Synthesis of MBA-Encoded Silver/Silica Core-Shell Nanoparticles as Novel SERS Tags for Biosensing Gibberellin A3 Based on Au@Fe3O4 as Substrate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Measurements
2.3. Synthesis of Ag Nanoparticles
2.4. Synthesis of AgNPs@SiO2 Core-Shell Nanoparticles
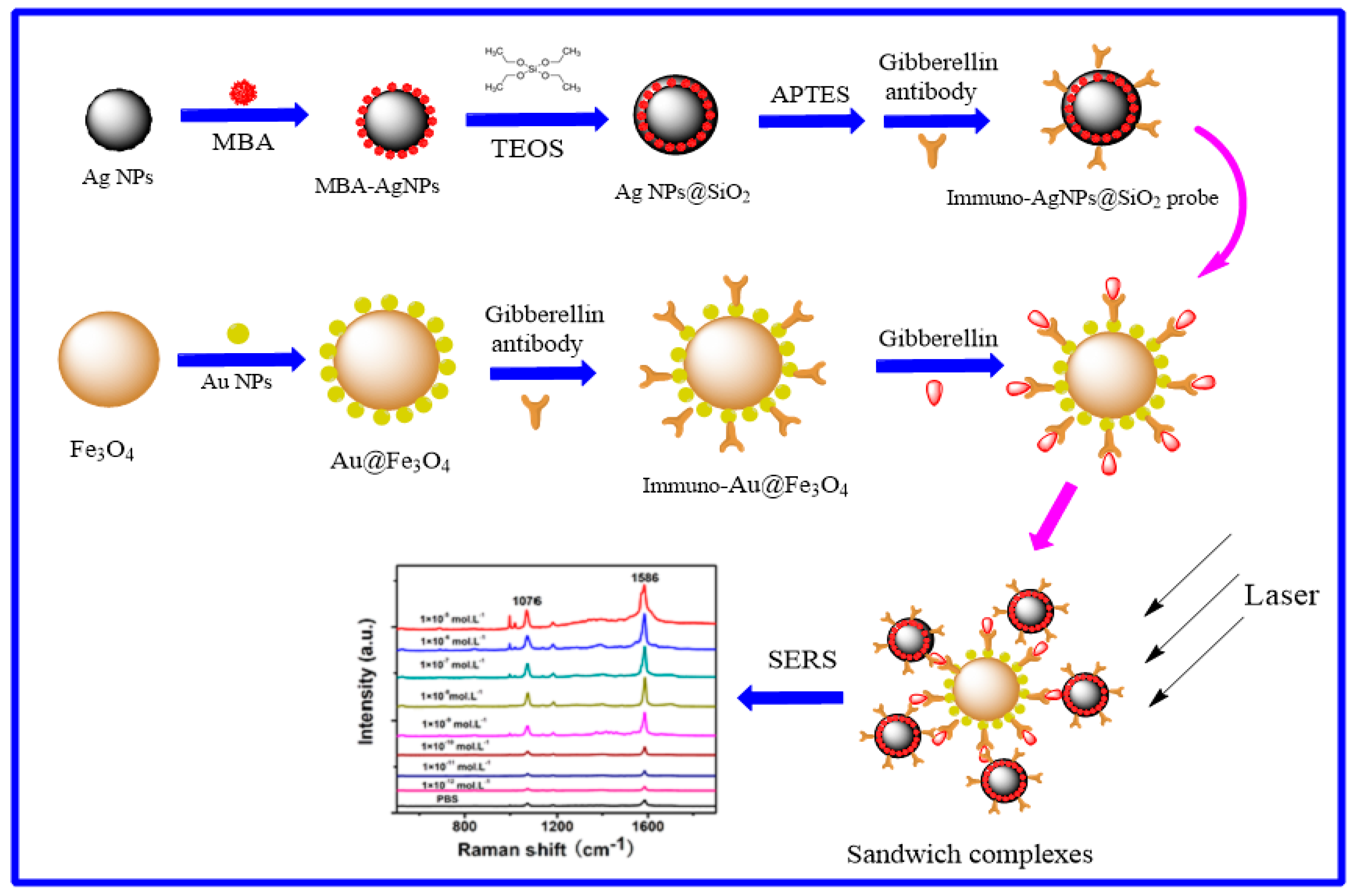
2.5. Construction of Immuno-Ag@SiO2 Core-Shell SERS Tags
2.6. Characterized of the Obtained Nanoparticles by SEM and TEM
2.7. Synthesis of Fe3O4 Nanoparticles
2.8. Synthesis of Au@Fe3O4 Nanoparticles
2.9. Assembly of Immuno-Au@Fe3O4 Nanoparticles
2.10. Immunoassay Detection of GA3 Based on SERS Tags and Magnetic Nanoparticles
3. Results and Discussion
3.1. UV–Vis Spectroscopy of AgNPs, MBA-AgNPs, Ag@SiO2 Core-Shell Nanoparticles
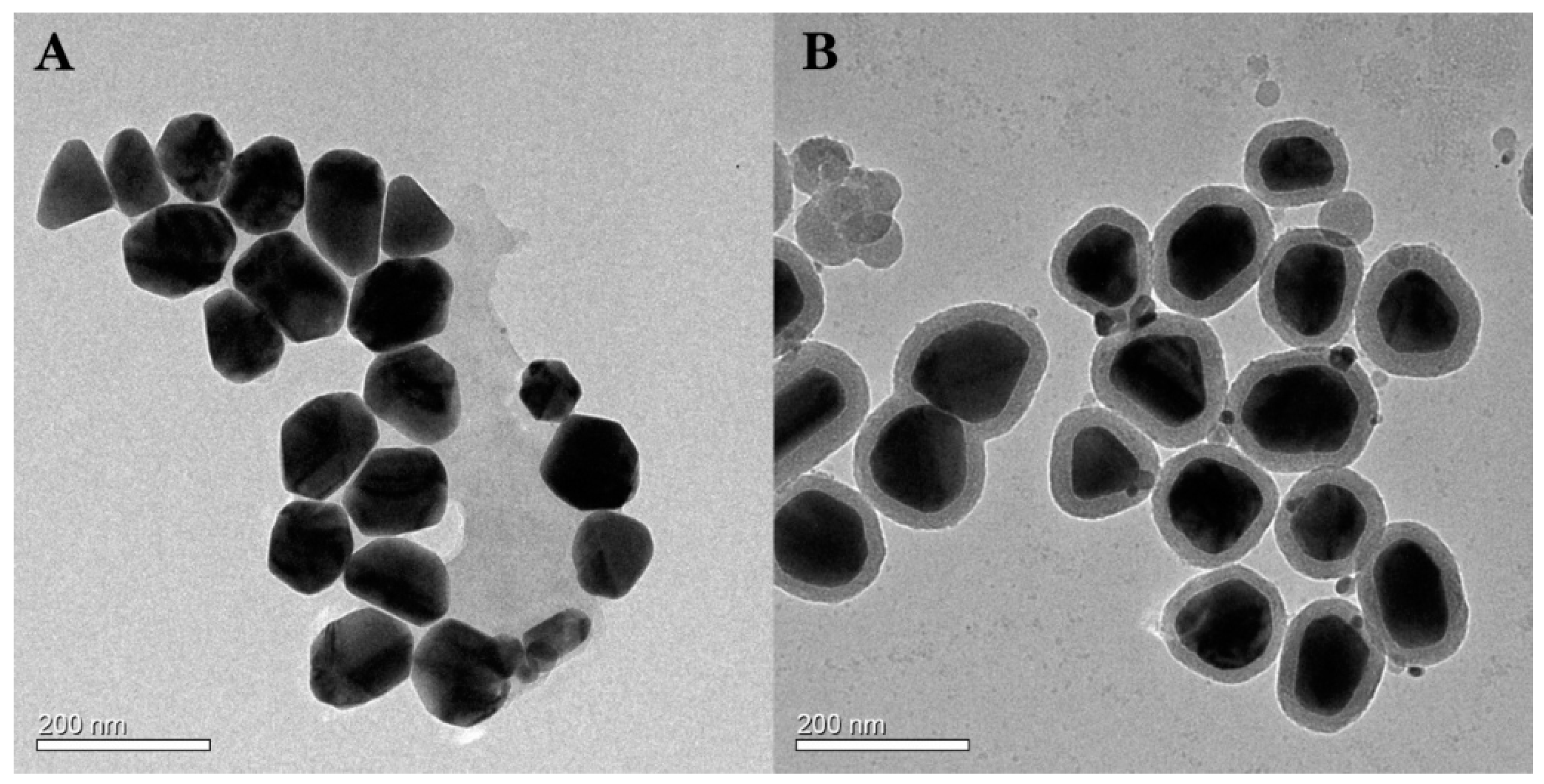
3.2. TEM of AgNPs and Ag@SiO2 Core-Shell Nanoparticles
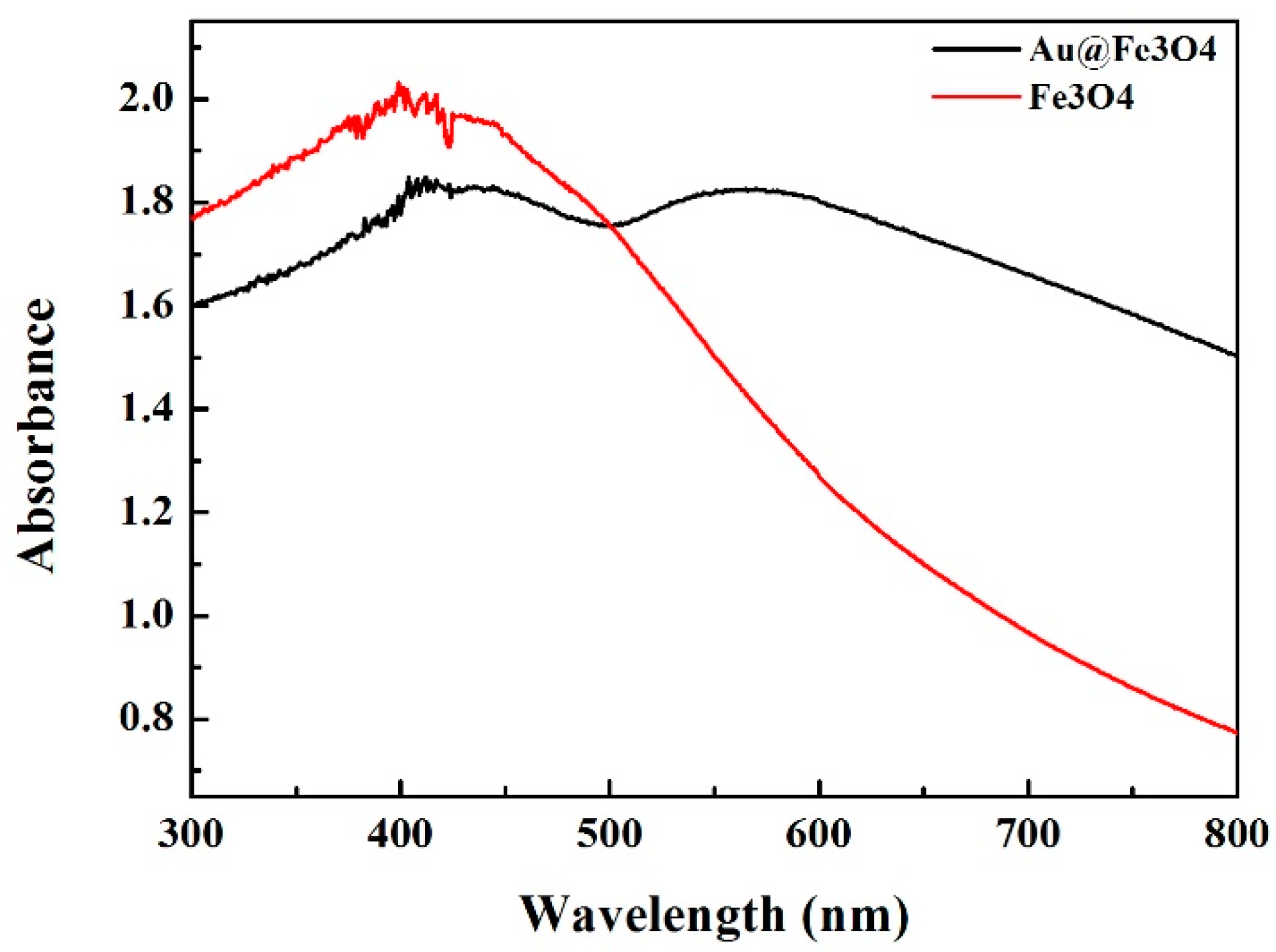
3.3. UV–Vis Spectroscopy of Fe3O4 and Au@Fe3O4 Nanoparticles
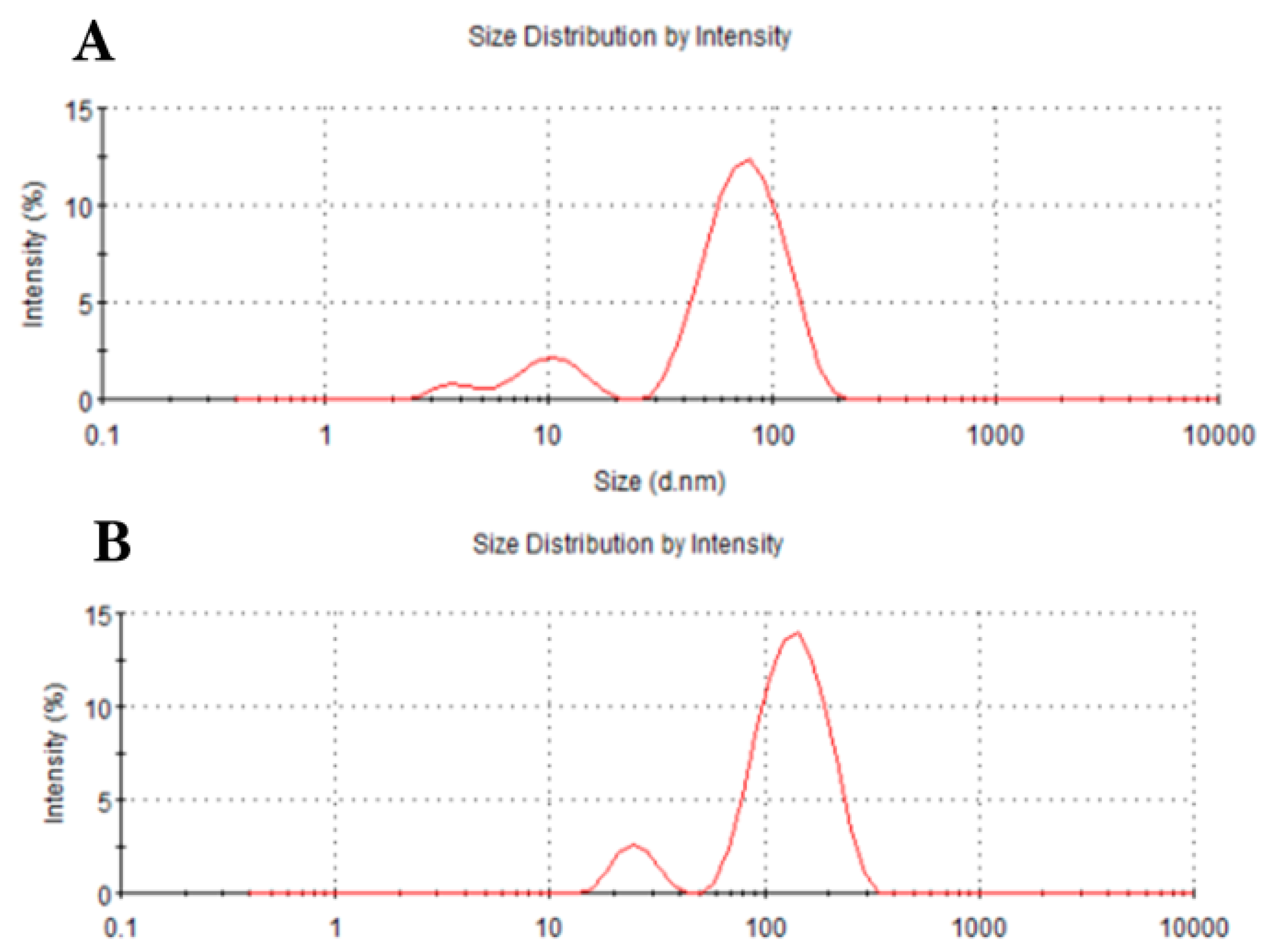
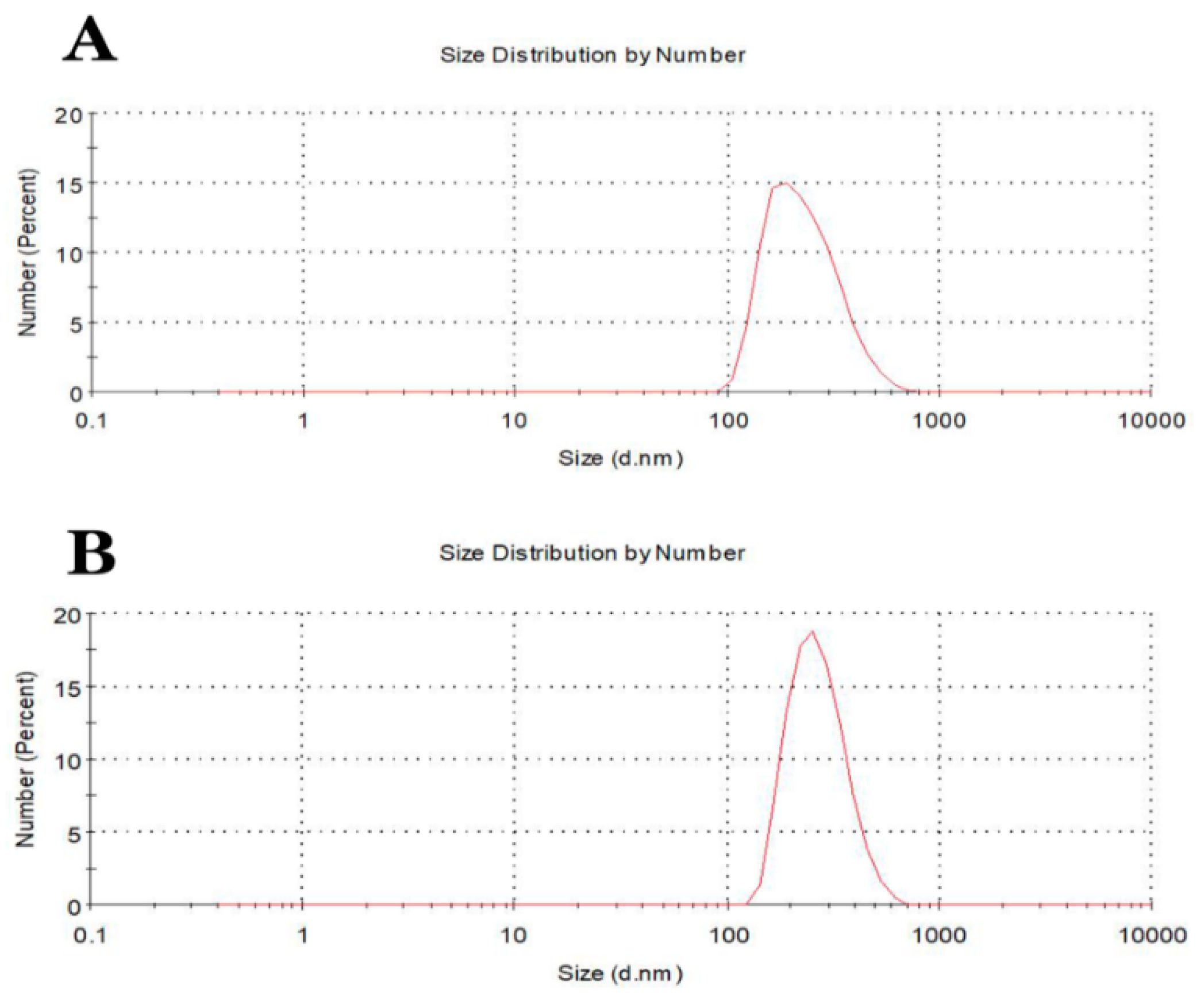
3.4. Dynamic Light Scattering of Fe3O4 and Au@Fe3O4 Nanoparticles
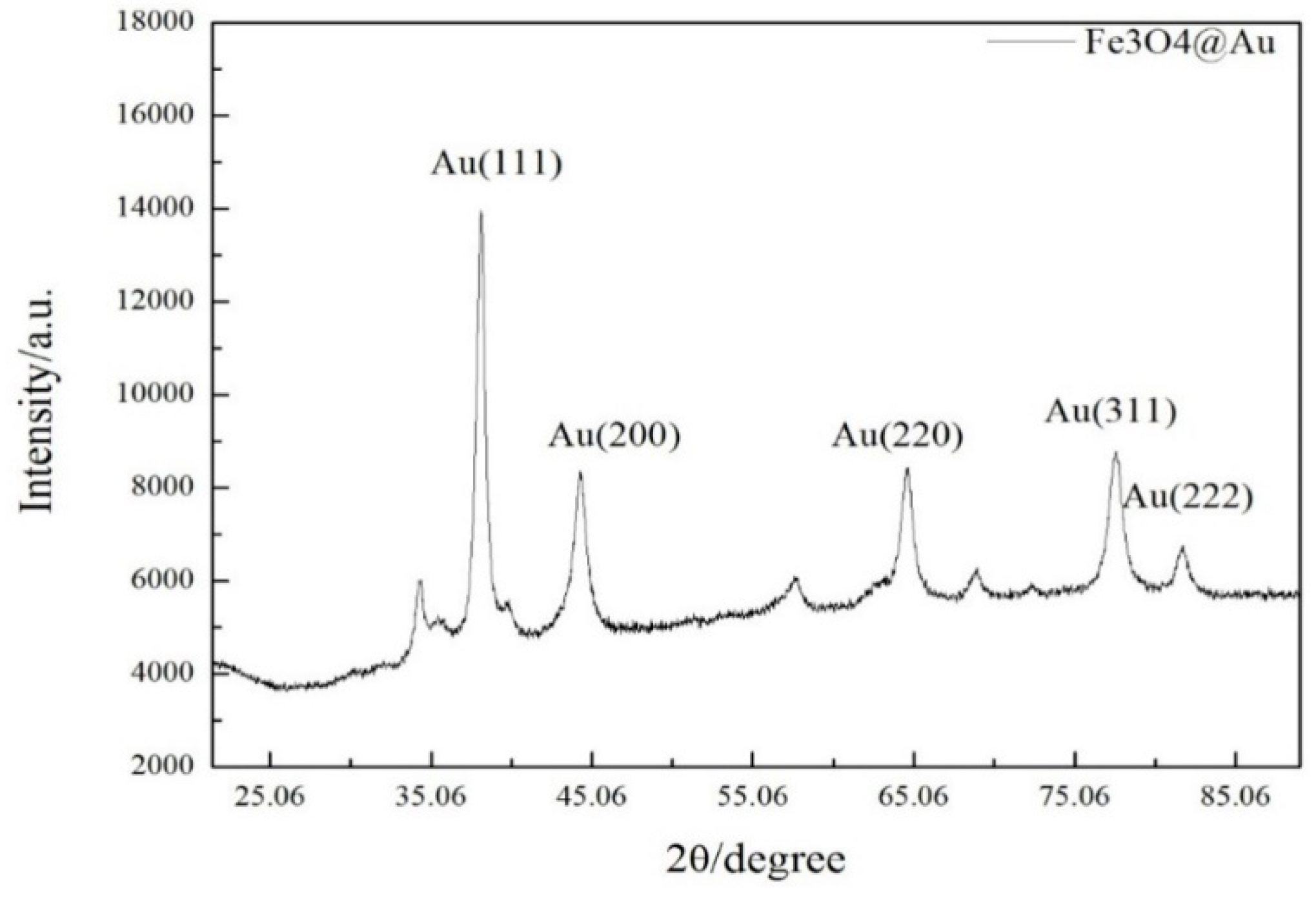
3.5. X-Ray Diffraction Characterization of Au@Fe3O4 Nanoparticles
3.6. SEM Characterization of Fe3O4 and Au@Fe3O4 Nanoparticles
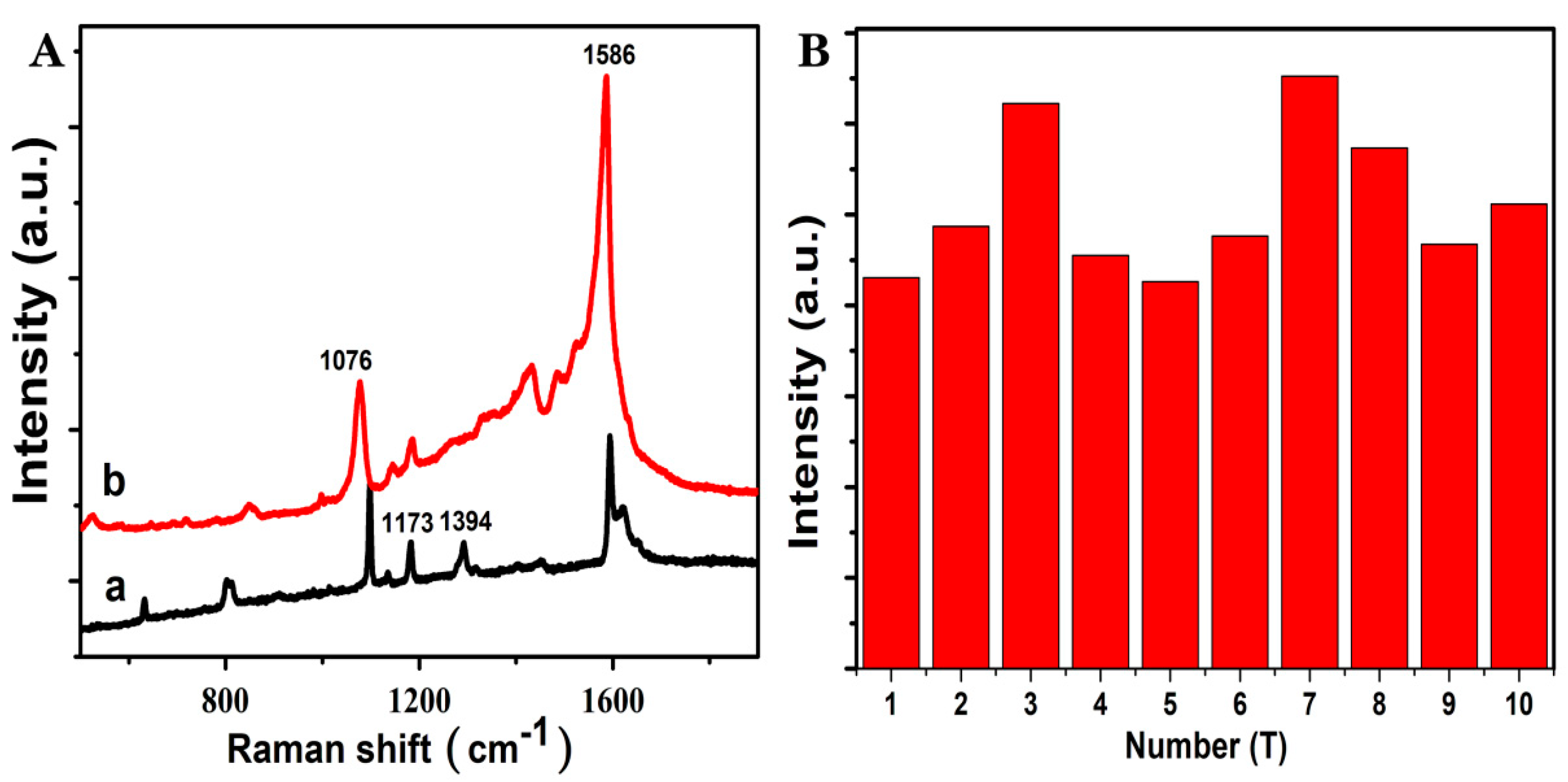
3.7. Raman Spectra of Solid MBA and AgNPs@SiO2 SERS Tags
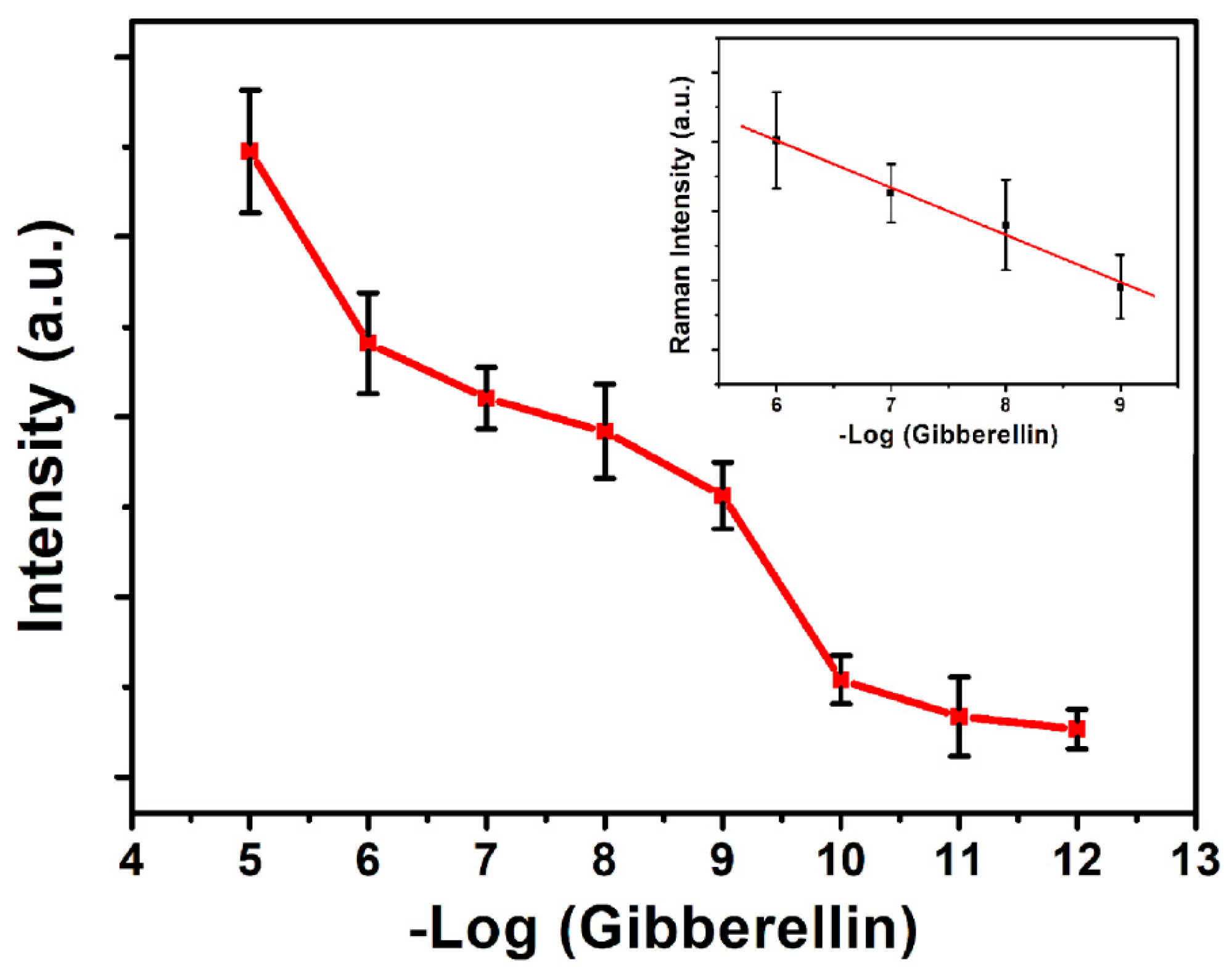
3.8. SERS Detection of GA3
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Olszewski, N.; Sun, T.-P.; Gubler, F. Gibberellin signaling: biosynthesis, catabolism, and response pathways. The Plant Cell 2002, 14, S61–S80. [Google Scholar] [CrossRef] [PubMed]
- Hedden, P.; Thomas, S.G. Gibberellin biosynthesis and its regulation. Biochem. J. 2012, 444, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Manzi, M.; Gómez-Cadenas, A.; Arbona, V. Rapid and reproducible determination of active gibberellins in citrus tissues by UPLC/ESI-MS/MS. Plant Physiol. Biochem. 2015, 94, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-H.; Cao, Y.-M.; Guo, X.-F.; Wang, H.; Zhang, H.-S. Determination of gibberellins using HPLC coupled with fluorescence detection. Anal. Methods 2016, 8, 1520–1526. [Google Scholar] [CrossRef]
- El-Mofty, M.; Sakr, S.; Rizk, A.; Moussa, E. Carcinogenic effect of gibberellin A3 in Swiss albino mice. Nutr. Cancer 1994, 21, 183–190. [Google Scholar] [CrossRef]
- El-Mofty, M.; Sakr, S. Induction of neoplasms in the Egyptian toad Bufo regularis by gibberellin A3. Oncology 1988, 45, 61–64. [Google Scholar] [CrossRef]
- Waqas, M.; Khan, A.L.; Kamran, M.; Hamayun, M.; Kang, S.-M.; Kim, Y.-H.; Lee, I.-J. Endophytic Fungi Produce Gibberellins and Indoleacetic Acid and Promotes Host-Plant Growth during Stress. Molecules 2012, 17, 10754–10773. [Google Scholar] [CrossRef]
- Nehela, Y.; Hijaz, F.; Elzaawely, A.A.; El-Zahaby, H.M.; Killiny, N. Phytohormone profiling of the sweet orange (Citrus sinensis (L.) Osbeck) leaves and roots using GC–MS-based method. J. Plant Physiol. 2016, 199, 12–17. [Google Scholar] [CrossRef]
- Hao, Y.-H.; Zhang, Z.; Wang, L.; Liu, C.; Lei, A.-W.; Yuan, B.-F.; Feng, Y.-Q. Stable isotope labeling assisted liquid chromatography–electrospray tandem mass spectrometry for quantitative analysis of endogenous gibberellins. Talanta 2015, 144, 341–348. [Google Scholar] [CrossRef]
- Pan, C.; Tan, S.N.; Yong, J.W.; Ge, L. Progress and development of analytical methods for gibberellins. J. Sep. Sci. 2017, 40, 346–360. [Google Scholar] [CrossRef]
- Wang, A.X.; Kong, X. Review of recent progress of plasmonic materials and nano-structures for surface-enhanced Raman scattering. Materials 2015, 8, 3024–3052. [Google Scholar] [CrossRef] [PubMed]
- Kneipp, K.; Wang, Y.; Kneipp, H.; Perelman, L.T.; Itzkan, I.; Dasari, R.; Feld, M.S. Single molecule detection using surface-enhanced Raman scattering (SERS). Phys. Rev. Lett. 1997, 78, 1667–1670. [Google Scholar] [CrossRef]
- Qian, X.M.; Peng, X.H.; Ansari, D.O.; Yin-Goen, Q.; Chen, G.Z.; Shin, D.M.; Yang, L.; Young, A.N.; Wang, M.D.; Nie, S.M. In vivo tumor targeting and spectroscopic detection with surface-enhanced Raman nanoparticle tags. Nat. Biotechnol. 2008, 26, 83–90. [Google Scholar] [CrossRef]
- Dies, H.; Raveendran, J.; Escobedo, C.; Docoslis, A. Rapid identification and quantification of illicit drugs on nanodendritic surface-enhanced Raman scattering substrates. Sens. Actuators, B Chem. 2018, 257, 382–388. [Google Scholar] [CrossRef]
- Alvarez-Puebla, R.A.; Liz-Marzan, L.M. Traps and cages for universal SERS detection. Chem. Soc. Rev. 2012, 41, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Pieczonka, N.P.W.; Aroca, R.F. Single molecule analysis by surfaced-enhanced Raman scattering. Chem. Soc. Rev. 2008, 37, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lin, D.; Li, M.; Yin, D.; Wang, S.; Wang, J. Ag@Au Core–Shell Porous Nanocages with Outstanding SERS Activity for Highly Sensitive SERS Immunoassay. Sensors 2019, 19, 1554. [Google Scholar] [CrossRef]
- Porter, M.D.; Lipert, R.J.; Siperko, L.M.; Wang, G.; Narayanana, R. SERS as a bioassay platform: Fundamentals, design, and applications. Chem. Soc. Rev. 2008, 37, 1001–1011. [Google Scholar] [CrossRef]
- Li, J.F.; Zhang, Y.J.; Ding, S.Y.; Panneerselvam, R.; Tian, Z.Q. Core-Shell Nanoparticle-Enhanced Raman Spectroscopy. Chem. Rev. 2017, 117, 5002–5069. [Google Scholar] [CrossRef]
- Kim, K.; Choi, N.; Jeon, J.H.; Rhie, G.-E.; Choo, J. SERS-Based Immunoassays for the Detection of Botulinum Toxins A and B Using Magnetic Beads. Sensors 2019, 19, 4081. [Google Scholar] [CrossRef]
- Deng, H.; Li, X.; Peng, Q.; Wang, X.; Chen, J.; Li, Y. Monodisperse Magnetic Single-Crystal Ferrite Microspheres. Angew. Chem. Int. Ed. 2005, 44, 2782–2785. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kong, X.; Lv, Z.; Zhou, S.; Du, X. Bifunctional quantum dot-decorated Ag@SiO2 nanostructures for simultaneous immunoassays of surface-enhanced Raman scattering (SERS) and surface-enhanced fluorescence (SEF). J. Mater. Chem. B 2013, 1, 2198–2204. [Google Scholar] [CrossRef]
- Lu, Y.; Yin, Y.; Li, Z.-Y.; Xia, Y. Synthesis and Self-Assembly of Au@SiO2 Core−Shell Colloids. Nano Lett. 2002, 2, 785–788. [Google Scholar] [CrossRef]
- Abdulrahman, H.B.; Krajczewski, J.; Aleksandrowska, D.; Kudelski, A. Silica-Protected Hollow Silver and Gold Nanoparticles: New Material for Raman Analysis of Surfaces. J. Phys. Chem. C 2015, 119, 20030–20038. [Google Scholar] [CrossRef]
- Orendorff, C.J.; Gole, A.; Sau, T.K.; Murphy, C.J. Surface-Enhanced Raman Spectroscopy of Self-Assembled Monolayers: Sandwich Architecture and Nanoparticle Shape Dependence. Anal. Chem. 2005, 77, 3261–3266. [Google Scholar] [CrossRef]
- Luo, Z.; Li, W.; Lu, D.; Chen, K.; He, Q.; Han, H.; Zou, M. A SERS-based immunoassay for porcine circovirus type 2 using multi-branched gold nanoparticles. Microchim. Acta 2013, 180, 1501–1507. [Google Scholar] [CrossRef]
- Yan, Z.; Nie, J.; Xu, G.; Li, H.; Li, J.; Li, Z.; Wu, Y.; Kuang, L. Simultaneous Determination of Plant Growth Regulators in Fruits Using a Modified QuEChERS Procedure and UPLC–MS/MS. Horticultural Plant J. 2016, 2, 203–208. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Q.; Lin, J.; Liu, F.; Wen, C.; Li, N.; Huang, G.; Luo, Z. Synthesis of MBA-Encoded Silver/Silica Core-Shell Nanoparticles as Novel SERS Tags for Biosensing Gibberellin A3 Based on Au@Fe3O4 as Substrate. Sensors 2019, 19, 5152. https://doi.org/10.3390/s19235152
Wei Q, Lin J, Liu F, Wen C, Li N, Huang G, Luo Z. Synthesis of MBA-Encoded Silver/Silica Core-Shell Nanoparticles as Novel SERS Tags for Biosensing Gibberellin A3 Based on Au@Fe3O4 as Substrate. Sensors. 2019; 19(23):5152. https://doi.org/10.3390/s19235152
Chicago/Turabian StyleWei, Qingmin, Jianjuan Lin, Fa Liu, Changchun Wen, Na Li, Guobao Huang, and Zhihui Luo. 2019. "Synthesis of MBA-Encoded Silver/Silica Core-Shell Nanoparticles as Novel SERS Tags for Biosensing Gibberellin A3 Based on Au@Fe3O4 as Substrate" Sensors 19, no. 23: 5152. https://doi.org/10.3390/s19235152



