Combustible Gas Classification Modeling using Support Vector Machine and Pairing Plot Scheme
Abstract
:1. Introduction
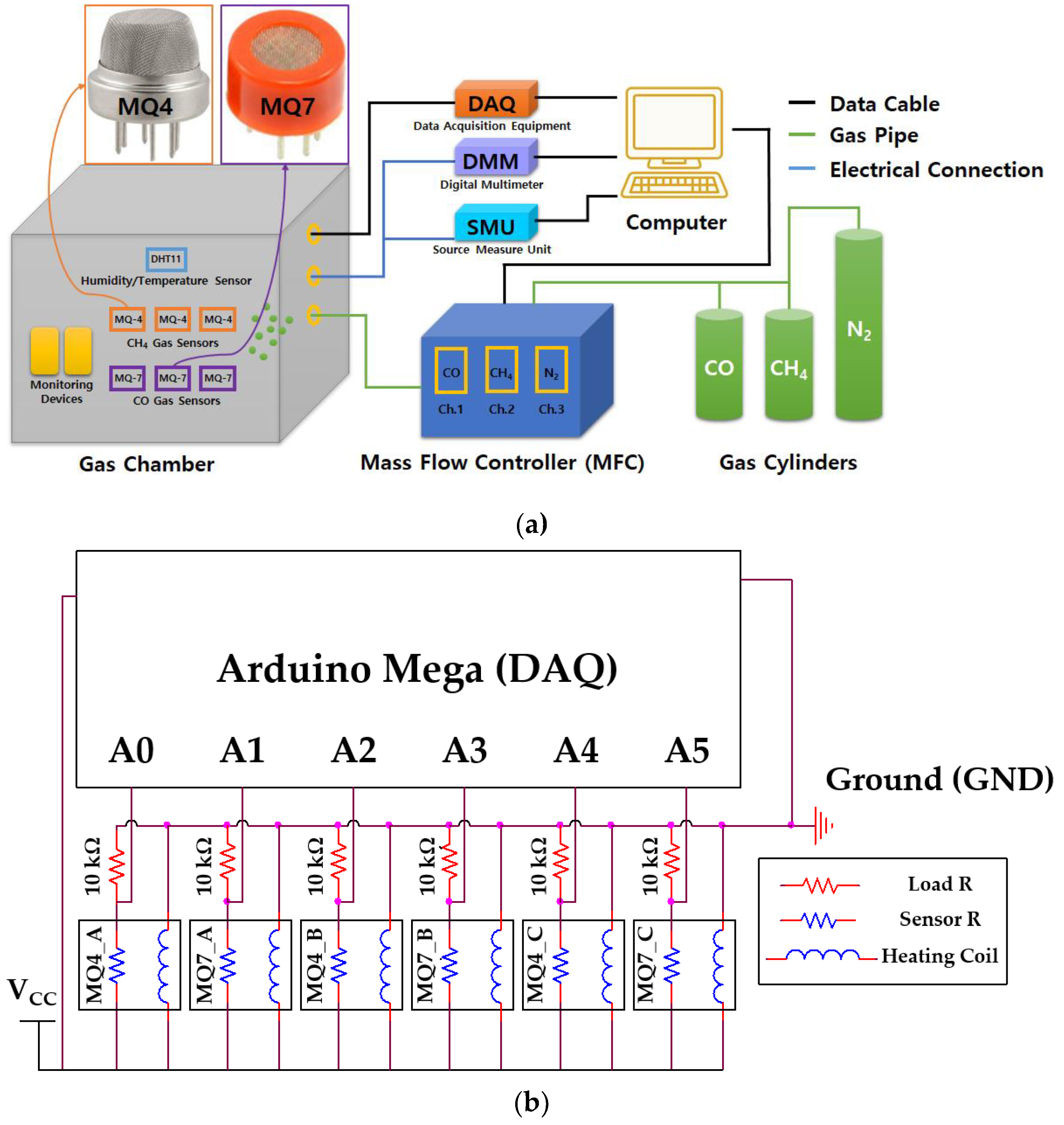
2. Materials and Methods
3. Results
3.1. Raw Data for Gas Sensing
3.2. Pairing Plot Scheme for Support Vector Machine
3.3. Gas Classification Model Using Non-Linear Support Vector Machine
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bjerketvedt, D.; Bakke, J.R.; Van Wingerden, K. Gas explosion handbook. J. Hazard. Mater. 1997, 52, 1–150. [Google Scholar] [CrossRef]
- Dobashi, R. Experimental study on gas explosion behavior in enclosure. J. Loss Prevent. Proc. 1997, 10, 83–89. [Google Scholar] [CrossRef]
- Schröder, V.; Molnarne, M. Flammability of gas mixtures: Part 1: Fire potential. J. Hazard. Mater. 2005, 121, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Molnarne, M.; Mizsey, P.; Schröder, V. Flammability of gas mixtures: Part 2: Influence of inert gases. J. Hazard. Mater. 2005, 121, 45–49. [Google Scholar] [CrossRef] [PubMed]
- MicroChem: Innovative Chemical Solutions for MEMS and Microelectronics. Available online: http://www.microchem.com (accessed on 16 August 2019).
- City Technology Ltd. Global Leaders in Gas Sensor Technology. Available online: http://www.citytech.com (accessed on 16 August 2019).
- Shahid, A.; Choi, J.H.; Rana, A.; Kim, H.S. Least Squares Neural Network-Based Wireless E-Nose System Using an SnO2 Sensor Array. Sensors 2018, 18, 1446. [Google Scholar] [CrossRef]
- Pardo, M.; Sberveglieri, G. Classification of electronic nose data with support vector machines. Sens. Actuators B Chem. 2005, 107, 730–737. [Google Scholar] [CrossRef]
- Han, L.; Yu, C.; Xiao, K.; Zhao, X. A New Method of Mixed Gas Identification Based on a Convolutional Neural Network for Time Series Classification. Sensors 2019, 19, 1960. [Google Scholar] [CrossRef]
- Ghaffarian, N.; Eslamloueyan, R.; Vaferi, B. Model identification for gas condensate reservoirs by using ANN method based on well test data. J. Petrol. Sci. Eng. 2014, 123, 20–29. [Google Scholar] [CrossRef]
- Jian, Y.; Huang, D.; Yan, J.; Lu, K.; Huang, Y.; Wen, T.; Zeng, T.; Zhong, S.; Xie, Q. A novel extreme learning machine classification model for e-Nose application based on the multiple kernel approach. Sensors 2017, 17, 1434. [Google Scholar] [CrossRef]
- Peng, P.; Zhao, X.; Pan, X.; Ye, W. Gas classification using deep convolutional neural networks. Sensors 2018, 18, 157. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, X.; Chen, Y.; Yang, Z. Research on a Mixed Gas Classification Algorithm Based on Extreme Random Tree. Appl. Sci. 2019, 9, 1728. [Google Scholar] [CrossRef]
- SparkFun Electronics. (Model: MQ4). Available online: https://cdn.sparkfun.com/datasheets/Sensors/Biometric/MQ-4%20Ver1.3%20-%20Manual.pdf (accessed on 16 August 2019).
- SparkFun Electronics. (Model: MQ7). Available online: https://cdn.sparkfun.com/datasheets/Sensors/Biometric/MQ-7%20Ver1.3%20-%20Manual.pdf (accessed on 16 August 2019).
- Harrison, P.G.; Willett, M.J. The mechanism of operation of tin (IV) oxide carbon monoxide sensors. Nature 1988, 332, 337–339. [Google Scholar] [CrossRef]
- Burresi, A.; Fort, A.; Rocchi, S.; Serrano, B.; Ulivieri, N.; Vignoli, V. Dynamic CO recognition in presence of interfering gases by using one MOX sensor and a selected temperature profile. Sens. Actuator B Chem. 2005, 106, 40–43. [Google Scholar] [CrossRef]
- Suematsu, K.; Ma, N.; Watanabe, K.; Yuasa, M.; Kida, T.; Shimanoe, K. Effect of humid aging on the oxygen adsorption in SnO2 gas sensors. Sensors 2018, 18, 254. [Google Scholar] [CrossRef] [PubMed]
- Schweizer-Berberich, M.; Zdralek, M.; Weimar, U.; Göpel, W.; Viard, T.; Martinez, D.; Seube, A.; Peyre-Lavigne, A. Pulsed mode of operation and artificial neural network evaluation for improving the CO selectivity of SnO2 gas sensors. Sens. Actuator B Chem. 2000, 65, 91–93. [Google Scholar] [CrossRef]
- Yu, J.H.; Choi, G.M. Selective CO gas detection of CuO-and ZnO-doped SnO2 gas sensor. Sens. Actuators B Chem. 2001, 75, 56–61. [Google Scholar] [CrossRef]
- Lentka, Ł.; Smulko, J.M.; Ionescu, R.; Granqvist, C.G.; Kish, L.B. Determination of gas mixture components using fluctuation enhanced sensing and the LS-SVM regression algorithm. Metrol. Meas. Syst. 2015, 22, 341–350. [Google Scholar] [CrossRef]
- Sedghi, S.M.; Mortazavi, Y.; Khodadadi, A. Low temperature CO and CH4 dual selective gas sensor using SnO2 quantum dots prepared by sonochemical method. Sens. Actuators B Chem. 2010, 145, 7–12. [Google Scholar] [CrossRef]
- Khemchandani, R.; Chandra, S. Twin support vector machines for pattern classification. IEEE Trans. Pattern Anal. Mach. Intell. 2007, 29, 905–910. [Google Scholar]
- Lin, Y.; Lee, Y.; Wahba, G. Support vector machines for classification in nonstandard situations. Mach. Learn. 2002, 46, 191–202. [Google Scholar] [CrossRef]
- Gold, C.; Sollich, P. Model selection for support vector machine classification. Neurocomputing 2003, 55, 221–249. [Google Scholar] [CrossRef]
- Furey, T.S.; Cristianini, N.; Duffy, N.; Bednarski, D.W.; Schummer, M.; Haussler, D. Support vector machine classification and validation of cancer tissue samples using microarray expression data. Bioinformatics 2000, 16, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Fung, G.M.; Mangasarian, O.L. A feature selection Newton method for support vector machine classification. Comput. Optim. Appl. 2004, 28, 185–202. [Google Scholar] [CrossRef]
- Chung, K.M.; Kao, W.C.; Sun, C.L.; Wang, L.L.; Lin, C.J. Radius margin bounds for support vector machines with the RBF kernel. Neural Comput. 2003, 15, 2643–2681. [Google Scholar] [CrossRef]
- Amari, S.I.; Wu, S. Improving support vector machine classifiers by modifying kernel functions. Neural Netw. 1999, 12, 783–789. [Google Scholar] [CrossRef]
- Landgrebe, T.C.; Duin, R.P. Efficient multiclass ROC approximation by decomposition via confusion matrix perturbation analysis. IEEE Trans. Pattern Anal. Mach. Intell. 2008, 30, 810–822. [Google Scholar] [CrossRef]
- Visa, S.; Ramsay, B.; Ralescu, A.L.; Van Der Knaap, E. Confusion Matrix-based Feature Selection. Mod. Artif. Intell. Cogn. Sci. 2011, 710, 120–127. [Google Scholar]
- Deng, X.; Liu, Q.; Deng, Y.; Mahadevan, S. An improved method to construct basic probability assignment based on the confusion matrix for classification problem. Inf. Sci. 2016, 340, 250–261. [Google Scholar] [CrossRef]
- Rodriguez, J.D.; Perez, A.; Lozano, J.A. Sensitivity analysis of k-fold cross validation in prediction error estimation. IEEE Trans. Pattern Anal. 2009, 32, 569–575. [Google Scholar] [CrossRef]
- Fushiki, T. Estimation of prediction error by using K-fold cross-validation. Stat. Comput. 2011, 21, 137–146. [Google Scholar] [CrossRef]
- Wong, T.T. Performance evaluation of classification algorithms by k-fold and leave-one-out cross validation. Pattern Recognit. 2015, 48, 2839–2846. [Google Scholar] [CrossRef]





| Parameter | Unit | CH4 | CO |
|---|---|---|---|
| Gas injection rate | sccm | 30 | 6 |
| Gas injection time | sec | 20 | 20 |
| Reaction time | min | 5 | 5 |
| Gas injection concentration | ppm | 100 | 20 |
| Total number of gas injections | - | 20 | 20 |
| Target gas concentration | ppm | 2000 | 400 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, K.-W.; Choi, J.-H.; Jeon, J.-H.; Kim, H.-S. Combustible Gas Classification Modeling using Support Vector Machine and Pairing Plot Scheme. Sensors 2019, 19, 5018. https://doi.org/10.3390/s19225018
Jang K-W, Choi J-H, Jeon J-H, Kim H-S. Combustible Gas Classification Modeling using Support Vector Machine and Pairing Plot Scheme. Sensors. 2019; 19(22):5018. https://doi.org/10.3390/s19225018
Chicago/Turabian StyleJang, Kyu-Won, Jong-Hyeok Choi, Ji-Hoon Jeon, and Hyun-Seok Kim. 2019. "Combustible Gas Classification Modeling using Support Vector Machine and Pairing Plot Scheme" Sensors 19, no. 22: 5018. https://doi.org/10.3390/s19225018





