Development of a Sensor with a Lipid/Polymer Membrane Comprising Na+ Ionophores to Evaluate the Saltiness Enhancement Effect
Abstract
:1. Introduction
2. Experimental Section
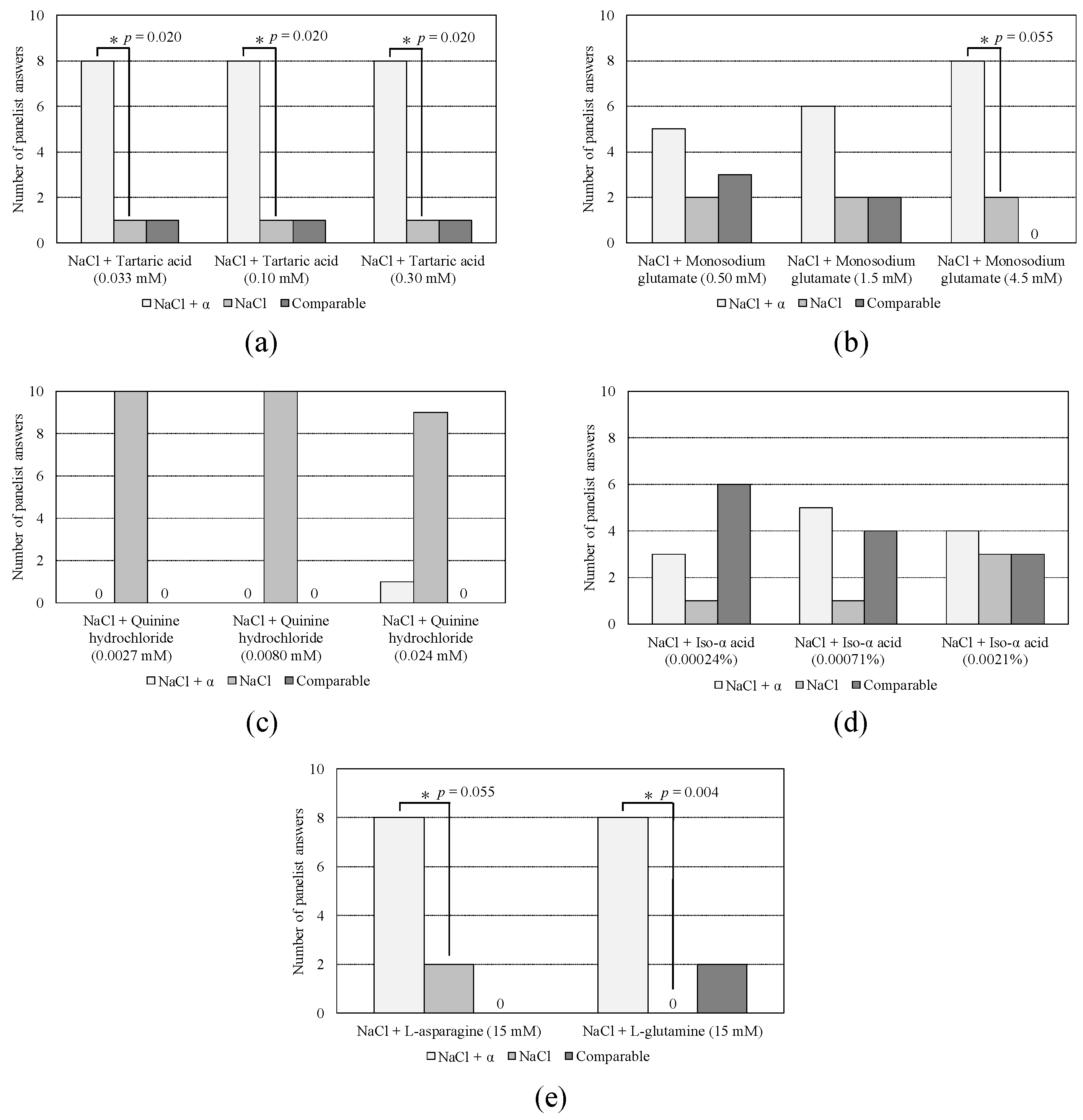
2.1. Sensory Test
2.2. Reagents
2.3. Lipid/Polymer Membrane
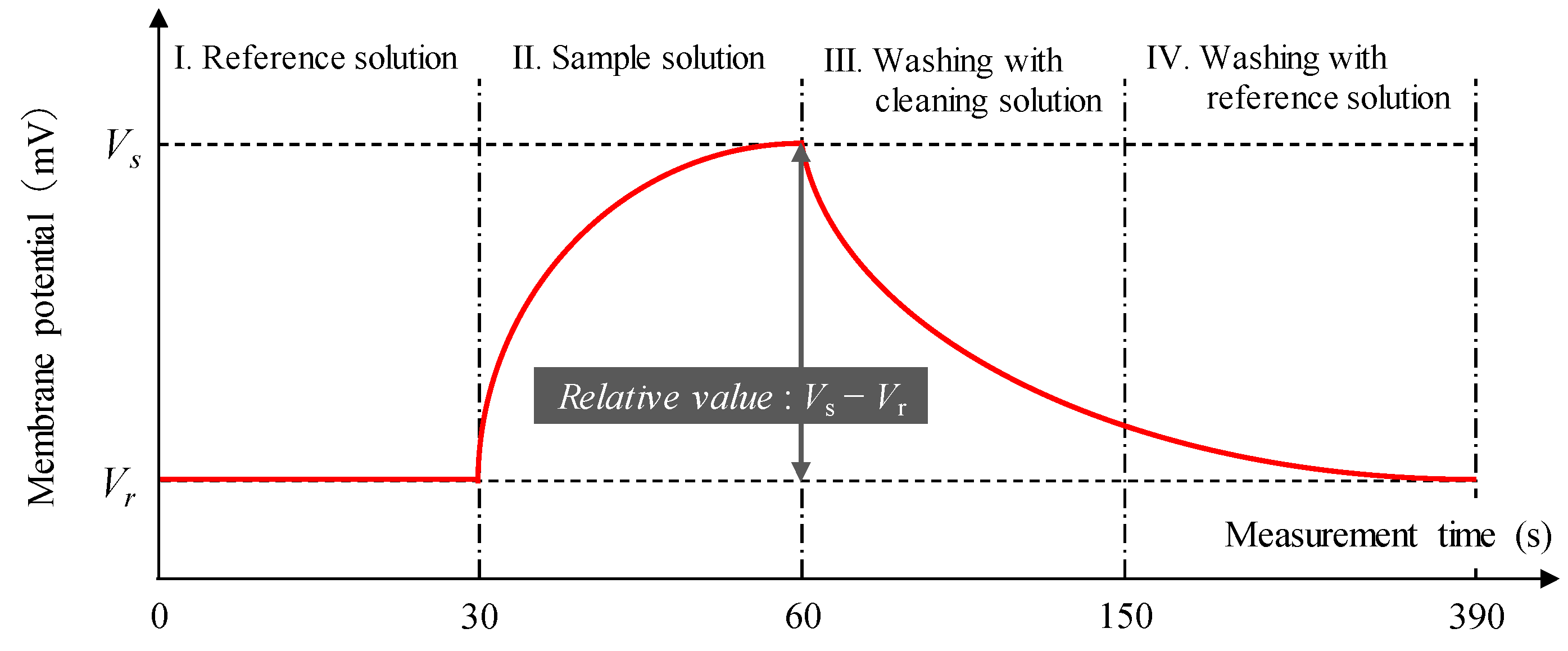
2.4. Measurement Procedure of Taste Sensor
2.5. Measurement of the Saltiness Enhancement Effect
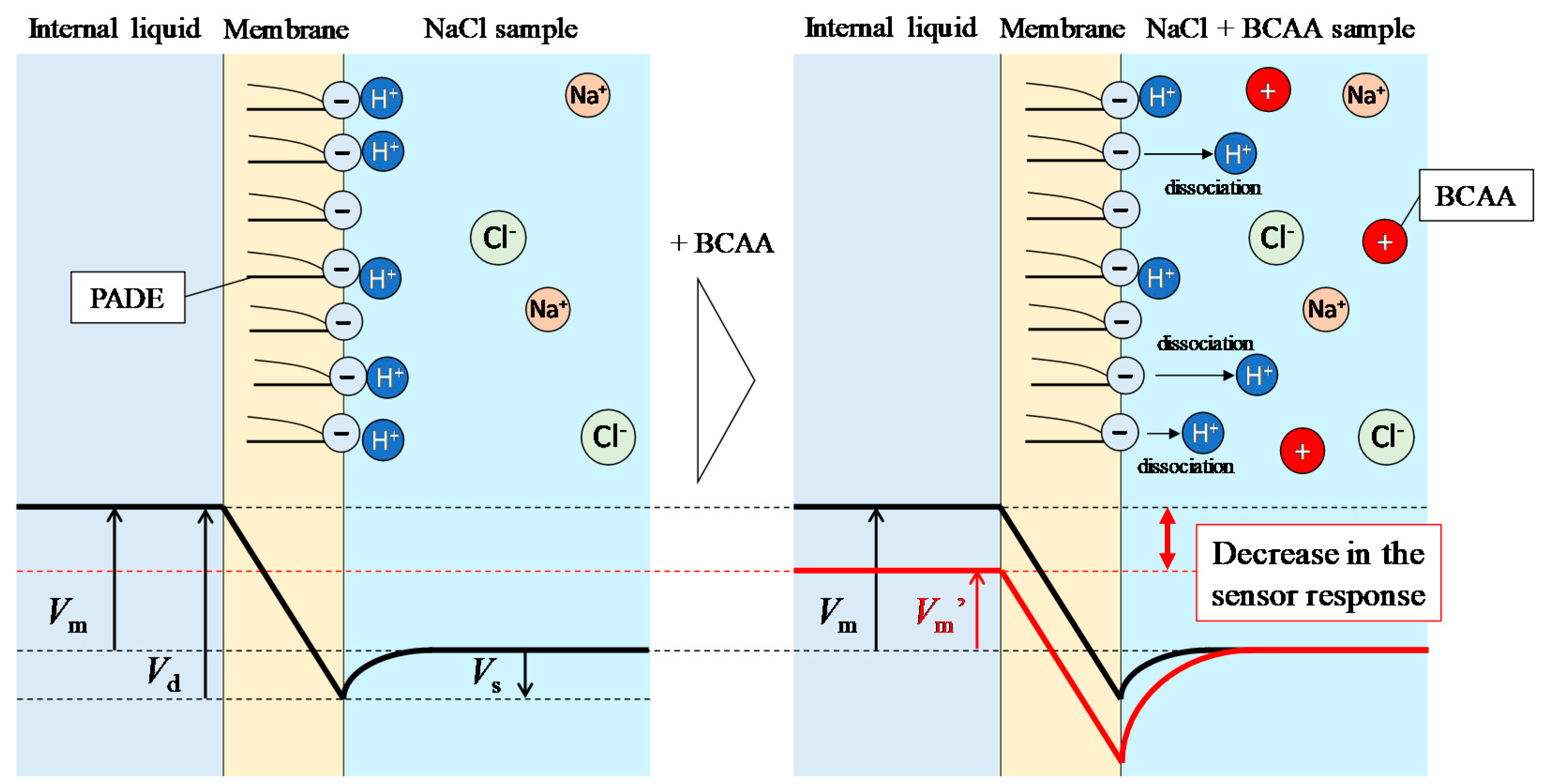
3. Results and Discussion
3.1. Sensory Test
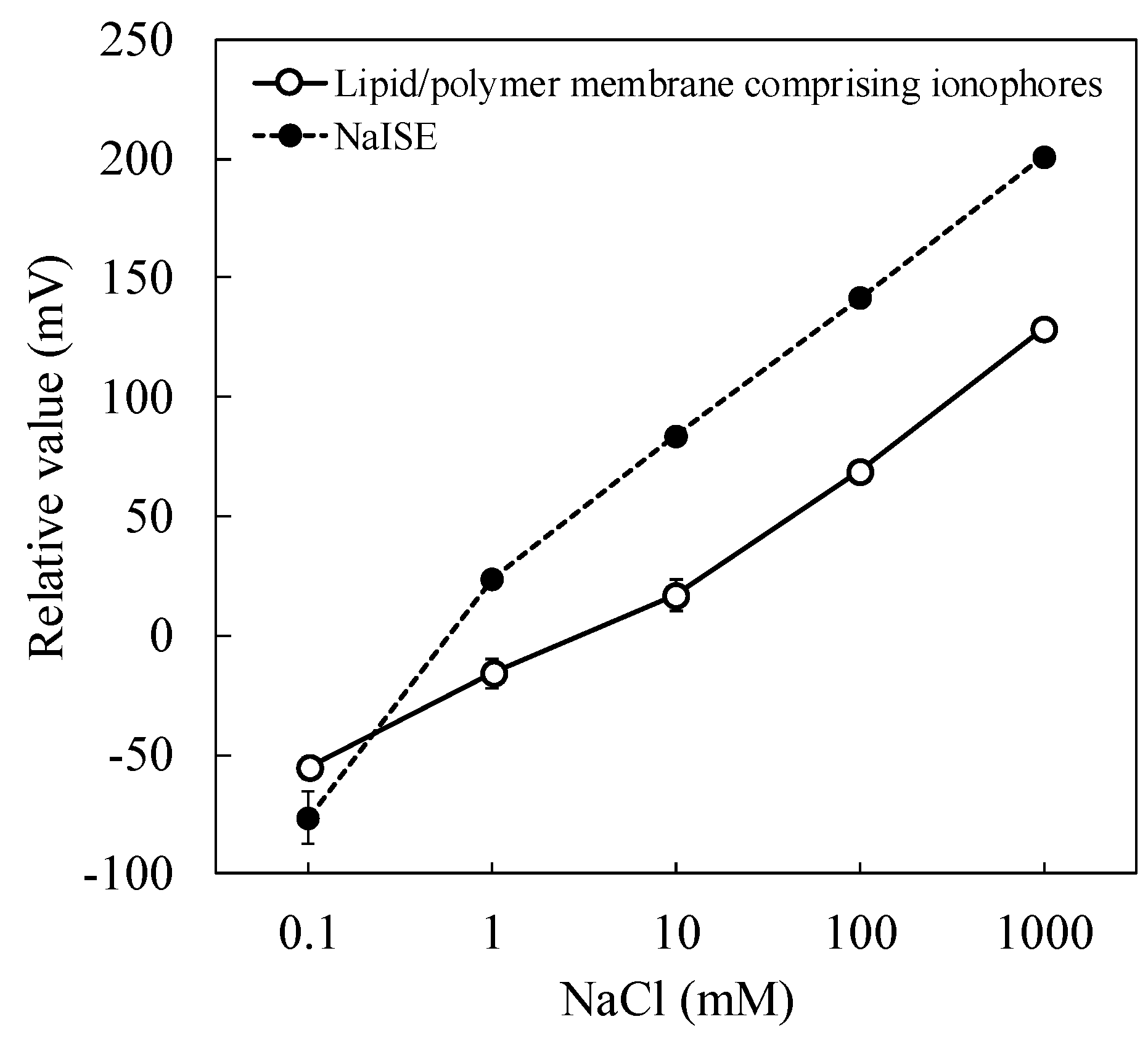
3.2. Response to NaCl
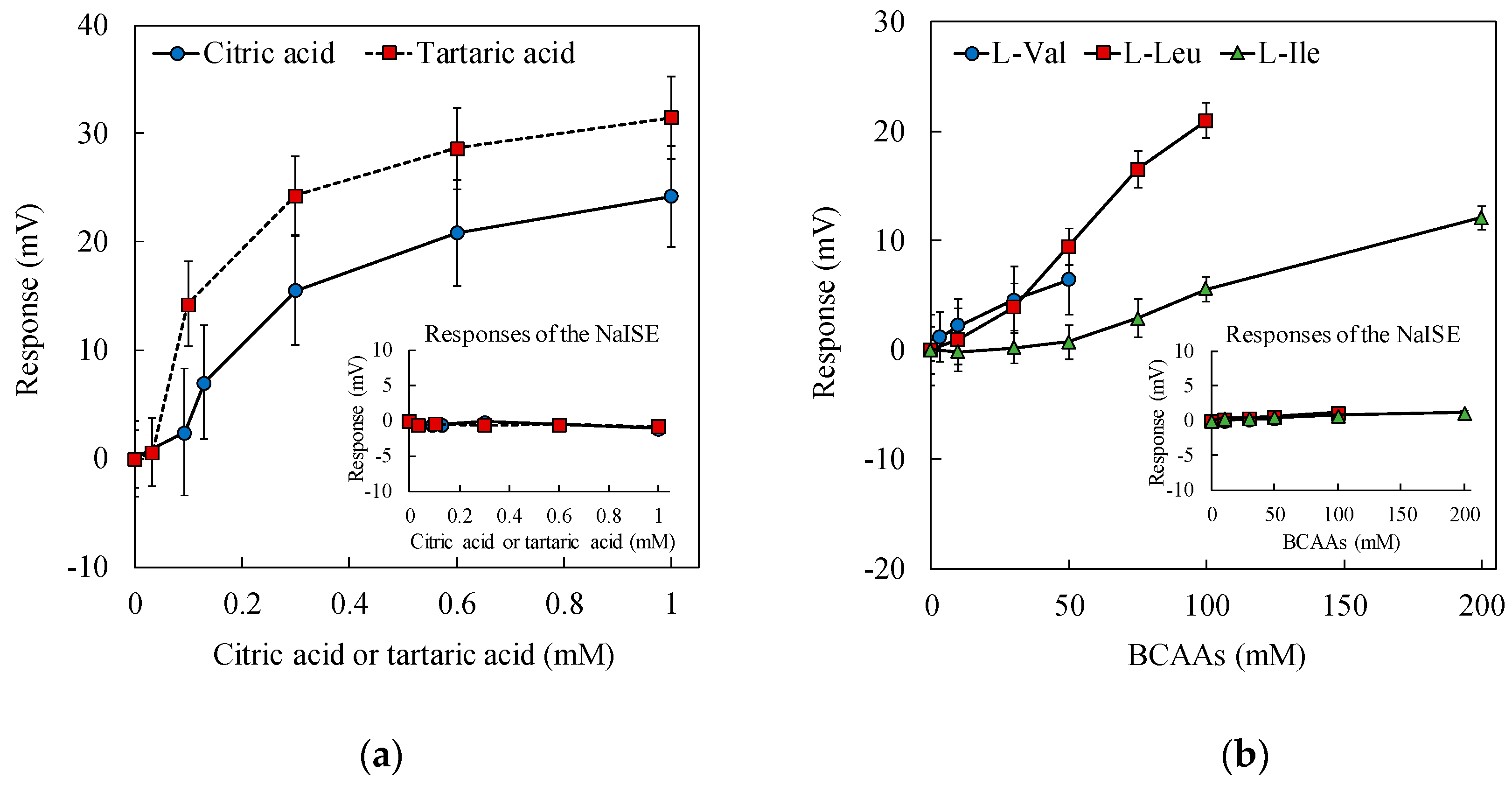
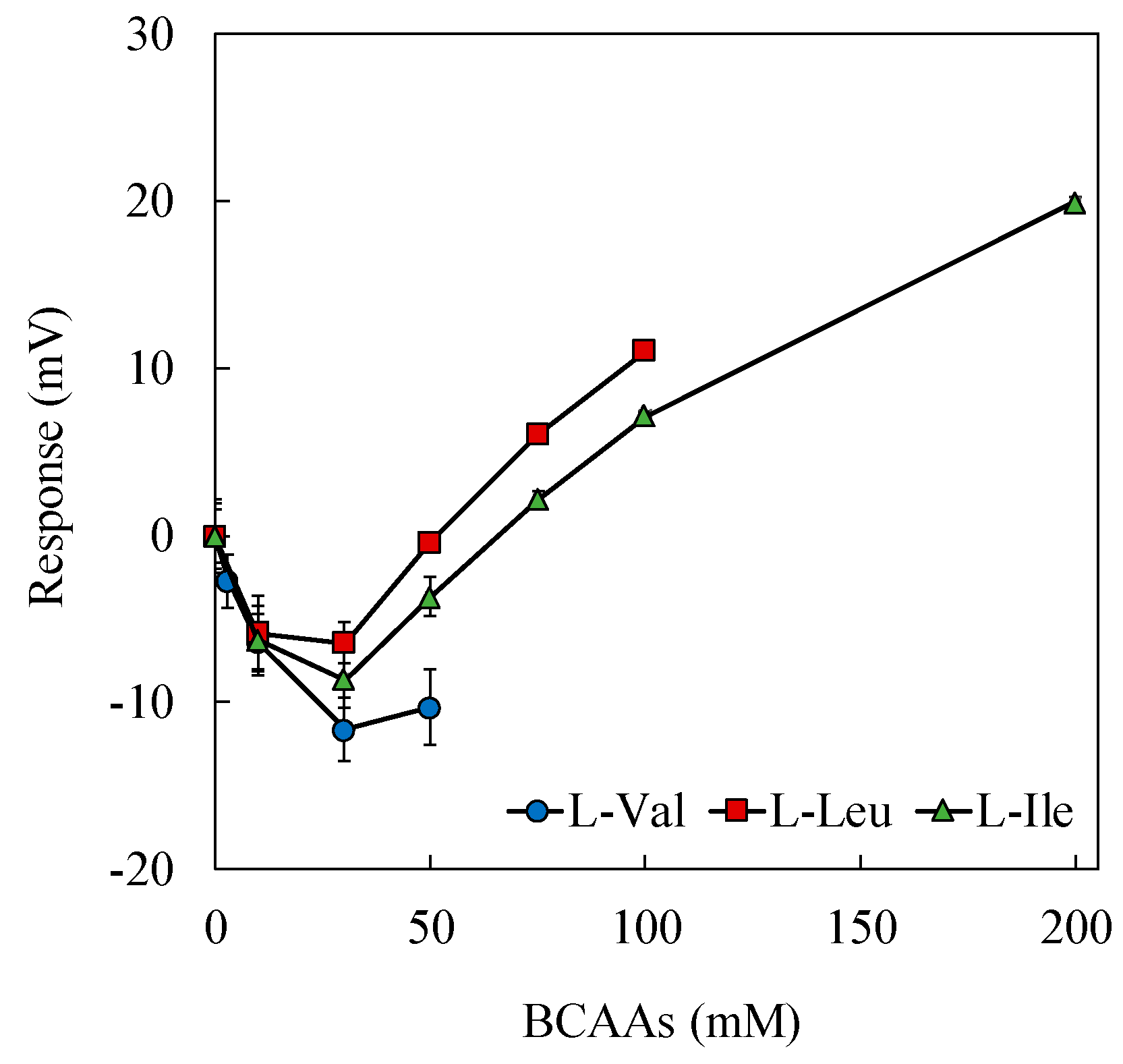
3.3. Response to 100 mM NaCl with Added Saltiness-Enhancing Substances
3.4. Response of Sensor with Low PADE Content
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dahl, L.K.; Love, R.A. Evidence for relationship sodium (chloride) intake and human essential hypertension. Arch. Intern. Med. 1954, 94, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Doyle, M.E.; Glass, K.A. Sodium reduction and its effect on food safety, food quality, and human health. Compr. Rev. Food Sci. Food Saf. 2010, 9, 44–56. [Google Scholar] [CrossRef]
- Tamura, M.; Seki, T.; Kawasaki, Y.; Tada, M.; Kikuchi, E.; Okai, H. An enhancing effect on the saltiness of sodium chloride of added amino acids and their esters. Agric. Biol. Chem. 1989, 53, 1625–1633. [Google Scholar]
- Japan Tobacco Inc. Seasoning Composition, Salty Taste-Like Taste Enhancer and Method for Enhancing Salty Taste-Like Taste of Food and Drink. E.P. Patent 2138053A4, 26 March 2008. [Google Scholar]
- Harada, Y.; Sakimori, M.; Nishimura, T. Utilization of L-leucine can reduce the salt content in food. Jpn. J. Tast. Smell Res. 2007, 14, 441–442. (In Japanese) [Google Scholar]
- Hiroshima University. Salty Taste Enhancer, Food and Drink and Method for Producing Food and Drink. U.S. Patent 20100075017A1, 28 March 2008. [Google Scholar]
- Kino, H.; Kakutani, M.; Hattori, K.; Tojo, H.; Komai, T.; Nammoku, T.; Kino, K. Screening of salt taste enhancing dipeptides based on a new strategy using L-amino acid ligase. Nippon Shokuhin Kagaku Kogaku Kaishi 2015, 62, 274–281. (In Japanese) [Google Scholar] [CrossRef]
- Ishikawa, K.; Takahashi, Y.; Endo, Y.; Sato, F.; Ogasawara, M.; Okuyama, K.; Kumagai, M.; Akiyama, Y.; Matsunaga, R. Novel development of ume salt enhanced saltiness. Bull. Soc. Sea Water Sci. Jpn. 2013, 67, 219–223. (In Japanese) [Google Scholar]
- Schindler, A.; Dunkel, A.; Stähler, F.; Backes, M.; Ley, J.; Meyerhof, W.; Hofmann, T. Discovery of salt taste enhancing arginyl dipeptides in protein digests and fermented fish sauces by means of a sensomics approach. J. Agric. Food Chem. 2011, 59, 12578–12588. [Google Scholar] [CrossRef]
- Nakagawa, T.; Kohori, J.; Koike, S.; Katsuragi, Y.; Shoji, T. Sodium aspartate as a specific enhancer of salty taste perception-sodium aspartate is a possible candidate to decrease excessive intake of dietary salt. Chem. Senses 2014, 39, 781–786. [Google Scholar] [CrossRef]
- Shimoda, M. Application of the linking response of taste and odor to salt-reduced food. Jpn. J. Tast. Smell Res. 2015, 22, 151–156. (In Japanese) [Google Scholar]
- Lawrence, G.; Salles, C.; Septier, C.; Busch, J.; Thomas-Danguin, T. Odour-taste interactions: A way to enhance saltiness in low-salt content solutions. Food Qual. Prefer. 2009, 20, 241–248. [Google Scholar] [CrossRef]
- Shimoda, M. Does shoyu odor strengthen salty flavor? Jpn. J. Tast. Smell Res. 2007, 14, 3–8. (In Japanese) [Google Scholar]
- Miyazawa, T. Enhancement effect of salt taste based on flavor technology. Jpn. J. Tast. Smell Res. 2015, 22, 5–10. (In Japanese) [Google Scholar]
- Kawai, T. The enhancing effect of saltiness in foods and the estimation. Nippon Jozokyokai Shi 2017, 112, 22–28. (In Japanese) [Google Scholar]
- Anand, V.; Kataria, M.; Kukkar, V.; Saharan, V.; Choudhury, P.K. The latest trends in the taste assessment of pharmaceuticals. Drug Discov. Today 2007, 12, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Riul, A., Jr.; Dantas, C.A.R.; Miyazaki, C.M.; Oliveira, O.N., Jr. Recent advances in electronic tongues. Analyst 2010, 135, 2481–2495. [Google Scholar] [CrossRef]
- Toko, K. Biomimetic Sensor Technology; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Kobayashi, Y.; Habara, M.; Ikezazki, H.; Chen, R.; Naito, Y.; Toko, K. Advanced taste sensors based on artificial lipids with global selectivity to basic taste qualities and high correlation to sensory scores. Sensors 2010, 10, 3411–3443. [Google Scholar] [CrossRef]
- Tahara, Y.; Toko, K. Electronic tongues-a review. IEEE Sens. J. 2013, 13, 3001–3011. [Google Scholar] [CrossRef]
- Zhang, H.; Zou, G.; Liu, X.; Xiao, Y.; Wang, W. Identification of Xinyang Maojian Tea Taste Using Electronic Tongue. Sens. Mater. 2019, 31, 2347–2356. [Google Scholar] [CrossRef]
- Haraguchi, T.; Uchida, T.; Yoshida, M.; Kojima, H.; Habara, M.; Ikezaki, H. The utility of the artificial taste sensor in evaluating the bitterness of drugs: Correlation with responses of human TASTE2 receptors (hTAS2Rs). Chem. Pharm. Bull. 2018, 66, 71–77. [Google Scholar] [CrossRef]
- Winquist, F. Voltammetric electronic tongues—Basic principles and applications. Microchim. Acta 2008, 163, 3–10. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, S.; Kumar, A.; Sharma, A.; Kumar, R.; Kaur, R.; Bhondekar, A.P. Development of lipid membrane based taste sensors for electronic tongue. Procedia Comput. Sci. 2015, 70, 146–152. [Google Scholar] [CrossRef]
- Ciosek, P.; Wróblewski, W. Sensor arrays for liquid sensing—electronic tongue systems. Analyst 2007, 132, 963–978. [Google Scholar] [CrossRef] [PubMed]
- Vlasov, Y.; Legin, A.; Rudnitskaya, A.; Di Natale, C.; D’Amico, A. Nonspecific sensor arrays (“electronic tongue”) for chemical analysis of liquids. Pure Appl. Chem. 2005, 77, 1965–1983. [Google Scholar] [CrossRef]
- Citterio, D.; Suzuki, K. Smart taste sensors. Anal. Chem. 2008, 80, 3965–3972. [Google Scholar]
- Nuñez, L.; Cetó, X.; Pividori, M.I.; Zanoni, M.V.B.; del Valle, M. Development and application of an electronic tongue for detection and monitoring of nitrate, nitrite and ammonium levels in waters. Microchem. J. 2013, 110, 273–279. [Google Scholar] [CrossRef]
- Podrazka, M.; Báczyńska, E.; Kundys, M.; Jeleń, P.S.; Nery, E.W. Electronic tongue—A tool for all tastes? Biosensors 2018, 8, 3. [Google Scholar] [CrossRef]
- Wu, X.; Onitake, H.; Haraguchi, T.; Tahara, Y.; Yatabe, R.; Yoshida, M.; Uchida, T.; Ikezaki, H.; Toko, K. Quantitative prediction of bitterness masking effect of high-potency sweeteners using taste sensor. Sens. Actuators B 2016, 235, 11–17. [Google Scholar] [CrossRef]
- Toko, K. Taste sensor. Sens. Actuators B 2000, 64, 205–215. [Google Scholar] [CrossRef]
- Chen, R.; Hidekazu, I.; Toko, K. Development of sensor with high selectivity for saltiness and its application in taste evaluation of table salt. Sens. Mater. 2010, 22, 313–325. [Google Scholar]
- Bakker, E.; Bühlmann, P.; Pretsch, E. Carrier-based ion-selective electrodes and bulk optodes. 1. General characteristics. Chem. Rev. 1997, 97, 3083–3132. [Google Scholar] [CrossRef]
- Kimura, K.; Yoshinaga, M.; Funaki, K.; Shibutani, Y.; Yakabe, K.; Shono, T.; Kasai, M.; Mizufune, H.; Tanaka, M. Effects of α-substituents on ion selectivity of bis(12-crown-4-methyl) malonates as neutral carriers for sodium ion-selective electrodes. Anal. Sci. 1996, 12, 67–70. [Google Scholar] [CrossRef]
- Indow, T. A general equi-distance scale of the four qualities of taste. Jpn. Psychol. Res. 1966, 8, 136–150. [Google Scholar] [CrossRef]
- Okai, H.; Tamura, M. An enhanced effect of saltiness of sodium chloride by peptides and their analogs. Nippon Shokuhin Kogyo Gakkaishi 1989, 36, 769–776. (In Japanese) [Google Scholar] [CrossRef]
- Habara, M.; Toko, K. Biomimetic membrane for taste sensing. In Bottom-Up Nanofabrication; Ariga, K., Nalwa, H.S., Eds.; American Scientific Publishers: Valencia, CA, USA, 2009; Volume 6, pp. 91–109. [Google Scholar]
- Kolpin, K.M. Human Bitterness Detection Thresholds of Hop Acids in Beer and Honey. Master’s Thesis, Oregon State University, Corvallis, OR, USA, June 2008. [Google Scholar]
- Shimomura, Y.; Yamamoto, Y.; Bajotto, G.; Sato, J.; Murakami, T.; Shimomura, N.; Kobayashi, H.; Mawatari, K. Nutraceutical effects of branched-chain amino acids on skeletal muscle. J. Nutr. 2006, 136, 529S–532S. [Google Scholar] [CrossRef] [PubMed]
- Marchesini, G.; Bianchi, G.; Merli, M.; Amodio, P.; Panella, C.; Loguercio, C.; Rossi Fanelli, F.; Abbiati, R. Nutritional supplementation with branched-chain amino acids in advanced cirrhosis: A double-blind, randomized trial. Gastroenterology 2003, 124, 1792–1801. [Google Scholar] [CrossRef]
- Träuble, H.; Teubner, M.; Woolley, P.; Eibl, H. Electrostatic interactions at charged lipid membranes: I. Effects of pH and univalent cations on membrane structure. Biophys. Chem. 1976, 4, 319–342. [Google Scholar] [CrossRef]


| Sample | Composition |
|---|---|
| Saltiness sample | NaCl (1%) |
| Sourness sample | Tartaric acid (0.033, 0.10, 0.30 mM) |
| Umami sample | Monosodium glutamate (0.50, 1.5, 4.5 mM) |
| Bitterness for medicine sample | Quinine hydrochloride dehydrate (0.0027, 0.0080, 0.024 mM) |
| Bitterness for food sample | Iso-α acid (0.00024, 0.00071, 0.0021%) |
| Amino acid sample | L-asparagine monohydrate (15 mM) L-glutamine (15 mM) |
| Composition | Concentration |
|---|---|
| NaCl | 100 mM NaCl |
| NaCl + L-Valine | 100 mM NaCl + L-valine (3, 10, 30, 50 mM) |
| NaCl + L-Leucine | 100 mM NaCl + L-leucine (10, 30, 50, 75, 100 mM) |
| NaCl + L-Isoleucine | 100 mM NaCl + L-isoleucine (10, 30, 50, 75, 100, 200 mM) |
| NaCl + Citric acid | 100 mM NaCl + Citric acid (0.094, 0.13, 0.3, 1 mM) |
| NaCl + Tartaric acid | 100 mM NaCl + Tartaric acid (0.033, 0.1, 0.3, 0.61 mM) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakatani, F.; Ienaga, T.; Wu, X.; Tahara, Y.; Ikezaki, H.; Sano, H.; Muto, Y.; Kaneda, Y.; Toko, K. Development of a Sensor with a Lipid/Polymer Membrane Comprising Na+ Ionophores to Evaluate the Saltiness Enhancement Effect. Sensors 2019, 19, 5251. https://doi.org/10.3390/s19235251
Nakatani F, Ienaga T, Wu X, Tahara Y, Ikezaki H, Sano H, Muto Y, Kaneda Y, Toko K. Development of a Sensor with a Lipid/Polymer Membrane Comprising Na+ Ionophores to Evaluate the Saltiness Enhancement Effect. Sensors. 2019; 19(23):5251. https://doi.org/10.3390/s19235251
Chicago/Turabian StyleNakatani, Futa, Tomofumi Ienaga, Xiao Wu, Yusuke Tahara, Hidekazu Ikezaki, Hiroyuki Sano, Yuki Muto, Yuya Kaneda, and Kiyoshi Toko. 2019. "Development of a Sensor with a Lipid/Polymer Membrane Comprising Na+ Ionophores to Evaluate the Saltiness Enhancement Effect" Sensors 19, no. 23: 5251. https://doi.org/10.3390/s19235251





