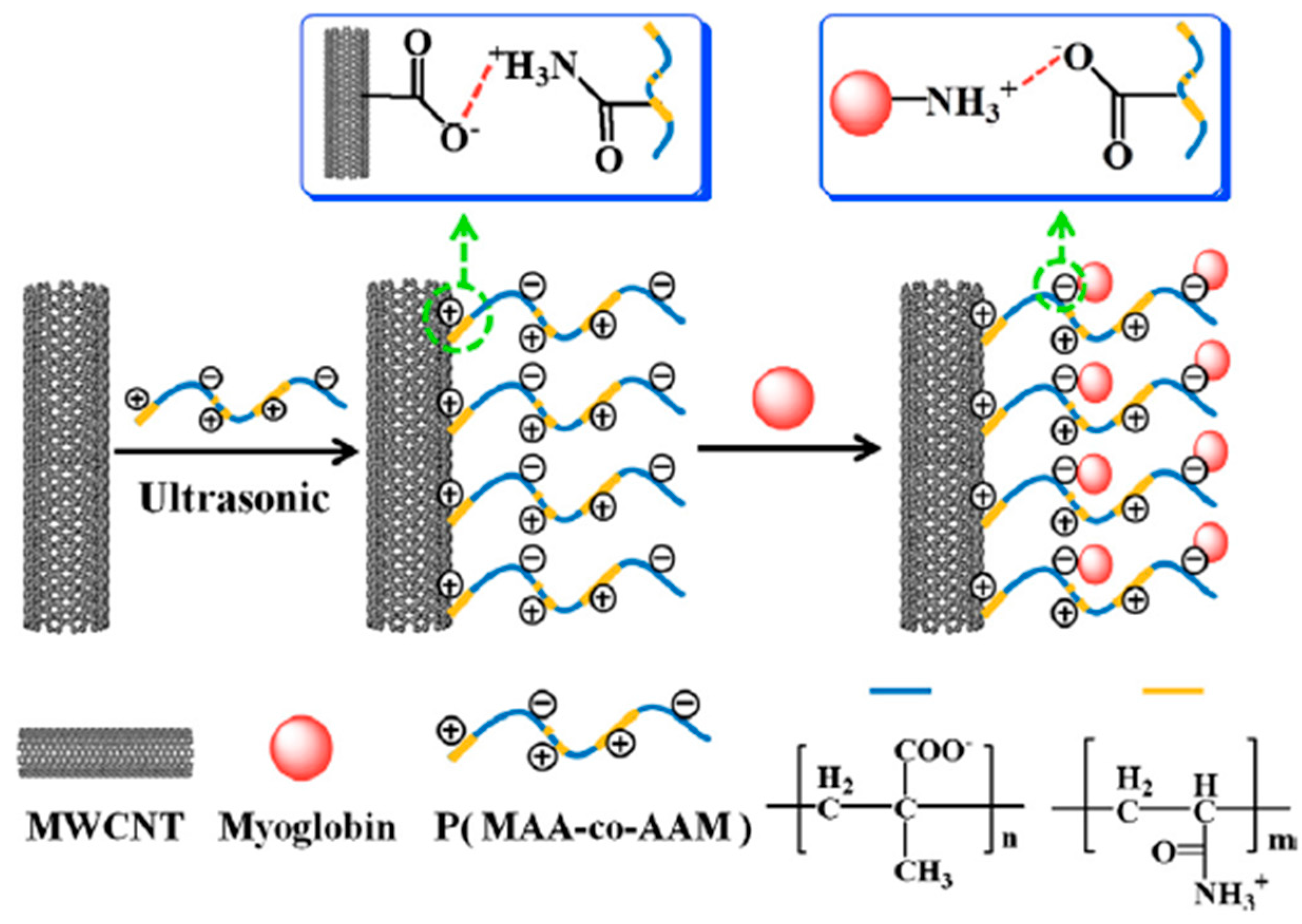
- Zhan, K.; Liu, H.; Zhang, H.; Chen, Y.; Ni, H.; Wu, M.; Sun, D.; Chen, Y. A facile method for the immobilization of myoglobin on multi-walled carbon nanotubes: Poly(methacrylic acid-co-acrylamide) nanocomposite and its application for direct bio-detection of H2O2. J. Electroanal. Chem. 2014, 724, 80–86. [Google Scholar] [CrossRef]
- Jia, F.; Shan, C.; Li, F.; Niu, L. Carbon nanotube/gold nanoparticles/polyethylenimine-functionalized ionic liquid thin film composites for glucose biosensing. Biosens. Bioelectron. 2008, 24, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Ivnitski, D.; Branch, B.; Atanassov, P.; Apblett, C. Glucose oxidase anode for biofuel cell based on direct electron transfer. Electrochem. Commun. 2006, 8, 1204–1210. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, D.C.; Zhang, W.D.; Jiang, X.; He, C.B.; Chung, T.S.; Goh, S.H.; Leong, K.W. Polyethylenimine-grafted multiwalled carbon nanotubes for secure noncovalent immobilization and efficient delivery of DNA. Angew. Chem. Int. Ed. Engl. 2005, 44, 4782–4785. [Google Scholar] [CrossRef] [PubMed]
- Sanz, V.; Borowiak, E.; Lukanov, P.; Galibert, A.M.; Flahaut, E.; Coley, H.M.; Silva, S.R.P.; McFadden, J. Optimising DNA binding to carbon nanotubes by non-covalent methods. Carbon 2011, 49, 1775–1781. [Google Scholar] [CrossRef]
- Liu, G.; Lin, Y. Amperometric glucose biosensor based on self-assembling glucose oxidase on carbon nanotubes. Electrochem. Commun. 2006, 8, 251–256. [Google Scholar] [CrossRef]
- Chen, Y.; Star, A.; Vidal, S. Sweet carbon nanostructures: Carbohydrate conjugates with carbon nanotubes and graphene and their applications. Chem. Soc. Rev. 2013, 42, 4532–4542. [Google Scholar] [CrossRef] [PubMed]
- Sartori, E.R.; Vicentini, F.C.; Fatibello-Filho, O. Indirect determination of sulfite using a polyphenol oxidase biosensor based on a glassy carbon electrode modified with multi-walled carbon nanotubes and gold nanoparticles within a poly(allylamine hydrochloride) film. Talanta 2011, 87, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Meng, L.; Lu, Q. Cell Behaviors on Polysaccharide Wrapped SWCNT A Quantitative Study of the Surface Properties of Biomimetic Nanofibrous Scaffolds. ACS Nano 2009, 3, 3200–3206. [Google Scholar] [CrossRef]
- Kumar, B.; Castro, M.; Feller, J.F. Controlled conductive junction gap for chitosan–carbon nanotube quantum resistive vapour sensors. J. Mater. Chem. 2012, 22, 10656–10664. [Google Scholar] [CrossRef]
- Wang, G.; Liu, X.; Yu, B.; Luo, G. Electrocatalytic response of norepinephrine at a β-cyclodextrin incorporated carbon nanotube modified electrode. J. Electroanal. Chem. 2004, 567, 227–231. [Google Scholar] [CrossRef]
- Yang, H.; Zhu, Y.; Chen, D.; Li, C.; Chen, S.; Ge, Z. Electrochemical biosensing platforms using poly-cyclodextrin and carbon nanotube composite. Biosens. Bioelectron. 2010, 26, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, D.; Mays, J.W.; Bratcher, M.S. Noncovalent and Nonspecific Molecular Interactions of Polymers with Multiwalled Carbon Nanotubes. Chem. Mater. 2005, 17, 3389–3397. [Google Scholar] [CrossRef]
- Su, L.; Gao, F.; Mao, L. Electrochemical Properties of Carbon Nanotube (CNT) Film Electrodes Prepared by Controllable Adsorption of CNTs onto an Alkanethiol Monolayer Self-Assembled on Gold Electrodes. Anal. Chem. 2006, 78, 2651–2657. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yan, J. A signal-on electrochemical biosensor for sensitive detection of silver ion based on alkanethiol–carbon nanotube-oligonucleotide modified electrodes. Sens. Actuators B Chem. 2014, 202, 1058–1064. [Google Scholar] [CrossRef]
- Mohamad, N.R.; Marzuki, N.H.; Buang, N.A.; Huyop, F.; Wahab, R.A. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Karajanagi, S.S.; Vertegel, A.A.; Kane, R.S.; Dordick, J.S. Structure and Function of Enzymes Adsorbed onto Single-Walled Carbon Nanotubes. Langmuir 2004, 20, 11594–11599. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.P.; Merchant, S.A.; Wang, Y.; Schmidtke, D.W. Amperometric Biosensors Based on Redox polymer-CNT-En composites. Anal. Chem. 2005, 77, 3183–3188. [Google Scholar] [CrossRef] [PubMed]
- Guiseppi-Elie, A.; Lei, C.; Baughman, R.H. Direct electron transfer of glucose oxidase on carbon nanotubes. Nanotechnology 2002, 13, 559–564. [Google Scholar] [CrossRef]
- Wang, J.; Li, M.; Shi, Z.; Li, N.; Gu, Z. Direct Electrochemistry of Cytochrome c at a Glassy Carbon Electrode Modified with Single-Wall Carbon Nanotubes. Anal. Chem. 2002, 74, 1993–1997. [Google Scholar] [CrossRef]
- Lin, Y.; Lu, F.; Tu, Y.; Ren, Z. Glucose Biosensors Based on Carbon Nanotube Nanoelectrode Ensembles. Nano Lett. 2004, 4, 191–195. [Google Scholar] [CrossRef]
- Gan, Z.-H.; Zhao, Q.; Gu, Z.-N.; Zhuang, Q.-K. Electrochemical studies of single-wall carbon nanotubes as nanometer-sized activators in enzyme-catalyzed reaction. Anal. Chim. Acta 2004, 511, 239–247. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Huang, Y.; Zhao, Z.; Qin, X.; Wang, Y.; Miao, Z.; Chen, Q.; Qiao, M. Noncovalently functionalized multi-wall carbon nanotubes in aqueous solution using the hydrophobin HFBI and their electroanalytical application. Biosens. Bioelectron. 2010, 26, 1104–1108. [Google Scholar] [CrossRef] [PubMed]
- Le Goff, A.; Gorgy, K.; Holzinger, M.; Haddad, R.; Zimmerman, M.; Cosnier, S. Tris(bispyrene-bipyridine)iron(II): A supramolecular bridge for the biofunctionalization of carbon nanotubes via pi-stacking and pyrene/beta-cyclodextrin host-guest interactions. Chemistry 2011, 17, 10216–10221. [Google Scholar] [CrossRef] [PubMed]
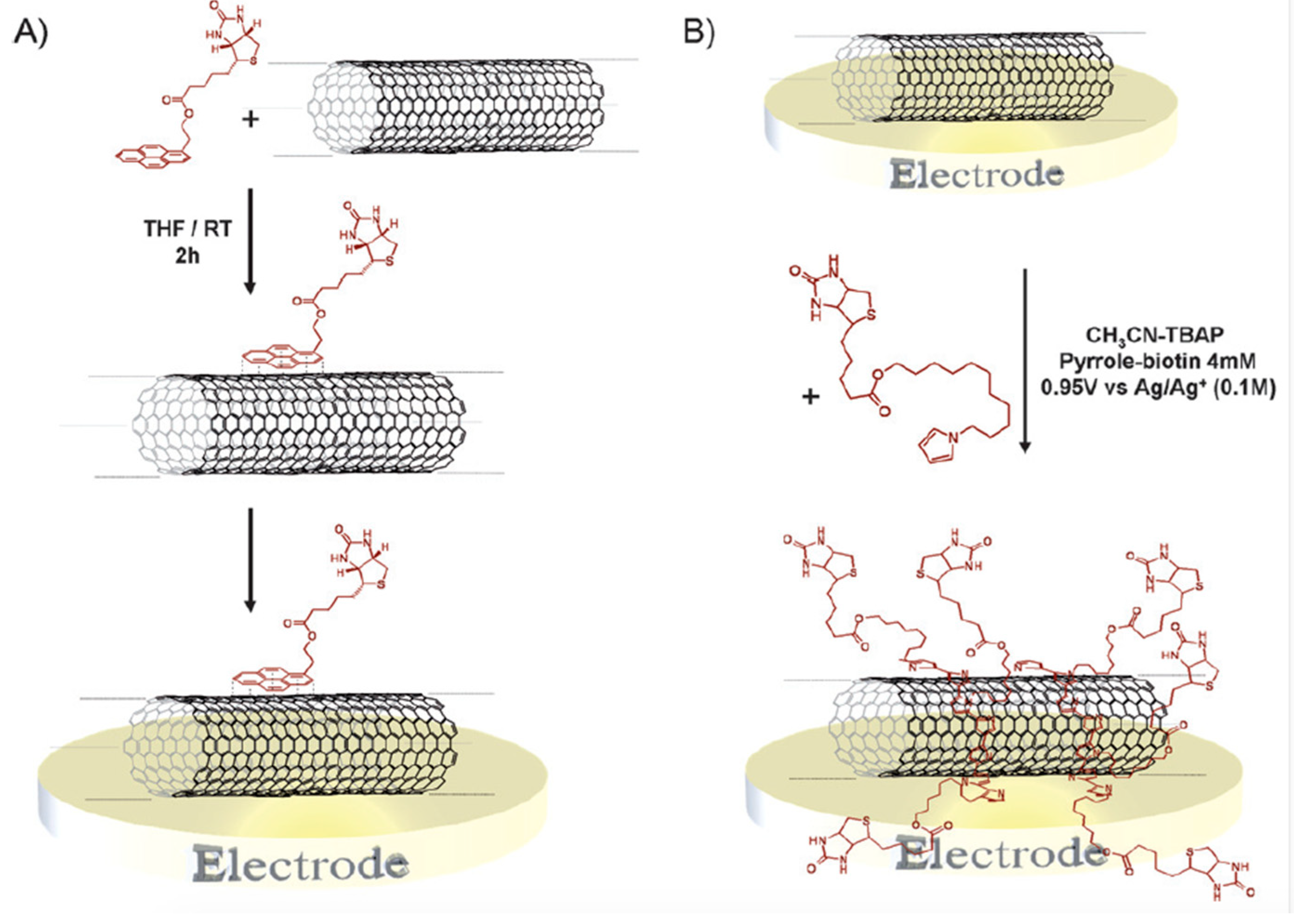
- Haddad, R.; Cosnier, S.; Maaref, A.; Holzinger, M. Non-covalent biofunctionalization of single-walled carbon nanotubes via biotin attachment by pi-stacking interactions and pyrrole polymerization. Analyst 2009, 134, 2412–2418. [Google Scholar] [CrossRef] [PubMed]
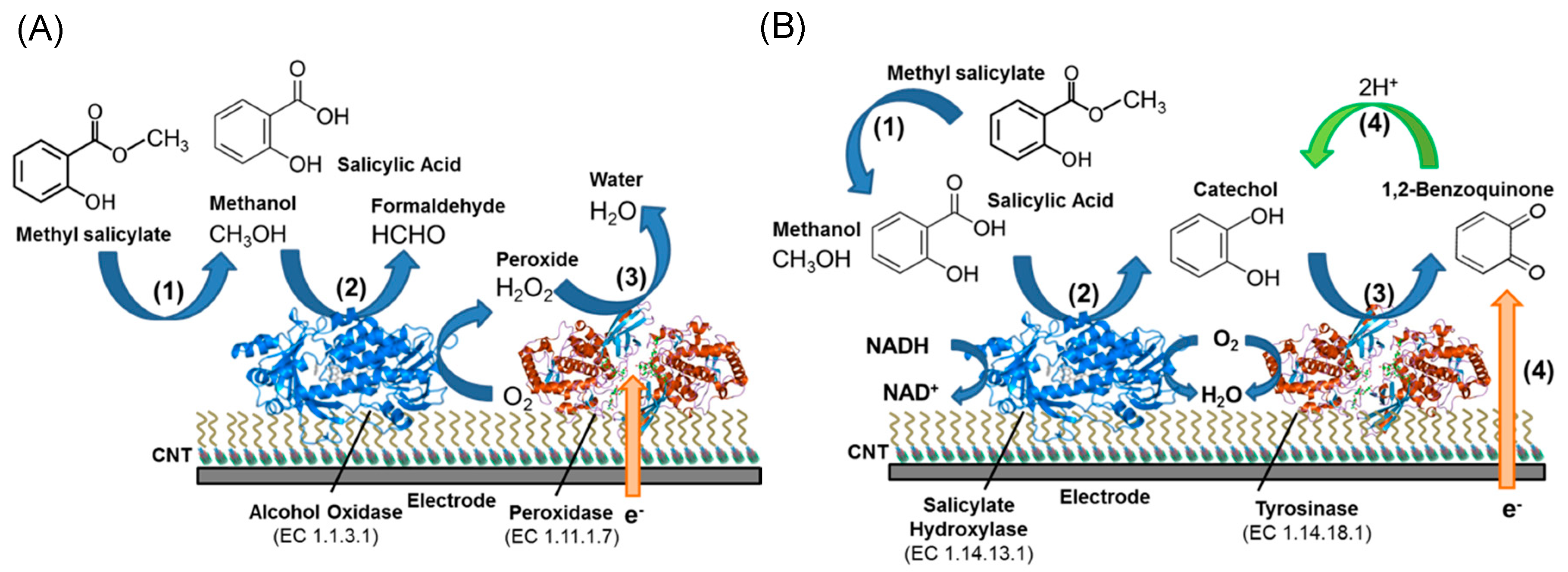
- Fang, Y.; Umasankar, Y.; Ramasamy, R.P. A novel bi-enzyme electrochemical biosensor for selective and sensitive determination of methyl salicylate. Biosens. Bioelectron. 2016, 81, 39–45. [Google Scholar] [CrossRef]
- Fang, Y.; Bullock, H.; Lee, S.A.; Sekar, N.; Eiteman, M.A.; Whitman, W.B.; Ramasamy, R.P. Detection of methyl salicylate using bi-enzyme electrochemical sensor consisting salicylate hydroxylase and tyrosinase. Biosens. Bioelectron. 2016, 85, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, B.A.G.; Trinidade, E.K.G.; Cabral, D.G.A.; Soares, E.C.L.; Menezes, C.E.L.; Ferreira, D.C.M.; Mendes, R.K.; Dutra, R.F. Nanomaterials for Advancing the Health Immunosensor. Biosens.-Micro Nanoscale Appl. 2015. [Google Scholar] [CrossRef]
- Sanchez, S.; Pumera, M.; Fabregas, E. Carbon nanotube/polysulfone screen-printed electrochemical immunosensor. Biosens. Bioelectron. 2007, 23, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.; Huang, H.; Yin, Z.; Gao, D.; Zhu, J.J. Horseradish peroxidase-functionalized gold nanoparticle label for amplified immunoanalysis based on gold nanoparticles/carbon nanotubes hybrids modified biosensor. Biosens. Bioelectron. 2008, 23, 1666–1673. [Google Scholar] [CrossRef]
- Zhou, Y.; Marar, A.; Kner, P.; Ramasamy, R.P. Charge-Directed Immobilization of Bacteriophage on Nanostructured Electrode for Whole-Cell Electrochemical Biosensors. Anal. Chem. 2017, 89, 5734–5741. [Google Scholar] [CrossRef]
- Zhou, Y.; Ramasamy, R.P. Phage-based Electrochemical Biosensors for Detection of Pathogenic Bacteria. ECS Trans. 2015, 69, 1–8. [Google Scholar] [CrossRef]
- Yu, T.; Gong, Y.; Lu, T.; Wei, L.; Li, Y.; Mu, Y.; Chen, Y.; Liao, K. Recognition of carbon nanotube chirality by phage display. RSC Adv. 2012, 2, 1466–1476. [Google Scholar] [CrossRef]
- Zhao, X.; Johnson, J.K. Simulation of Adsorption of DNA on Carbon Nanotubes. J. Am. Chem. Soc. 2007, 129, 10438–10445. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Jagota, A.; Strano, M.S.; Santos, A.P.; Barone, P.; Chou, S.G.; Diner, B.A.; Dresselhaus, M.S.; Mclean, R.S.; Onoa, G.B.; et al. Structure based cntsorting by sequence dependent DNA assembly. Science 2003, 302, 1545–1548. [Google Scholar] [CrossRef] [PubMed]
- Gigliotti, B.; Sakizzie, B.; Bethune, D.S.; Shelby, R.M.; Cha, J.N. Sequence-Independent Helical Wrapping of Single-Walled Carbon Nanotubes by Long Genomic DNA. Nano Lett. 2006, 6, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jiao, K.; Liu, S.; Hu, Y. Readily Reusable Electrochemical DNA Hybridization Biosensor Based on the Interaction of DNA with Single-Walled Carbon Nanotubes. Anal. Chem. 2009, 81, 6006–6012. [Google Scholar] [CrossRef] [PubMed]
- Kalogianni, D.P.; Koraki, T.; Christopoulos, T.K.; Ioannou, P.C. Nanoparticle-based DNA biosensor for visual detection of genetically modified organisms. Biosens. Bioelectron. 2006, 21, 1069–1076. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Amini, M.; Rezaei, B. Biosensor based on ds-DNA decorated chitosan modified multiwall carbon nanotubes for voltammetric biodetection of herbicide amitrole. Colloids Surf. B Biointerfaces 2013, 109, 45–51. [Google Scholar] [CrossRef]






| Crosslinker | Biomolecule | Type of CNT | Application | Reference |
|---|---|---|---|---|
| EDC/PLL * | HRP | COOH-SWCNT | H2O2 sensing | [35] |
| EDC-NHS | GOx and HRP | COOH-CNT | Glucose biosensor | [36] |
| EDC-NHS | Antibody | COOH-MWCNT | Aflatoxin detection | [37] |
| GA | Antibody | PEI-MWCNT | Carcinoembryonic antigen detection | [38] |
| GA | GOx | Gelatin-MWCNT | Glucose biosensor | [39] |
| Compound Name | Structure | CNT Structure | Application | References |
|---|---|---|---|---|
| 1-Pyrenebutanoic acid, succinimidyl ester (PBSE) |  | SWCNT MWCNT | Bioelectrocatalysis of oxygen | [27,45,50,51,52,53,54] |
| 1-(2-Anthraquinonylamino-methyl)pyrene | 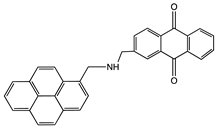 | MWCNT | Bioelectrocatalysis of oxygen | [54] |
| [bis(2-anthraquinonyl)-aminomethyl]pyrene |  | MWCNT | Bioelectrocatalysis of oxygen | [54] |
| 5,7,12,14-Tetramethyl-dibenzo-1,4,8,11-tetraazacyclotetradeca-3,5,7,10,-12,14-hexaene (TMTAA) | 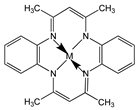 | SWCNT | Not available | [60] |
| Adamantane-pyrene | 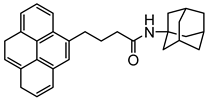 | SWCNT | Glucose biosensor | [61] |
| Biotin–pyrene | 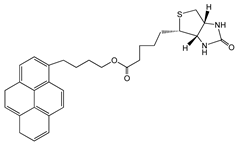 | SWCNT | Glucose biosensor | [61] |
| Nitrilotriacetic acid (NTA)–pyrene | 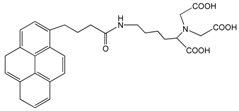 | SWCNT | Glucose biosensor | [61] |
| Ferrocene | 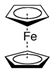 | SWCNT | Glucose biosensor/ L -glutamate detection | [59,62,63] |
| Anthracene |  | SWCNT | Bioelectrocatalysis of oxygen | [64,65] |
| Anthrarobin | 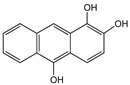 | SWCNT | Not available | [65] |
| 9,10-Dibromoanthracene | 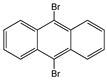 | SWCNT | Not available | [65] |
| 9,10-Anthracene-dicarbonitrile | 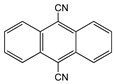 | SWCNT | Not available | [65] |
| 9-Anthracenemethanol |  | SWCNT | Not available | [65] |
| Thionine | 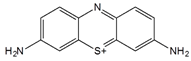 | MWCNT | Glucose and uric acid biosensors/ Hydrogen peroxide detection | [66,67,68,69,70,71] |
| Methylene blue (MB) |  | SWCNT | Not available | [72,73] |
| Naphthalen-1-methyl- phosphonic acid (NYPA) | 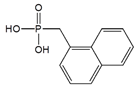 | SWCNT | Hydrogen peroxide detection | [74] |
| 1,10-Phenanthroline-5,6-dione (PD) | 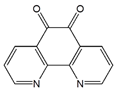 | MWCNT | Ethanol biosensor | [75] |
| Triphenylene (TP) |  | MWCNT | TNT detection | [76] |
| Porphyrin | 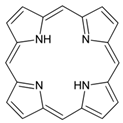 | SWCNT | Not available | [77] |
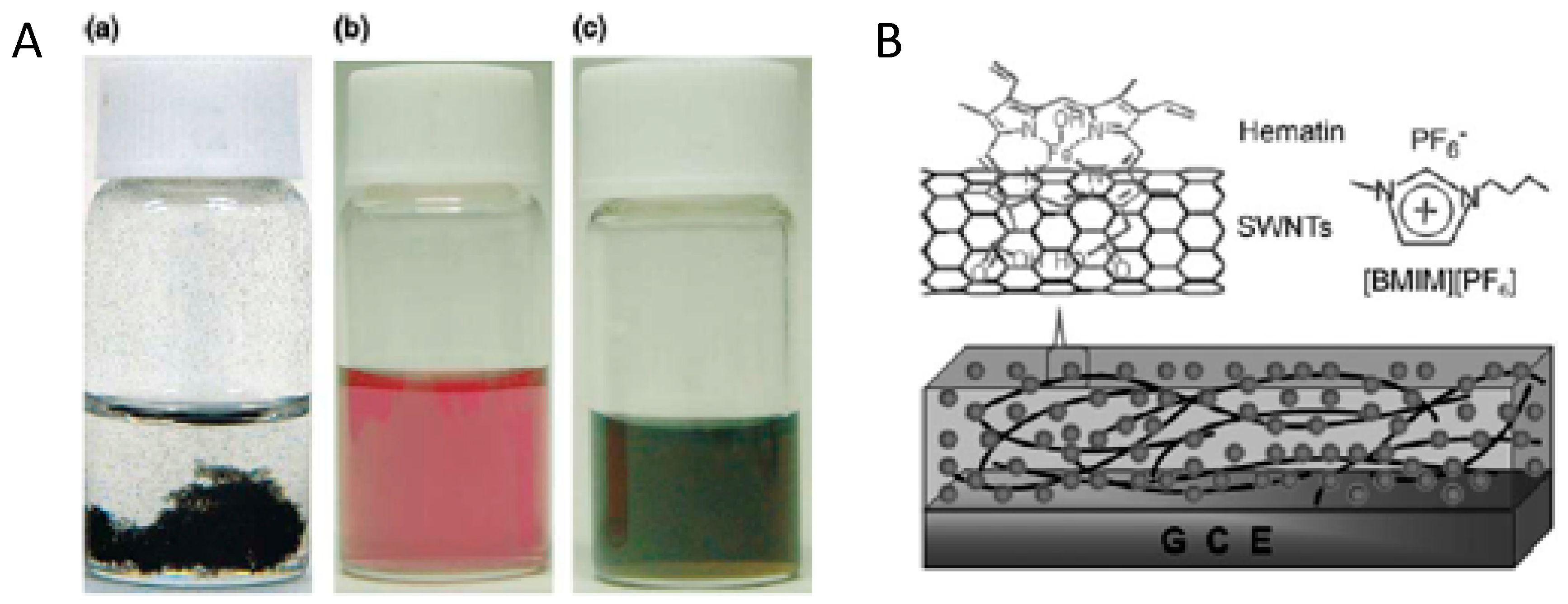
| Hydroxyferriproto- porphyrin (hematin) |  | SWCNT | Trichloroacetic acid biosensor | [78] |
| Compound Name | Structure | CNT Structure | Application | References |
|---|---|---|---|---|
| Polypyrrole (PPy) |  | SWCNT | Nitrite detection/ anti-goat IgGs detection | [94,95,97] |
| Adamantane-pyrrole | 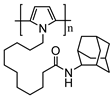 | SWCNT | Glucose biosensor | [98] |
| Poly[3-(3-N,N-diethyl-aminopropoxy)thio-phene] (PDAOT) |  | SWCNT | Glucose biosensor | [99] |
| Poly(methacrylic acid-co-acrylamide) (P(MAA-co-AAM)) | 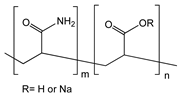 | MWCNT | Hydrogen peroxide detection | [100] |
| Polyaniline (PANI) |  | MWCNT | Carbamate pesticides detection | [93] |
| Glycodendrimer |  | SWCNT | Metal detection | [27,52] |
| Polyethyleneimine | 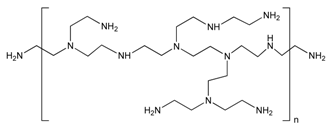 | MWCNT | Glucose biosensor/DNA delivery/ Carcinoembryonic antigen detection | [38,101,102,103,104] |
| Poly diallyl dimethyl ammonium chloride (PDDA) | 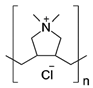 | SWCNT | Glucose biosensor | [105] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Fang, Y.; Ramasamy, R.P. Non-Covalent Functionalization of Carbon Nanotubes for Electrochemical Biosensor Development. Sensors 2019, 19, 392. https://doi.org/10.3390/s19020392
Zhou Y, Fang Y, Ramasamy RP. Non-Covalent Functionalization of Carbon Nanotubes for Electrochemical Biosensor Development. Sensors. 2019; 19(2):392. https://doi.org/10.3390/s19020392
Chicago/Turabian StyleZhou, Yan, Yi Fang, and Ramaraja P. Ramasamy. 2019. "Non-Covalent Functionalization of Carbon Nanotubes for Electrochemical Biosensor Development" Sensors 19, no. 2: 392. https://doi.org/10.3390/s19020392
APA StyleZhou, Y., Fang, Y., & Ramasamy, R. P. (2019). Non-Covalent Functionalization of Carbon Nanotubes for Electrochemical Biosensor Development. Sensors, 19(2), 392. https://doi.org/10.3390/s19020392






