The Response of UV/Blue Light and Ozone Sensing Using Ag-TiO2 Planar Nanocomposite Thin Film
Abstract
:1. Introduction
2. Materials and Methods
3. Results
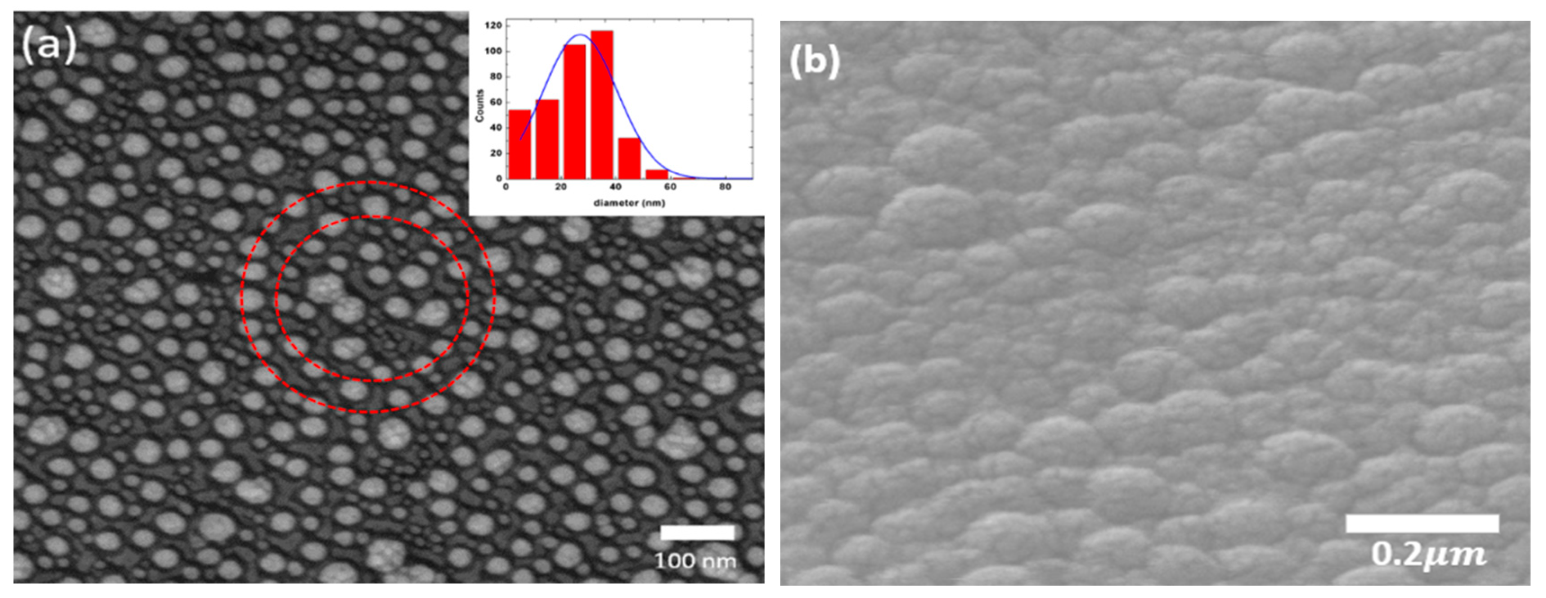
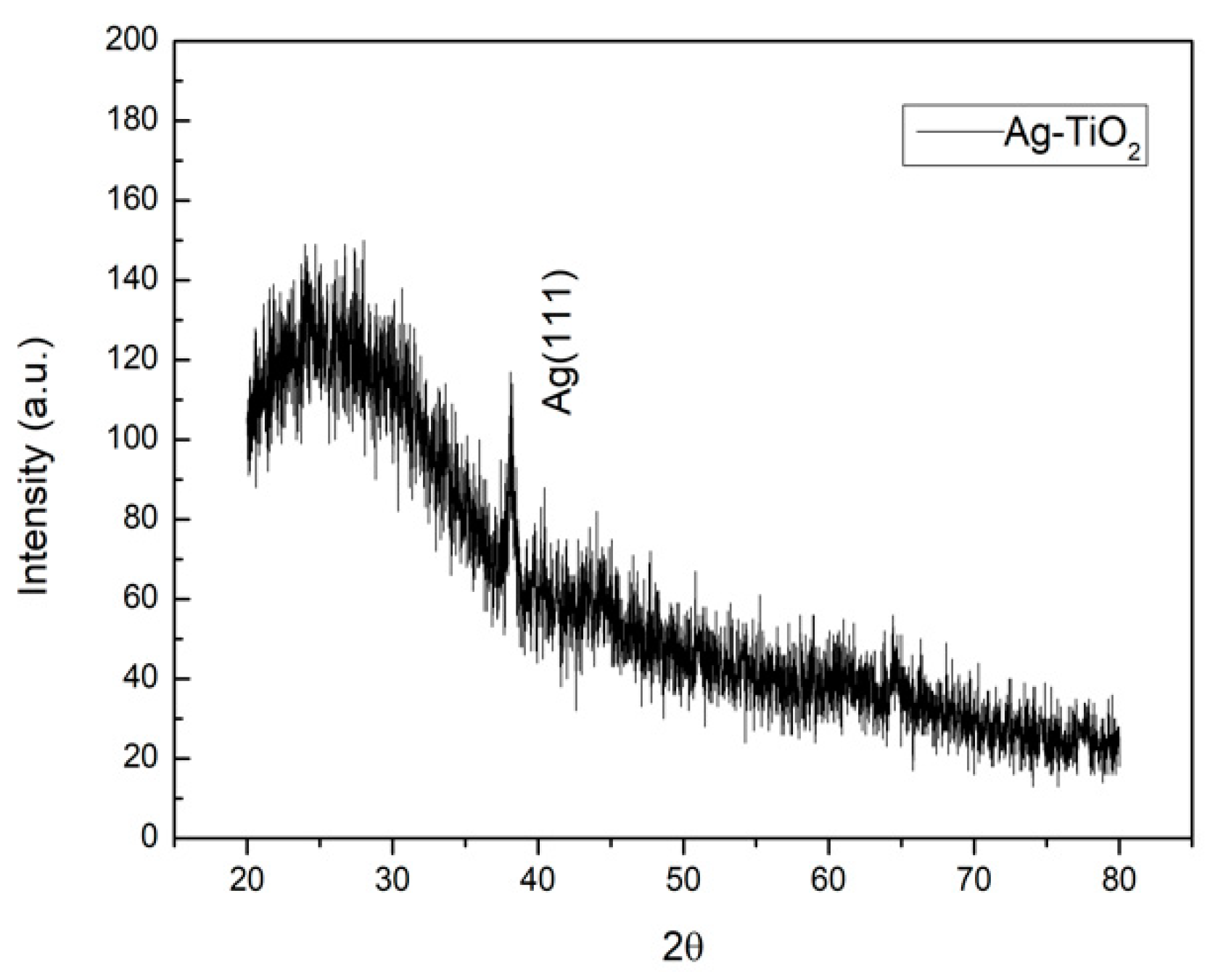
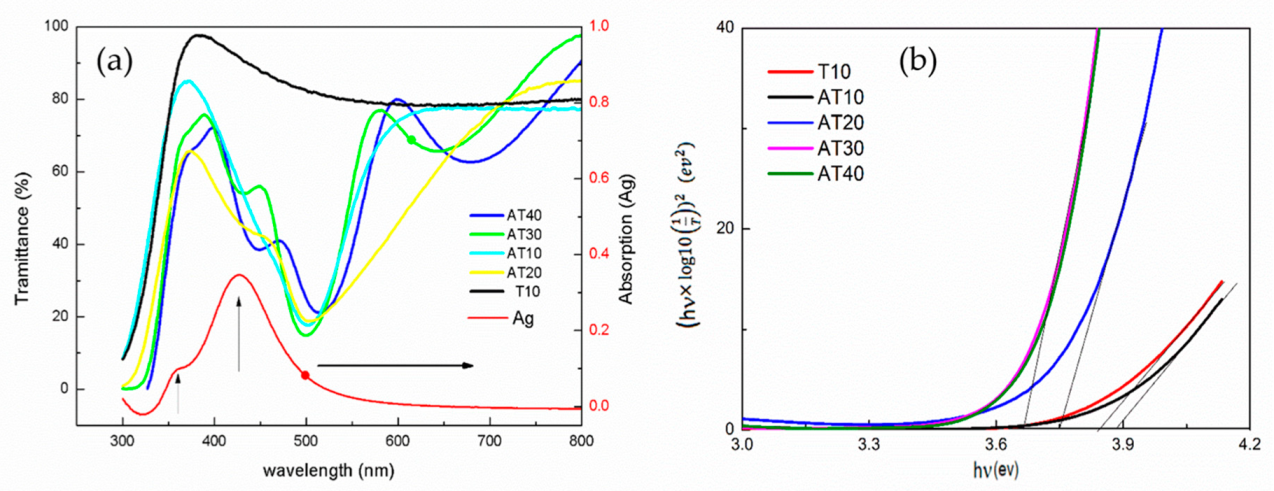
3.1. Chacteristics of Ag-TiO2
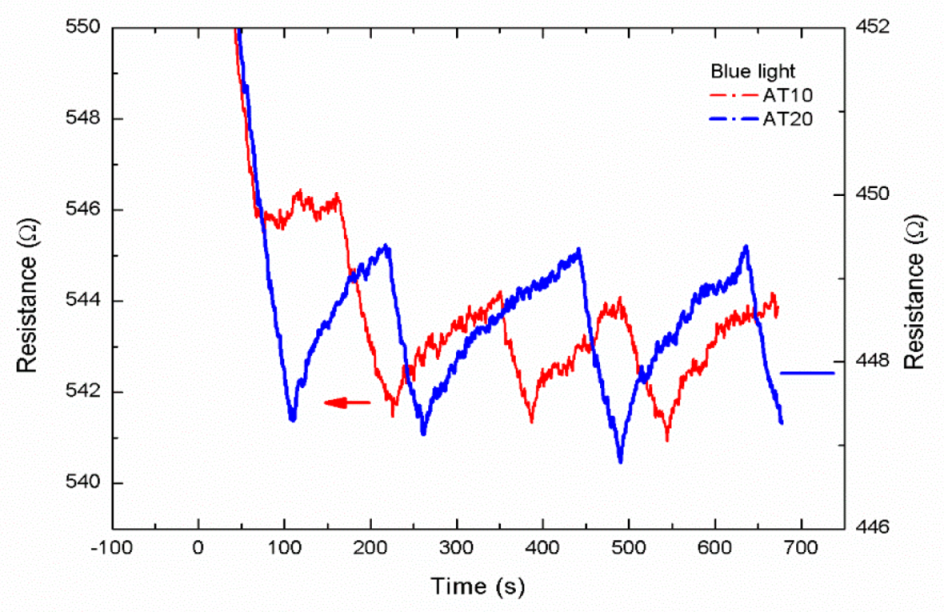
3.2. UV and Blue Light Response
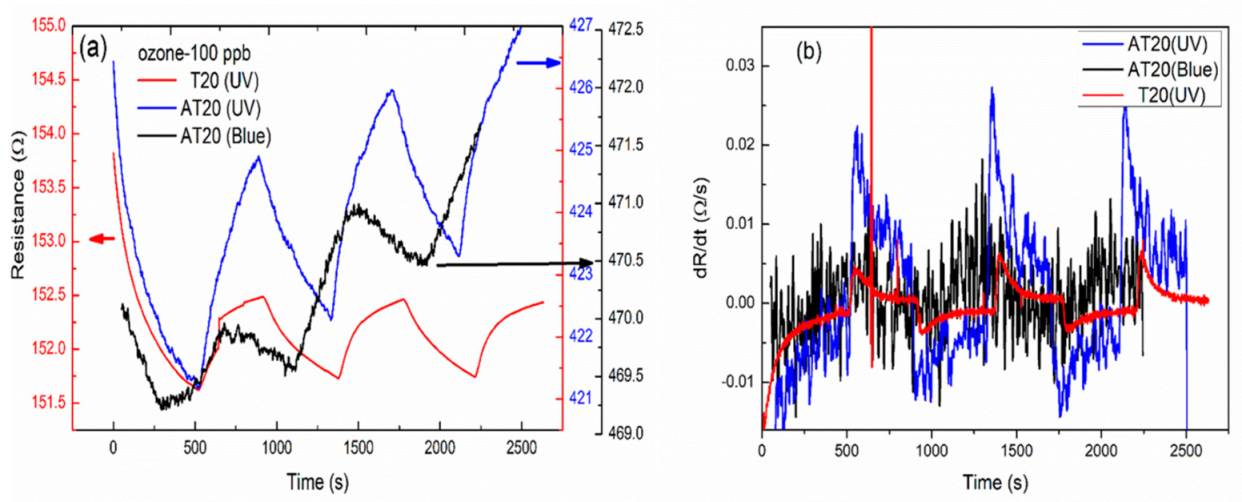
3.3. Gas Sensing
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, X.; Xie, J.; Jiang, C.; Yu, J.; Zhang, P. Review on design and evaluation of environmental photocatalysts. Front. Environ. Sci. Eng. 2018, 12, 14. [Google Scholar] [CrossRef]
- Anpo, M.; Kamat, P.V. Environmentally Benign Photocatalysts: Applications of Titanium Oxide-Based Materials; Springer: New York, NY, USA, 2010. [Google Scholar]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.G.; Devi, L.G. Review on Modified TiO2 Photocatalysis under UV/Visible Light: Selected Results and Related Mechanisms on Interfacial Charge Carrier Transfer Dynamics. J. Phys. Chem. A 2011, 115, 13211–13241. [Google Scholar] [CrossRef] [PubMed]
- Shehzad, N.; Tahir, M.; Johari, K.; Murugesan, T.; Hussain, M. A critical review on TiO2 based photocatalytic CO2 reduction system: Strategies to improve efficiency. J. CO2 Util. 2018, 26, 98–122. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.; Hamilton, J.W.; Byrne, J.A.; O’shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Ayati, A.; Ahmadpour, A.; Bamoharram, F.F.; Tanhaei, B.; Mänttäri, M.; Sillanpää, M. A review on catalytic applications of Au/TiO2 nanoparticles in the removal of water pollutant. Chemosphere 2014, 107, 163–174. [Google Scholar] [CrossRef]
- Maijenburg, A.W.; Veerbeek, J.; de Putter, R.; Veldhuis, S.A.; Zoontjes, M.G.; Mul, G.; Montero-Moreno, J.M.; Nielsch, K.; Schäfer, H.; Steinhart, M.; et al. Electrochemical synthesis of coaxial TiO2-Ag nanowires and their application in photocatalytic water splitting. J. Mater. Chem. A 2014, 2, 2648–2656. [Google Scholar] [CrossRef]
- Zieli´nska-Jurek, A.; Zaleska, A. Ag/Pt-modified TiO2 nanoparticles for toluene photooxidation in the gas phase. Catal. Today 2014, 230, 104–111. [Google Scholar] [CrossRef]
- He, C.; Xiong, Y.; Chen, J.; Zha, C.; Zhu, X. Photoelectrochemical performance of Ag-TiO2/ITO film and photoelectrocatalytic activity towards the oxidation of organic pollutants. J. Photochem. Photobiol. A Chem. 2013, 157, 71–79. [Google Scholar] [CrossRef]
- Yu, D.H.; Yu, X.; Wang, C.; Liu, X.C.; Xing, Y. Synthesis of natural cellulose-templated TiO2/Ag nanosponge composites and photocatalytic properties. ACS Appl. Mater. Interfaces 2012, 4, 2781–2787. [Google Scholar] [CrossRef]
- Ge, M.Z.; Cao, C.Y.; Li, S.H.; Tang, Y.X.; Wang, L.N.; Qi, N.; Huang, J.Y.; Zhang, K.Q.; Al-Deyab, S.S.; Lai, Y.K. In situ plasmonic Ag nanoparticle anchored TiO2 nanotube arrays as visible-light-driven photocatalysts for enhanced water splitting. Nanoscale 2016, 8, 5226–5234. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Extremely sensitive and selective sub-ppm CO detection by the synergistic effect of Au nanoparticles and core–shell nanowires. Sens. Actuators B Chem. 2017, 249, 177–188. [Google Scholar] [CrossRef]
- Genix, A.C.; Schmitt-Pauly, C.; Alauzun, J.G.; Bizien, T.; Mutin, P.H.; Oberdisse, J. Tuning Local Nanoparticle Arrangements in TiO2-Polymer Nanocomposites by Grafting of Phosphonic Acids. Macromolecules 2017, 50, 7721–7729. [Google Scholar] [CrossRef]
- Hirakawa, T.; Kamat, P.V. Charge separation and catalytic activity of Ag@TiO2–core-shell composite clusters under UV-irradiation. J. Am. Chem. Soc. 2005, 127, 3928–3934. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Chang, J.L.; Wu, R.J. Fast ozone detection by using a core–shell Au@TiO2sensor at room temperature. Sens. Actuators B Chem. 2015, 214, 56–62. [Google Scholar] [CrossRef]
- Singh, J.; Sahu, K. Thermal annealing induced strong photoluminescence enhancement in Ag-TiO2 plasmonic nanocomposite thin films. J. Alloys Compd. 2019, 786, 750–757. [Google Scholar] [CrossRef]
- Shao, X.; Li, B.; Zhang, B.; Shao, L.; Wu, Y. Au@ZnO core–shell nanostructures with plasmon-induced visible-light photocatalytic and photoelectrochemical properties. Inorg. Chem. Front. 2016, 3, 934–943. [Google Scholar] [CrossRef]
- Wu, W.Y.; Hsu, C.F.; Wu, M.J.; Chen, C.N.; Huang, J.J. Ag–TiO2 composite photoelectrode for dye-sensitized solar cell. Appl. Phys. A 2017, 123, 357. [Google Scholar] [CrossRef]
- Lou, Z.; Han, H.; Zhou, M.; Wan, J.; Sun, Q.; Zhou, X.; Gu, N. Fabrication of Magnetic Conjugation Clusters via Intermolecular Assembling for Ultrasensitive Surface Plasmon Resonance (SPR) Detection in a Wide Range of Concentrations. Anal. Chem. 2017, 89, 13472–13479. [Google Scholar] [CrossRef]
- Lou, Z.; Han, H.; Mao, D.; Jiang, Y.; Song, J. Qualitative and Quantitative Detection of PrPSc Based on the Controlled Release Property of Magnetic Microspheres Using Surface Plasmon Resonance (SPR). Nanomaterials 2018, 8, 107. [Google Scholar] [CrossRef]
- Lou, Z.; Wan, J.; Zhang, X.; Zhang, H.; Zhou, X.; Cheng, S.; Gu, N. Quick and sensitive SPR detection of prion disease-associated isoform(PrPSc) based on its self-assembling behavior on bare gold film andspecific interactions with aptamer-graphene oxide (AGO). Colloids Surf. B Biointerfaces 2017, 157, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.Y.; Chen, K.L.; Wu, C.H. Improving the SERS signals of biomolecules using a stacked biochip containing Fe2O3/Au nanoparticles and a DC magnetic field. Sci. Rep. 2019, 9, 9566. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.L.; Yeh, Y.W.; Chen, J.M.; Hong, Y.J.; Huang, T.L.; Deng, Z.Y.; Wu, C.H.; Liao, S.H.; Wang, L.M. Influence of magnetoplasmonic γ-Fe2O3/Au core/shell nanoparticles on low-field nuclear magnetic resonance. Sci. Rep. 2016, 6, 35477. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cao, S.; Zhang, Z.; Wang, Y.; Liao, C.; Wang, Y. Temperature Sensor Based on Side-Polished Fiber SPR Device Coated with Polymer. Sensors 2019, 19, 4063. [Google Scholar] [CrossRef]
- Qi, F.; Wang, C.; Cheng, N.; Liu, P.; Xiao, Y.; Li, F.; Sun, X.; Liu, W.; Guo, S.; Zhao, X.Z. Improving the performance through SPR effect by employing Au@SiO2 core-shell nanoparticles incorporated TiO2 scaffold in efficient hole transport material free perovskite solar cells. Electrochim. Acta 2018, 282, 10–15. [Google Scholar] [CrossRef]
- Sun, S.-Q.; Sun, B.; Zhang, W.; Wang, D. Preparation and antibacterial activity of Ag-TiO2 composite film by liquid phase deposition (LPD) method. Bull. Mater. Sci. 2008, 31, 61–66. [Google Scholar] [CrossRef]
- Wang, H.; Faria, J.L.; Dong, S.; Chang, Y. Mesoporous Au/TiO2 composites preparation, characterization, and photocatalytic properties. Mater. Sci. Eng. B 2012, 177, 913–919. [Google Scholar] [CrossRef]
- Binyu, Y.; Man, L.K.; Qiuquan, G.; Ming, L.W.; Jun, Y. Synthesis of Ag-TiO2 composite nano thin film for antimicrobial application. Nanotechnology 2011, 22, 115603. [Google Scholar]
- Wang, F.X.; Hwangbo, C.K.; Jung, B.Y.; Lee, J.H.; Park, B.H.; Kim, N.Y. optical and structural properties of TiO2 films deposited from Ti3O5 by electron beam. Surf. Coat. Technol. 2007, 201, 5367–5370. [Google Scholar] [CrossRef]
- Shei, S.-C. Optical and Structural Properties of Titanium Dioxide Films from TiO2 and Ti3O5 Starting Materials Annealed at Various Temperatures. Adv. Mater. Sci. Eng. 2013, 2013, 545076. [Google Scholar] [CrossRef]
- Lin, C.W.; Huang, K.L.; Chang, K.W.; Chen, J.H.; Chen, K.L.; Wu, C.H. Ultraviolet photodetector and gas sensor based on amorphous In-Ga-Zn-O film. Thin Solid Film. 2016, 618, 73–76. [Google Scholar] [CrossRef]
- Wu, C.H.; Jiang, G.J.; Chiu, C.C.; Paul, C.; Jeng, C.C.; Wu, R.J.; Chen, J.H. Fast gas concentration sensing by analyzing the rate of resistance change. Sens. Actuators B 2015, 209, 906–910. [Google Scholar] [CrossRef]
- Wu, C.H.; Chang, K.W.; Li, Y.N.; Deng, Z.Y.; Chen, K.L.; Jeng, C.C.; Wu, R.J.; Chen, J.H. Improving the sensitive and selective of trace amount ozone sensor on Indium-Gallium-Zinc Oxide thin film by ultraviolet irradiation. Sens. Actuators B 2018, 273, 1713–1718. [Google Scholar] [CrossRef]
- Liu, X.; Li, D.; Sun, X.; Li, Z.; Song, H.; Jiang, H.; Chen, Y. Tunable Dipole Surface Plasmon Resonances of Silver Nanoparticles by Cladding Dielectric Layers. Sci. Rep. 2015, 5, 12555. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.C.; Lin, S.J.; Lin, C.H.; Tsai, C.S.; Lu, Y.J. Size effect of Ag nanoparticles on surface plasmon resonance. Surf. Coat. Technol. 2008, 202, 5339–5342. [Google Scholar] [CrossRef]
- Lee, S.Y.; Cho, E.S.; Kwon, S.J. The optical analyses of the multilayer transparent electrode and the formation of ITO/Mesh-Ag/ITO multilayers for enhancing an optical transmittance. Appl. Surf. Sci. 2019, 487, 990–999. [Google Scholar] [CrossRef]
- Sanzone, G.; Zimbone, M.; Cacciato, G.; Ruffino, F.; Carles, R.; Privitera, V.; Grimaldi, M.G. Ag/TiO2 nanocomposite for visible light-driven photocatalysis. Superlattices Microstruct. 2018, 123, 394–402. [Google Scholar] [CrossRef]
- Jiang, M.M.; Chen, Y.H.; Li, H.B.; Liu, W.K.; Shan, X.C.; Shen, Z.D. Hybrid quadrupolar resonances stimulated at short wavelengths using coupled plasmonic silver nanoparticle aggregation Hybrid quadrupolar resonances stimulated at short wavelengths using coupled plasmonic silver nanoparticle aggregation. J. Mater. Chem. C 2014, 2, 56–63. [Google Scholar] [CrossRef]
- Hooshmand, N.; Mostafa, A. El-Sayed, Collective multipole oscillations direct the plasmonic coupling at the nanojunction interfaces. Proc. Natl. Acad. Sci. USA 2019, 116, 19299–19304. [Google Scholar] [CrossRef] [Green Version]
- Miroshnichenko, A.E.; Kivshar, Y.S. Fano Resonances in All-Dielectric Oligomers. Nano Lett. 2012, 12, 6459–6463. [Google Scholar] [CrossRef]
- Yakovlev, A.B.; Hedayati, M.; Silveirinha, M.G.; Hanson, G.W. Local thickness-dependent permittivity model for nonlocal bounded wire-medium structures. Phys. Rev. B 2016, 94, 155442. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.L.; Liu, R.S.; Tsai, D.P. Plasmonic photocatalysis. Rep. Prog. Phys. 2013, 76, 046401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Méndez-Medrano, M.G.; Kowalska, E.; Lehoux, A.; Herissan, A.; Ohtani, B.; Bahena, D.; Briois, V.; Colbeau-Justin, C.; Rodríguez-López, J.L.; Remita, H. Surface Modification of TiO2 with Ag Nanoparticles and CuO Nanoclusters for Application in Photocatalysis. J. Phys. Chem. C 2016, 120, 5143–5154. [Google Scholar] [CrossRef]
- Khore, S.K.; Kadam, S.R.; Naik, S.D.; Kale, B.B.; Sonawane, R.S. Solar light active plasmonic Au@TiO2 nanocomposite with superior photocatalytic performance for H2 production and pollutant degradation. New J. Chem. 2018, 42, 10958–10968. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, R.; Bhattacharyya, B.; Husale, S. Hot electron induced NIR detection in CdS films. Sci. Rep. 2016, 6, 22939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buso, D.; Post, M.; Cantalini, C.; Mulvaney, P.; Martucci, A. Gold Nanoparticle-Doped TiO2 Semiconductor Thin Films: Gas Sensing Properties. Adv. Funct. Mater. 2008, 18, 3843–3849. [Google Scholar] [CrossRef] [Green Version]
- Rai, P.; Khan, R.; Raj, S.; Majhi, S.M.; Park, K.K.; Yu, Y.T.; Lee, I.H.; Sekhar, P.K. Au@Cu2O core–shell nanoparticles as chemiresistors for gas sensor applications: Effect of potential barrier modulation on the sensing performance. Nanoscale 2014, 6, 581–588. [Google Scholar] [CrossRef]
- Avansi, W., Jr.; Catto, A.C.; da Silva, L.F.; Fiorido, T.; Bernardini, S.; Mastelaro, V.R.; Aguir, K.; Arenal, R. One-Dimensional V2O5/TiO2 Heterostructures for Chemiresistive Ozone Sensors. ACS Appl. Nano Mater. 2019, 2, 4756–4764. [Google Scholar] [CrossRef]
- Onofre, Y.J.; Catto, A.C.; Bernardini, S.; Fiorido, T.; Aguir, K.; Longo, E.; Mastelaro, V.R.; da Silva, L.F.; de Godoy, M.P. Highly selective ozone gas sensor based on nanocrystalline Zn0.95Co0.05O thin film obtained via spray pyrolysis technique. Appl. Surf. Sci. 2019, 478, 347–354. [Google Scholar] [CrossRef]
- Catto, A.C.; Fiorido, T.; Souza, É.L.; Avansi, W., Jr.; Andres, J.; Aguir, K.; Longo, E.; Cavalcante, L.S.; Da Silva, L.F. Improving the ozone gas-sensing properties of CuWO4 nanoparticles. J. Alloys Compd. 2018, 748, 411–417. [Google Scholar] [CrossRef] [Green Version]
- Wu, R.J.; Chen, C.Y.; Chen, M.H.; Sun, Y.L. Photoreduction measurement of ozone usingPt/TiO2–SnO2 material at room temperature. Sens. Actuators B Chem. 2007, 123, 1077–1082. [Google Scholar] [CrossRef]
- Nunes, D.; Pimentel, A.; Gonçalves, A.; Pereira, S.; Branquinho, R.; Barquinha, P.; Fortunato, E.; Martins, R. Metal oxide nanostructures for sensor applications. Semicond. Sci. Technol. 2019, 34, 043001. [Google Scholar] [CrossRef] [Green Version]

| T10 | AT10 | T20 | AT20 | |
|---|---|---|---|---|
| UV | 0.2% | 1.7% | 0.1% | 1.2% |
| Blue | X | 0.45% | X | 0.46% |
| T20 | AT20 | |||
|---|---|---|---|---|
| Blue | UV | Blue | UV | |
| Ozone | X | 0.35% | 0.38% | 0.8% |
| Materials | Ozone (ppb) | Operating Temperature | Response | Reference |
|---|---|---|---|---|
| core–shell Au@TiO2 | 500 | R. T | 1.15 * | [16] |
| V2O5/TiO2 | 1000 | 300 °C | 1.4 # | [49] |
| Zn0.95Co0.05O | 20 | 250 °C | 0.4 # | [50] |
| CuWO4 | 15 | 250 °C | ~2 * | [51] |
| Pt/TiO2-SnO2 | 2500 | R.T.(UV) | 1100 * | [52] |
| Ag/TiO2 | 100 | R.T. (Blue) | 1.004 * | Present study |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo, T.-H.; Shih, P.-Y.; Wu, C.-H. The Response of UV/Blue Light and Ozone Sensing Using Ag-TiO2 Planar Nanocomposite Thin Film. Sensors 2019, 19, 5061. https://doi.org/10.3390/s19235061
Lo T-H, Shih P-Y, Wu C-H. The Response of UV/Blue Light and Ozone Sensing Using Ag-TiO2 Planar Nanocomposite Thin Film. Sensors. 2019; 19(23):5061. https://doi.org/10.3390/s19235061
Chicago/Turabian StyleLo, Tzu-Hsuan, Pen-Yuan Shih, and Chiu-Hsien Wu. 2019. "The Response of UV/Blue Light and Ozone Sensing Using Ag-TiO2 Planar Nanocomposite Thin Film" Sensors 19, no. 23: 5061. https://doi.org/10.3390/s19235061




