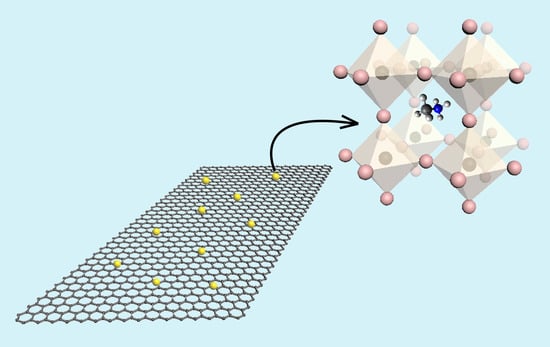
Gas Sensing Properties of Perovskite Decorated Graphene at Room Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Perovskite Synthesis and Graphene Preparation
2.2. Material Characterization
2.3. Sensor Fabrication
2.4. Gas sensing Measurements
3. Results
3.1. Material Characterization
3.2. Gas Sensing Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. 7 Million Premature Deaths Annually Linked to Air Pollution. Available online: https://www.who.int/mediacentre/news/releases/2014/air-pollution/en/ (accessed on 12 March 2019).
- Hansen, J.; Sato, M.; Ruedy, R.; Lacis, A.; Oinas, V. Global warming in the Twenty-First century: An alternative scenario. Proc. Natl. Acad. Sci. USA 2000, 97, 9875–9880. [Google Scholar] [CrossRef] [PubMed]
- Ibañez, F.J.; Zamborini, F.P. Chemiresistive Sensing with Chemically Modified Metal and Alloy Nanoparticles. Small 2012, 8, 174–202. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, A.; Leonardi, S.G.; Neri, G. Detection of hazardous volatile organic compounds (VOCs) by metal oxide nanostructures-based gas sensors: A review. Ceram. Int. 2016, 42, 15119–15141. [Google Scholar] [CrossRef]
- Zaidi, N.A.; Tahir, M.W.; Vellekoop, M.J.; Lang, W.; Zaidi, N.A.; Tahir, M.W.; Vellekoop, M.J.; Lang, W. A Gas Chromatographic System for the Detection of Ethylene Gas Using Ambient Air as a Carrier Gas. Sensors 2017, 17, 2283. [Google Scholar] [CrossRef]
- Meng, F.-L.; Guo, Z.; Huang, X.-J. Graphene-Based hybrids for chemiresistive gas sensors. TrAC Trends Anal. Chem. 2015, 68, 37–47. [Google Scholar] [CrossRef]
- Miller, D.R.; Akbar, S.A.; Morris, P.A. Nanoscale metal Oxide-Based heterojunctions for gas sensing: A review. Sens. Actuators B Chem. 2014, 204, 250–272. [Google Scholar] [CrossRef]
- Scott, S.M.; James, D.; Ali, Z. Data analysis for electronic nose systems. Microchim. Acta 2006, 156, 183–207. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, L.; Herranz, M.Á.; Martín, N. The chemistry of pristine graphene. Chem. Commun. 2013, 49, 3721–3735. [Google Scholar] [CrossRef]
- Prezioso, S.; Perrozzi, F.; Giancaterini, L.; Cantalini, C.; Treossi, E.; Palermo, V.; Nardone, M.; Santucci, S.; Ottaviano, L. Graphene Oxide as a Practical Solution to High Sensitivity Gas Sensing. J. Phys. Chem. C 2013, 117, 10683–10690. [Google Scholar] [CrossRef]
- Lipatov, A.; Varezhnikov, A.; Wilson, P.; Sysoev, V.; Kolmakov, A.; Sinitskii, A. Highly selective gas sensor arrays based on thermally reduced graphene oxide. Nanoscale 2013, 5, 5426–5434. [Google Scholar] [CrossRef] [Green Version]
- Varghese, S.S.; Lonkar, S.; Singh, K.K.; Swaminathan, S.; Abdala, A. Recent advances in graphene based gas sensors. Sens. Actuators B Chem. 2015, 218, 160–183. [Google Scholar] [CrossRef]
- Rodner, M.; Puglisi, D.; Ekeroth, S.; Helmersson, U.; Shtepliuk, I.; Yakimova, R.; Skallberg, A.; Uvdal, K.; Schütze, A.; Eriksson, J.; et al. Graphene Decorated with Iron Oxide Nanoparticles for Highly Sensitive Interaction with Volatile Organic Compounds. Sensors 2019, 19, 918. [Google Scholar] [CrossRef] [PubMed]
- Kaniyoor, A.; Jafri, R.I.; Arockiadoss, T.; Ramaprabhu, S. Nanostructured Pt decorated graphene and multi walled carbon nanotube based room temperature hydrogen gas sensor. Nanoscale 2009, 1, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Seekaew, Y.; Lokavee, S.; Phokharatkul, D.; Wisitsoraat, A.; Kerdcharoen, T.; Wongchoosuk, C. Low-Cost and flexible printed graphene–PEDOT: PSS gas sensor for ammonia detection. Org. Electron. 2014, 15, 2971–2981. [Google Scholar] [CrossRef]
- Kang, M.-A.; Ji, S.; Kim, S.; Park, C.-Y.; Myung, S.; Song, W.; Lee, S.S.; Lim, J.; An, K.-S. Highly sensitive and wearable gas sensors consisting of chemically functionalized graphene oxide assembled on cotton yarn. RSC Adv. 2018, 8, 11991–11996. [Google Scholar] [CrossRef] [Green Version]
- Fine, G.F.; Cavanagh, L.M.; Afonja, A.; Binions, R. Metal oxide Semi-Conductor gas sensors in environmental monitoring. Sensors 2010, 10, 5469–5502. [Google Scholar] [CrossRef]
- Sun, D.; Luo, Y.; Debliquy, M.; Zhang, C. Graphene-Enhanced metal oxide gas sensors at room temperature: A review. Beilstein J. Nanotechnol. 2018, 9, 2832–2844. [Google Scholar] [CrossRef]
- Llobet, E. Gas sensors using carbon nanomaterials: A review. Sens. Actuators B 2013, 179, 32–45. [Google Scholar] [CrossRef]
- Correa-Baena, J.P.; Abate, A.; Saliba, M.; Tress, W.; Jesper Jacobsson, T.; Grätzel, M.; Hagfeldt, A. The rapid evolution of highly efficient perovskite solar cells. Energy Environ. Sci. 2017, 10, 710–727. [Google Scholar] [CrossRef]
- Sun, S.; Salim, T.; Mathews, N.; Duchamp, M.; Boothroyd, C.; Xing, G.; Sum, T.C.; Lam, Y.M. The origin of high efficiency in Low-Temperature Solution-Processable bilayer organometal halide hybrid solar cells. Energy Environ. Sci. 2014, 7, 399–407. [Google Scholar] [CrossRef]
- Juarez-Perez, E.J.; Ono, L.K.; Maeda, M.; Jiang, Y.; Hawash, Z.; Qi, Y. Photodecomposition and thermal decomposition in methylammonium halide lead perovskites and inferred design principles to increase photovoltaic device stability. J. Mater. Chem. A 2018, 6, 9604–9612. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Zhang, M.; Bo, R.; Barugkin, C.; Zheng, J.; Ma, Q.; Huang, S.; Ho-Baillie, A.W.Y.; Catchpole, K.R.; Tricoli, A. Superior Self-Powered Room-Temperature Chemical Sensing with Light-Activated Inorganic Halides Perovskites. Small 2018, 14, 1702571. [Google Scholar] [CrossRef] [PubMed]
- Kakavelakis, G.; Gagaoudakis, E.; Petridis, K.; Petromichelaki, V.; Binas, V.; Kiriakidis, G.; Kymakis, E. Solution Processed CH3NH3PbI3-xClx Perovskite Based Self-Powered Ozone Sensing Element Operated at Room Temperature. ACS Sens. 2018, 3, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Bao, C.; Yang, J.; Zhu, W.; Zhou, X.; Gao, H.; Li, F.; Fu, G.; Yu, T.; Zou, Z. A resistance change effect in perovskite CH3NH3PbI3 films induced by ammonia. Chem. Commun. 2015, 51, 15426–15429. [Google Scholar] [CrossRef]
- Zhuang, Y.; Yuan, W.; Qian, L.; Chen, S.; Shi, G. High-Performance gas sensors based on a thiocyanate ion-doped organometal halide perovskite. Phys. Chem. Chem. Phys. 2017, 19, 12876–12881. [Google Scholar] [CrossRef]
- Samorì, P.; Orgiu, E.; Liscio, F.; Gobbi, M.; Stoeckel, M.-A.; Ferlauto, L.; Bonacchi, S. Reversible, Fast, and Wide-Range Oxygen Sensor Based on Nanostructured Organometal Halide Perovskite. Adv. Mater. 2017, 29, 1702469. [Google Scholar]
- Zhu, R.; Zhang, Y.; Zhong, H.; Wang, X.; Xiao, H.; Chen, Y.; Li, X. High-Performance Room-Temperature NO2 sensors based on CH3NH3PbBr3 semiconducting films: Effect of surface capping by alkyl chain on sensor performance. J. Phys. Chem. Solids 2019, 129, 270–276. [Google Scholar] [CrossRef]
- Acik, M.; Darling, S.B. Graphene in perovskite solar cells: Device design, characterization and implementation. J. Mater. Chem. A 2016, 4, 6185–6235. [Google Scholar] [CrossRef]
- Christians, J.A.; Miranda Herrera, P.A.; Kamat, P.V. Transformation of the excited state and photovoltaic efficiency of CH3NH3PbI3 perovskite upon controlled exposure to humidified air. J. Am. Chem. Soc. 2015, 137, 1530–1538. [Google Scholar] [CrossRef]
- Leguy, A.M.A.; Hu, Y.; Campoy-Quiles, M.; Alonso, M.I.; Weber, O.J.; Azarhoosh, P.; van Schilfgaarde, M.; Weller, M.T.; Bein, T.; Nelson, J.; et al. Reversible Hydration of CH3NH3PbI3 in Films, Single Crystals, and Solar Cells. Chem. Mater. 2015, 27, 3397–3407. [Google Scholar] [CrossRef]
- O’keeffe, P.; Catone, D.; Paladini, A.; Toschi, F.; Turchini, S.; Avaldi, L.; Martelli, F.; Agresti, A.; Pescetelli, S.; Del, A.E.; et al. Graphene-Induced Improvements of Perovskite Solar Cell Stability: Effects on Hot-Carriers. Nano Lett. 2019, 19, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Berhe, T.A.; Su, W.N.; Chen, C.H.; Pan, C.J.; Cheng, J.H.; Chen, H.M.; Tsai, M.C.; Chen, L.Y.; Dubale, A.A.; Hwang, B.J. Organometal halide perovskite solar cells: Degradation and stability. Energy Environ. Sci. 2016, 9, 323–356. [Google Scholar] [CrossRef]
- Lee, G.Y.; Yang, M.Y.; Kim, D.; Lim, J.; Byun, J.; Choi, D.S.; Lee, H.J.; Nam, Y.S.; Kim, I.; Kim, S.O. Nitrogen-Dopant-Induced Organic–Inorganic Hybrid Perovskite Crystal Growth on Carbon Nanotubes. Adv. Funct. Mater. 2019, 29, 1902489. [Google Scholar] [CrossRef]
- Fu, X.; Jiao, S.; Dong, N.; Lian, G.; Zhao, T.; Lv, S.; Wang, Q.; Cui, D. A CH3NH3PbI3 film for a Room-Temperature NO2 gas sensor with quick response and high selectivity. RSC Adv. 2018, 8, 390–395. [Google Scholar] [CrossRef]
- Gupta, N.; Nanda, O.; Grover, R.; Saxena, K. A new inorganic-organic hybrid halide perovskite thin film based ammonia sensor. Org. Electron. 2018, 58, 202–206. [Google Scholar] [CrossRef]
- European Union. Air Quality Standards. Available online: https://ec.europa.eu/environment/air/quality/standards.htm (accessed on 12 October 2019).
- Environmental Protection Agency. NAAQS Table. Available online: https://www.epa.gov/criteria-air-pollutants/naaqs-table (accessed on 12 October 2019).
- European Union. Commission Directive 2000/39/EC. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:02000L0039-20100108&from=EN (accessed on 12 October 2019).
- Centers for Disease Control and Prevention. Ammonia. Available online: https://www.cdc.gov/niosh/npg/npgd0028.html (accessed on 12 October 2019).
- Schmidt, L.C.; Pertegás, A.; González-Carrero, S.; Malinkiewicz, O.; Agouram, S.; Mínguez Espallargas, G.; Bolink, H.J.; Galian, R.E.; Pérez-Prieto, J. Nontemplate Synthesis of CH3NH3PbBr3 Perovskite Nanoparticles. J. Am. Chem. Soc. 2014, 136, 850–853. [Google Scholar] [CrossRef]
- Yang, G.; Kim, B.-J.; Kim, K.; Woo Han, J.; Kim, J. Energy and dose dependence of Proton-Irradiation damage in graphene. RSC Adv. 2015, 5, 31861–31865. [Google Scholar] [CrossRef]
- D’Acunto, G.; Ripanti, F.; Postorino, P.; Betti, M.G.; Scardamaglia, M.; Bittencourt, C.; Mariani, C. Channelling and induced defects at ion-bombarded aligned multiwall carbon nanotubes. Carbon 2018, 139, 768–775. [Google Scholar] [CrossRef]
- Johra, F.T.; Lee, J.-W.; Jung, W.-G. Facile and safe graphene preparation on solution based platform. J. Ind. Eng. Chem. 2014, 20, 2883–2887. [Google Scholar] [CrossRef]
- Ganesan, K.; Ghosh, S.; Krishna, N.G.; Ilango, S.; Kamruddin, M.; Tyagi, A.K. A comparative study on defect estimation using XPS and Raman spectroscopy in few layer nanographitic structures. Phys. Chem. Chem. Phys. 2016, 18, 22160. [Google Scholar] [CrossRef]
- Roy, S.; Das, T.; Ming, Y.; Chen, X.; Yue, C.Y.; Hu, X. Specific functionalization and polymer grafting on multiwalled carbon nanotubes to fabricate advanced nylon 12 composites. J. Mater. Chem. A 2014, 2, 3961–3970. [Google Scholar] [CrossRef]
- Datsyuk, V.; Kalyva, M.; Papagelis, K.; Parthenios, J.; Tasis, D.; Siokou, A.; Kallitsis, I.; Galiotis, C. Chemical oxidation of multiwalled carbon nanotubes. Carbon 2008, 46, 833–840. [Google Scholar] [CrossRef]
- Kumar, P.V.; Bernardi, M.; Grossman, J.C. The Impact of Functionalization on the Stability, Work Function, and Photoluminescence of Reduced Graphene Oxide. ACS Nano 2013, 7, 1638–1645. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Durstock, M.; Dai, L. Graphene oxide derivatives as Hole- and Electron-Extraction layers for High-Performance polymer solar cells. Energy Environ. Sci. 2014, 7, 1297–1306. [Google Scholar] [CrossRef]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of Graphene: Covalent and Non-Covalent Approaches, Derivatives and Applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Lu, Y.; Xu, W.; Mu, H.; Chen, C.; Qiao, H.; Song, J.; Li, S.; Sun, B.; et al. Hybrid Graphene-Perovskite Phototransistors with Ultrahigh Responsivity and Gain. Adv. Opt. Mater. 2015, 3, 1389–1396. [Google Scholar] [CrossRef]
- Lv, C.; Hu, C.; Luo, J.; Liu, S.; Qiao, Y.; Zhang, Z.; Song, J.; Shi, Y.; Cai, J.; Watanabe, A. Recent Advances in Graphene-Based Humidity Sensors. Nanomater 2019, 9, 422. [Google Scholar] [CrossRef]
- Casanova-Cháfer, J.; Navarrete, E.; Noirfalise, X.; Umek, P.; Bittencourt, C.; Llobet, E. Gas Sensing with Iridium Oxide Nanoparticle Decorated Carbon Nanotubes. Sensors 2019, 19, 113. [Google Scholar] [CrossRef]
- Fang, H.H.; Adjokatse, S.; Wei, H.; Yang, J.; Blake, G.R.; Huang, J.; Even, J.; Loi, M.A. Ultrahigh sensitivity of methylammonium lead tribromide perovskite single crystals to environmental gases. Sci. Adv. 2016, 2, e1600534. [Google Scholar] [CrossRef]








| NO2 | NH3 | R.H. Effect | Degradation | Ref | |
|---|---|---|---|---|---|
| Graphene- MAPbBr3 NCs | 14.5 | 3.8 | ↓ 5% | No | This work |
| MAPbI3 film- SCN− ions | 0.53 | 3.9 | ↓ 11% | No | [26] |
| MAPbBr3 film | 0.13 | NT | NT | Yes | [28] |
| NCNT- MAPbBr3 | 0.88 | NT | NT | NT | [34] |
| MAPbI3 film | 0.62 | 11 | ↓ 16% | NT | [35] |
| EDPIC * | NT | 4.3 | NT | NT | [36] |
| Averaging Time | European Union | United States | |
|---|---|---|---|
| NO2 | 1 h | 200 ppb | 100 ppb |
| 1 year | 40 ppb | 53 ppb | |
| NH3 | ST | 50 ppm | 35 ppm |
| TWA | 20 ppm | 25 ppm |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casanova-Cháfer, J.; García-Aboal, R.; Atienzar, P.; Llobet, E. Gas Sensing Properties of Perovskite Decorated Graphene at Room Temperature. Sensors 2019, 19, 4563. https://doi.org/10.3390/s19204563
Casanova-Cháfer J, García-Aboal R, Atienzar P, Llobet E. Gas Sensing Properties of Perovskite Decorated Graphene at Room Temperature. Sensors. 2019; 19(20):4563. https://doi.org/10.3390/s19204563
Chicago/Turabian StyleCasanova-Cháfer, Juan, Rocío García-Aboal, Pedro Atienzar, and Eduard Llobet. 2019. "Gas Sensing Properties of Perovskite Decorated Graphene at Room Temperature" Sensors 19, no. 20: 4563. https://doi.org/10.3390/s19204563
APA StyleCasanova-Cháfer, J., García-Aboal, R., Atienzar, P., & Llobet, E. (2019). Gas Sensing Properties of Perovskite Decorated Graphene at Room Temperature. Sensors, 19(20), 4563. https://doi.org/10.3390/s19204563