Hydrophobic Paper-Based SERS Sensor Using Gold Nanoparticles Arranged on Graphene Oxide Flakes
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Reagents
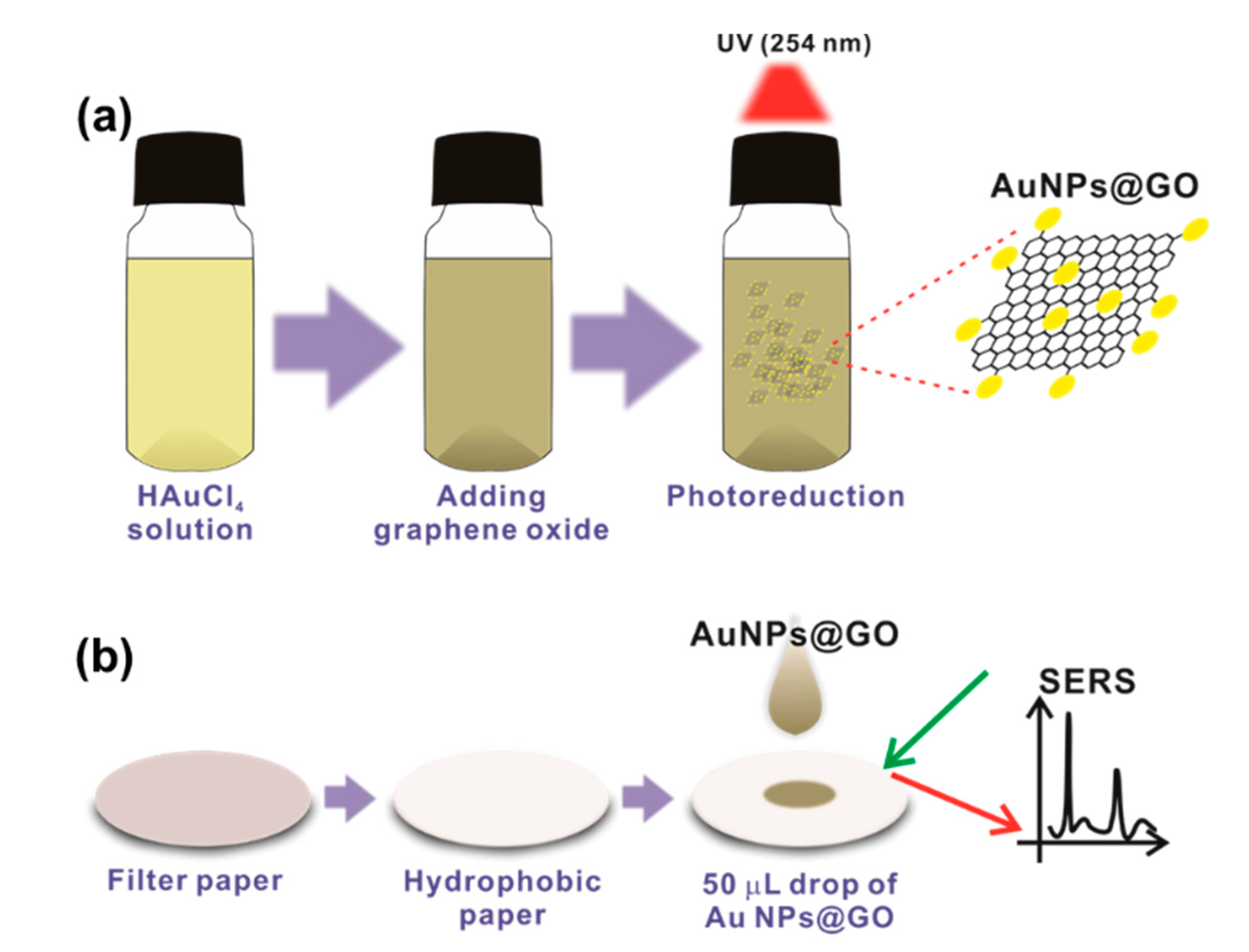
2.2. Photosynthesis of AuNPs@GO
2.3. Fabrication of the h-Paper-Based SERS Sensor
2.4. Characterization and Measurements
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Kim, W.; Lee, S.H.; Kim, J.H.; Ahn, Y.J.; Kim, Y.H.; Yu, J.S.; Choi, S. Paper-Based Surface-Enhanced Raman Spectroscopy for Diagnosing Prenatal Diseases in Women. ACS Nano 2018, 12, 7100–7108. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.Y.; Man, P.H.; Huo, Y.Y.; Ning, T.Y.; Li, C.H.; Man, B.Y.; Yang, C. Synthesis of the 3D AgNF/AgNP arrays for the paper-based surface enhancement Raman scattering application. Sens. Actuators B Chem. 2018, 265, 302–309. [Google Scholar] [CrossRef]
- Wei, W.; Du, Y.X.; Zhang, L.M.; Yang, Y.; Gao, Y.F. Improving SERS hot spots for on- site pesticide detection by combining silver nanoparticles with nanowires. J. Mater. Chem. C 2018, 6, 8793–8803. [Google Scholar] [CrossRef]
- Willets, K.A.; Duyne, R.P.V. Localized Surface Plasmon Resonance Spectroscopy and Sensing. Annu. Rev. Phys. Chem. 2007, 58, 267–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cialla-May, D.; Zheng, X.S.; Weber, K.; Popp, J. Recent progress in surface-enhanced Raman spectroscopy for biological and biomedical applications: From cells to clinics. Chem. Soc. Rev. 2017, 46, 3945–3961. [Google Scholar] [CrossRef]
- Lai, H.S.; Xu, F.G.; Zhang, Y.; Wang, L. Recent progress on graphene-based substrates for surface-enhanced Raman scattering applications. J. Mater. Chem. B 2018, 6, 4008–4028. [Google Scholar] [CrossRef]
- Xu, K.; Zhou, R.; Takei, K.; Hong, M. Toward Flexible Surface-Enhanced Raman Scattering (SERS) Sensors for Point-of-Care Diagnostics. Adv. Sci. 2019, 6, 1900925. [Google Scholar] [CrossRef]
- Wang, C.; Wong, K.W.; Wang, Q.; Zhou, Y.F.; Tang, C.Y.; Fan, M.K.; Mei, J.; Lau, W.M. Silver-nanoparticles-loaded chitosan foam as a flexible SERS substrate for active collecting analytes from both solid surface and solution. Talanta 2019, 191, 241–247. [Google Scholar] [CrossRef]
- Chen, J.; Huang, M.Z.; Kong, L.L.; Lin, M.S. Jellylike flexible nanocellulose SERS substrate for rapid in-situ non-invasive pesticide detection in fruits/vegetables. Carbohydr. Polym. 2019, 205, 596–600. [Google Scholar] [CrossRef]
- Wang, Y.C.; Jin, Y.H.; Xiao, X.Y.; Zhang, T.F.; Yang, H.T.; Zhao, Y.D.; Wang, J.P.; Jiang, K.L.; Fan, S.S.; Li, Q.Q. Flexible, transparent and highly sensitive SERS substrates with cross-nanoporous structures for fast on-site detection. Nanoscale 2018, 10, 15195–15204. [Google Scholar] [CrossRef]
- Wang, K.H.; Huang, M.Z.; Chen, J.; Lin, L.L.; Kong, L.L.; Liu, X.; Wang, H.; Lin, M.S. A “drop-wipe-test” SERS method for rapid detection of pesticide residues in fruits. J. Raman Spectrosc. 2018, 49, 493–498. [Google Scholar] [CrossRef]
- Wang, C.M.; Roy, P.K.; Juluri, B.K.; Chattopadhyaya, S. A SERS tattoo for in situ, ex situ, and multiplexed detection of toxic food additives. Sens. Actuators B Chem. 2018, 261, 218–225. [Google Scholar] [CrossRef]
- Kim, K.; Choi, N.; Jeon, J.H.; Rhie, G.E.; Choo, J. SERS-Based Immunoassays for the Detection of Botulinum Toxins A and B Using Magnetic Beads. Sensors 2019, 19, 4081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linh, V.T.N.; Moon, J.; Mun, C.; Devaraj, V.; Oh, J.W.; Park, S.G.; Kim, D.H.; Choo, J.; Lee, Y.I.; Jung, H.S. A facile low-cost paper-based SERS substrate for label-free molecular detection. Sens. Actuators B Chem. 2019, 291, 369–377. [Google Scholar] [CrossRef]
- Oh, K.; Lee, M.; Lee, S.G.; Jung, D.H.; Lee, H.L. Cellulose nanofibrils coated paper substrate to detect trace molecules using surface-enhanced Raman scattering. Cellulose 2018, 25, 3339–3350. [Google Scholar] [CrossRef]
- Lee, M.; Oh, K.; Choi, H.K.; Lee, S.G.; Youn, H.J.; Lee, H.L.; Jeong, D.H. Subnanomolar Sensitivity of Filter Paper-Based SERS Sensor for Pesticide Detection by Hydrophobicity Change of Paper Surface. ACS Sens. 2018, 3, 151–159. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Du, H.F.; Zhang, Q.; Zhao, J.; Weng, G.J.; Li, J.J.; Zhao, J.W. SERS detection of glucose using graphene-oxide-wrapped gold nanobones with silver coating. J. Mater. Chem. C 2019, 7, 3322–3334. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.Y.; Zhang, L.C.; Chu, B.H.; Liu, Q.Y.; Lu, Y.L. Graphene sandwiched platform for surface-enhanced Raman scattering. RSC Adv. 2017, 7, 49303–49308. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zou, Y.X.; Liu, F.; Xu, Y.T.; Wang, X.W.; Li, Y.J.; Liang, H.; Chen, L.; Chen, Z.; Tan, W.H. Stable Graphene-Isolated-Au-Nanocrystal for Accurate and Rapid Surface Enhancement Raman Scattering Analysis. Anal. Chem. 2016, 88, 10611–10616. [Google Scholar] [CrossRef]
- Qu, L.L.; Wang, N.; Zhu, G.; Yadav, T.P.; Shuai, X.T.; Bao, D.D.; Yang, G.H.; Li, D.W.; Li, H.T. Facile fabrication of ternary TiO2-gold nanoparticle-graphene oxide nanocomposites for recyclable surface enhanced Raman scattering. Talanta 2018, 186, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Goul, R.; Das, S.; Liu, Q.F.; Xin, M.; Lu, R.T.; Hui, R.; Wu, J.Z. Quantitative analysis of surface enhanced Raman spectroscopy of Rhodamine 6G using a composite graphene and plasmonic Au nanoparticle substrate. Carbon 2017, 111, 386–392. [Google Scholar] [CrossRef]
- Ghosh, P.; Paria, D.; Balasubramanian, K.; Ghosh, A.; Narayanan, R.; Raghavan, S. Directed Microwave-Assisted Self-Assembly of Au-Graphene-Au Plasmonic Dimers for SERS Applications. Adv. Mater. Interfaces 2019, 6, 1900629. [Google Scholar] [CrossRef]
- Ali, A.; Hwang, E.Y.; Choo, J.; Lim, D.W. PEGylated nanographene-mediated metallic nanoparticle clusters for surface enhanced Raman scattering-based biosensing. Analyst 2018, 143, 2604–2615. [Google Scholar] [CrossRef]
- Lin, D.H.; Qin, T.Q.; Wang, Y.Q.; Sun, X.Y.; Chen, L.X. Graphene Oxide Wrapped SERS Tags: Multifunctional Platforms toward Optical Labeling, Photothermal Ablation of Bacteria, and the Monitoring of Killing Effect. ACS Appl. Mater. Interfaces 2014, 6, 1320–1329. [Google Scholar] [CrossRef]
- Fu, X.L.; Wang, Y.Q.; Liu, Y.M.; Liu, H.T.; Fu, L.W.; Wen, J.H.; Li, J.W.; Wei, P.H.; Chen, L.X. A graphene oxide/gold nanoparticle-based amplification method for SERS immunoassay of cardiac troponin I. Analyst 2019, 144, 1582–1589. [Google Scholar] [CrossRef]
- Hernández-Sánchez, D.; Villabona-Leal, G.; Saucedo-Orozco, I.; Bracamonte, V.; Pérez, E.; Bittencourt, C.; Quintana, M. Stable graphene oxide–gold nanoparticle platforms for biosensing applications. Phys. Chem. Chem. Phys. 2018, 20, 1685–1692. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Lee, D.; Kim, D.Y. Paper-Based, Hand-Painted Strain Sensor Based on ITO Nanoparticle Channels for Human Motion Monitoring. IEEE Access 2019, 7, 77200–77207. [Google Scholar] [CrossRef]
- Boyen, H.G.; Ethirajan, A.; Kästle, G.; Weigl, F.; Ziemann, P.; Schmid, G.; Garnier, M.G.; Büttner, M.; Oelhafen, P. Alloy Formation of Supported Gold Nanoparticles at Their Transition from Clusters to Solids: Does Size Matter? Phys. Rev. Lett. 2005, 94, 016804. [Google Scholar] [CrossRef]
- Yang, L.; Hu, J.; He, L.; Tang, J.; Zhou, Y.; Li, J.; Ding, K. One-pot synthesis of multifunctional magnetic N-doped graphene composite for SERS detection, adsorption separation and photocatalytic degradation of Rhodamine 6G. Chem. Eng. J. 2017, 327, 694–704. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.-J.; Kim, D.Y. Hydrophobic Paper-Based SERS Sensor Using Gold Nanoparticles Arranged on Graphene Oxide Flakes. Sensors 2019, 19, 5471. https://doi.org/10.3390/s19245471
Lee D-J, Kim DY. Hydrophobic Paper-Based SERS Sensor Using Gold Nanoparticles Arranged on Graphene Oxide Flakes. Sensors. 2019; 19(24):5471. https://doi.org/10.3390/s19245471
Chicago/Turabian StyleLee, Dong-Jin, and Dae Yu Kim. 2019. "Hydrophobic Paper-Based SERS Sensor Using Gold Nanoparticles Arranged on Graphene Oxide Flakes" Sensors 19, no. 24: 5471. https://doi.org/10.3390/s19245471
APA StyleLee, D. -J., & Kim, D. Y. (2019). Hydrophobic Paper-Based SERS Sensor Using Gold Nanoparticles Arranged on Graphene Oxide Flakes. Sensors, 19(24), 5471. https://doi.org/10.3390/s19245471




