Hg2+ Optical Fiber Sensor Based on LSPR Generated by Gold Nanoparticles Embedded in LBL Nano-Assembled Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis Method of the PAA-Capped AuNPs
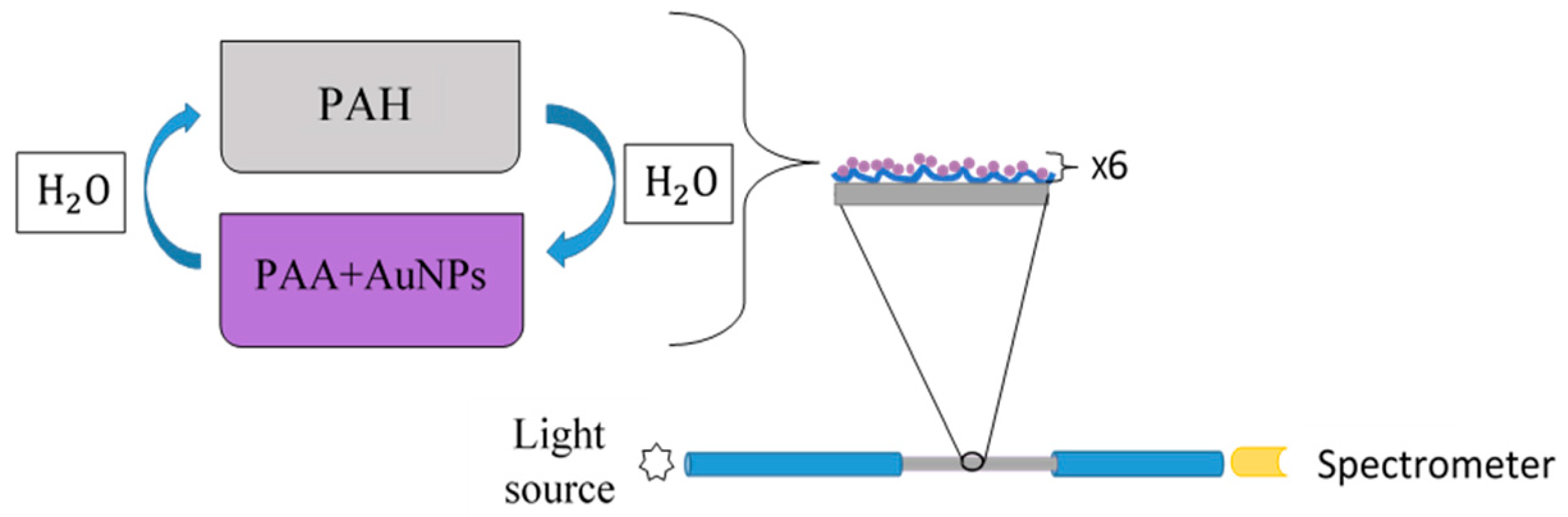
2.3. Optical Detection Setup
2.4. Layer-By-Layer Nano-Assembly
2.5. Mercury Samples
2.6. Sensors Regeneration
2.7. Data Processing
2.8. Cross-Sensitivity to Other Metals
3. Results and Discussion
3.1. Effects of Hg0 on AuNPs in Dispersion
3.2. Obtaining the AuNPs LSPR onto the Fiber Optics
3.3. Detection of Mercury Ions with Fiber Optic Sensor
3.4. Sensor Regeneration
3.5. Cross Sensitivity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zou, Y.; Zhang, Y.; Xie, Z.; Luo, S.; Zeng, Y.; Chen, Q.; Liu, G.; Tian, Z. Improved sensitivity and reproducibility in electrochemical detection of trace mercury (II) by bromide ion & electrochemical oxidation. Talanta 2019, 203, 186–193. [Google Scholar]
- Gong, J.; Zhou, T.; Song, D.; Zhang, L. Monodispersed Au nanoparticles decorated graphene as an enhanced sensing platform for ultrasensitive stripping voltammetric detection of mercury (II). Sens. Actuators B Chem. 2010, 150, 491–497. [Google Scholar] [CrossRef]
- Deshmukh, M.A.; Celiesiute, R.; Ramanaviciene, A.; Shirsat, M.D.; Ramanavicius, A. EDTA_PANI/SWCNTs nanocomposite modified electrode for electrochemical determination of copper (II), lead (II) and mercury (II) ions. Electrochim. Acta 2018, 259, 930–938. [Google Scholar] [CrossRef]
- Bansod, B.K.; Kumar, T.; Thakur, R.; Rana, S.; Singh, I. A review on various electrochemical techniques for heavy metal ions detection with different sensing platforms. Biosens. Bioelectron. 2017, 94, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.Y.; Lee, S.C.; Okazaki, T.; Kuramit, H.; Abd-Rahman, F. Detection of mercury (II) ions in water by polyelectrolyte–gold nanoparticles coated long period fiber grating sensor. Opt. Commun. 2018, 419, 18–24. [Google Scholar] [CrossRef]
- Priyadarshini, E.; Pradhan, N. Gold nanoparticles as efficient sensors in colorimetric detection of toxic metal ions: A review. Sens. Actuators B Chem. 2017, 238, 888–902. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Arora, V.; Sapra, S.; Gupta, B.D. Localized Surface Plasmon Resonance-Based Fiber Optic U-Shaped Biosensor for the Detection of Blood Glucose. Plasmonics 2012, 7, 261–268. [Google Scholar] [CrossRef]
- Rycenga, M.; Cobley, C.M.; Zeng, J.; Li, W.; Moran, C.H.; Zhang, Q.; Qin, D.; Xia, Y. Controlling the synthesis and assembly of silver nanostructures for plasmonic applications. Chem. Rev. 2011, 111, 3669–3712. [Google Scholar] [CrossRef] [PubMed]
- Rivero, P.J.; Hernaez, M.; Goicoechea, J.; Matías, I.R.; Arregui, F.J. A comparative study in the sensitivity of optical fiber refractometers based on the incorporation of gold nanoparticles into layer-by-layer films. Int. J. Smart Sens. Intell. Syst. 2015, 8, 822–841. [Google Scholar] [CrossRef]
- Jia, S.; Bian, C.; Sun, J.; Tong, J.; Xia, S. A wavelength-modulated localized surface plasmon resonance (LSPR) optical fiber sensor for sensitive detection of mercury(II) ion by gold nanoparticles-DNA conjugates. Biosens. Bioelectron. 2018, 114, 15–21. [Google Scholar] [CrossRef]
- Haynes, C.L.; van Duyne, R.P. Nanosphere lithography: A versatile nanofabrication tool for studies of size-dependent nanoparticle optics. J. Phys. Chem. B 2001, 105, 5599–5611. [Google Scholar] [CrossRef]
- Shukla, G.M.; Punjabi, N.; Kundu, T.; Mukherji, S. Optimization of Plasmonic U-shaped Optical Fiber Sensor for Mercury Ions Detection using Glucose Capped Silver Nanoparticles. IEEE Sens. J. 2019, 19, 3224–3231. [Google Scholar] [CrossRef]
- Sadani, K.; Nag, P.; Mukherji, S. LSPR based optical fiber sensor with chitosan capped gold nanoparticles on BSA for trace detection of Hg (II) in water, soil and food samples. Biosens. Bioelectron. 2019, 134, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Sai, V.V.R.; Kundu, T.; Mukherji, S. Novel U-bent fiber optic probe for localized surface plasmon resonance based biosensor. Biosens. Bioelectron. 2009, 24, 2804–2809. [Google Scholar] [CrossRef]
- Abu-Ali, H.; Nabok, A.; Smith, T.J. Development of novel and highly specific ssDNA-aptamer-based electrochemical biosensor for rapid detection of mercury (II) and lead (II) ions in water. Chemosensors 2019, 7, 27. [Google Scholar] [CrossRef]
- Fayazi, M.; Taher, M.A.; Afzali, D.; Mostafavi, A. Fe3O4 and MnO2 assembled on halloysite nanotubes: A highly efficient solid-phase extractant for electrochemical detection of mercury(II) ions. Sens. Actuators B Chem. 2016, 228, 1–9. [Google Scholar] [CrossRef]
- Paulauskas, A.; Selskis, A.; Bukauskas, V.; Vaicikauskas, V.; Ramanavicius, A.; Balevicius, Z. Real time study of amalgam formation and mercury adsorption on thin gold film by total internal reflection ellipsometry. Appl. Surf. Sci. 2018, 427, 298–303. [Google Scholar] [CrossRef]
- Vasjari, M.; Shirshov, Y.M.; Samoylov, A.V.; Mirsky, V.M. SPR investigation of mercury reduction and oxidation on thin gold electrodes. J. Electroanal. Chem. 2007, 605, 73–76. [Google Scholar] [CrossRef]
- Sabri, N.; Aljunid, S.A.; Salim, M.S.; Fouad, S. Fiber Optic Sensors: Short Review and Applications. In Recent Trends in Physics of Material Science and Technology; Gaol, F.L., Shrivastava, K., Akhtar, J., Eds.; Springer: Singapore, 2015; pp. 299–311. [Google Scholar]
- Elosua, C.; Arregui, F.J.; del Villar, I.; Ruiz-Zamarreño, C.; Corres, J.M.; Bariain, C.; Goicoechea, J.; Hernaez, M.; Rivero, P.J.; Socorro, A.B.; et al. Micro and nanostructured materials for the development of optical fibre sensors. Sensors 2017, 17, 2312. [Google Scholar] [CrossRef]
- Jia, S.; Bian, C.; Tong, J.H.; Sun, J.Z.; Xia, S.H. A Fiber-optic Sensor Based on Plasmon Coupling Effects in Gold Nanoparticles Core-satellites Nanostructure for Determination of Mercury Ions (II). Chin. J. Anal. Chem. 2017, 45, 785–790. [Google Scholar] [CrossRef]
- Schopf, C.; Martín, A.; Schmidt, M.; Iacopino, D. Investigation of Au-Hg amalgam formation on substrate-immobilized individual Au nanorods. J. Mater. Chem. C 2015, 3, 8865–8872. [Google Scholar] [CrossRef]
- Goicoechea, J.; Rivero, P.J.; Sada, S.; Arregui, F.J. Self-Referenced Optical Fiber Sensor for Hydrogen Peroxide Detection based on LSPR of Metallic Nanoparticles in Layer-by-Layer Films. Sensors 2019, 19, 3872. [Google Scholar] [CrossRef] [PubMed]
- Decher, G.; Eckle, M.; Schmitt, J.; Struth, B. Layer-by-layer assembled multicomposite films. Curr. Opin. Colloid Interface Sci. 1998, 3, 32–39. [Google Scholar] [CrossRef]
- Fuzzy, D.G. Nanoassemblies: Toward layered polymeric multicomposites. Science 1997, 277, 1232–1237. [Google Scholar]
- Baba, Y.; Ohe, K.; Kawasaki, Y.; Kolev, S.D. Adsorption of mercury(II) from hydrochloric acid solutions on glycidylmethacrylate-divinylbenzene microspheres containing amino groups. React. Funct. Polym. 2006, 66, 1158–1164. [Google Scholar] [CrossRef]
- Jenkins, D.W. Biological Monitoring of Toxic Trace Metals; EPA Report 600/S3-80-090; United States Environmental Protection Agency: Washington, DC, USA, 1980; pp. 1–9.
- Wang, T.; Kang, D.H.; Yu, Y.J.; Gu, J.H.; Yang, L.R. Determination of macro and trace elements in rare earth magnesium cast iron by inductively coupled plasma atomic emission spectrometry. Yejin Fenxi/Metall. Anal. 2012, 32, 66–69. [Google Scholar]
- USEPA. National Recommended Water Quality Criteria; 4304T; United States Environmental Protection Agency: Washington, DC, USA, 2009; Volume 1.
- European Commission. Council Directive 98/83/EC of 3 November 1998 on the Quality of Water Intended for Human Consumption. Off. J. Eur. Communities 1998, 41, 34–54. [Google Scholar]









© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Hernández, M.E.; Goicoechea, J.; Arregui, F.J. Hg2+ Optical Fiber Sensor Based on LSPR Generated by Gold Nanoparticles Embedded in LBL Nano-Assembled Coatings. Sensors 2019, 19, 4906. https://doi.org/10.3390/s19224906
Martínez-Hernández ME, Goicoechea J, Arregui FJ. Hg2+ Optical Fiber Sensor Based on LSPR Generated by Gold Nanoparticles Embedded in LBL Nano-Assembled Coatings. Sensors. 2019; 19(22):4906. https://doi.org/10.3390/s19224906
Chicago/Turabian StyleMartínez-Hernández, María Elena, Javier Goicoechea, and Francisco J. Arregui. 2019. "Hg2+ Optical Fiber Sensor Based on LSPR Generated by Gold Nanoparticles Embedded in LBL Nano-Assembled Coatings" Sensors 19, no. 22: 4906. https://doi.org/10.3390/s19224906
APA StyleMartínez-Hernández, M. E., Goicoechea, J., & Arregui, F. J. (2019). Hg2+ Optical Fiber Sensor Based on LSPR Generated by Gold Nanoparticles Embedded in LBL Nano-Assembled Coatings. Sensors, 19(22), 4906. https://doi.org/10.3390/s19224906





