Feasibility Study of Enhancing Microwave Brain Imaging Using Metamaterials
Abstract
:1. Introduction
2. Materials and Methods
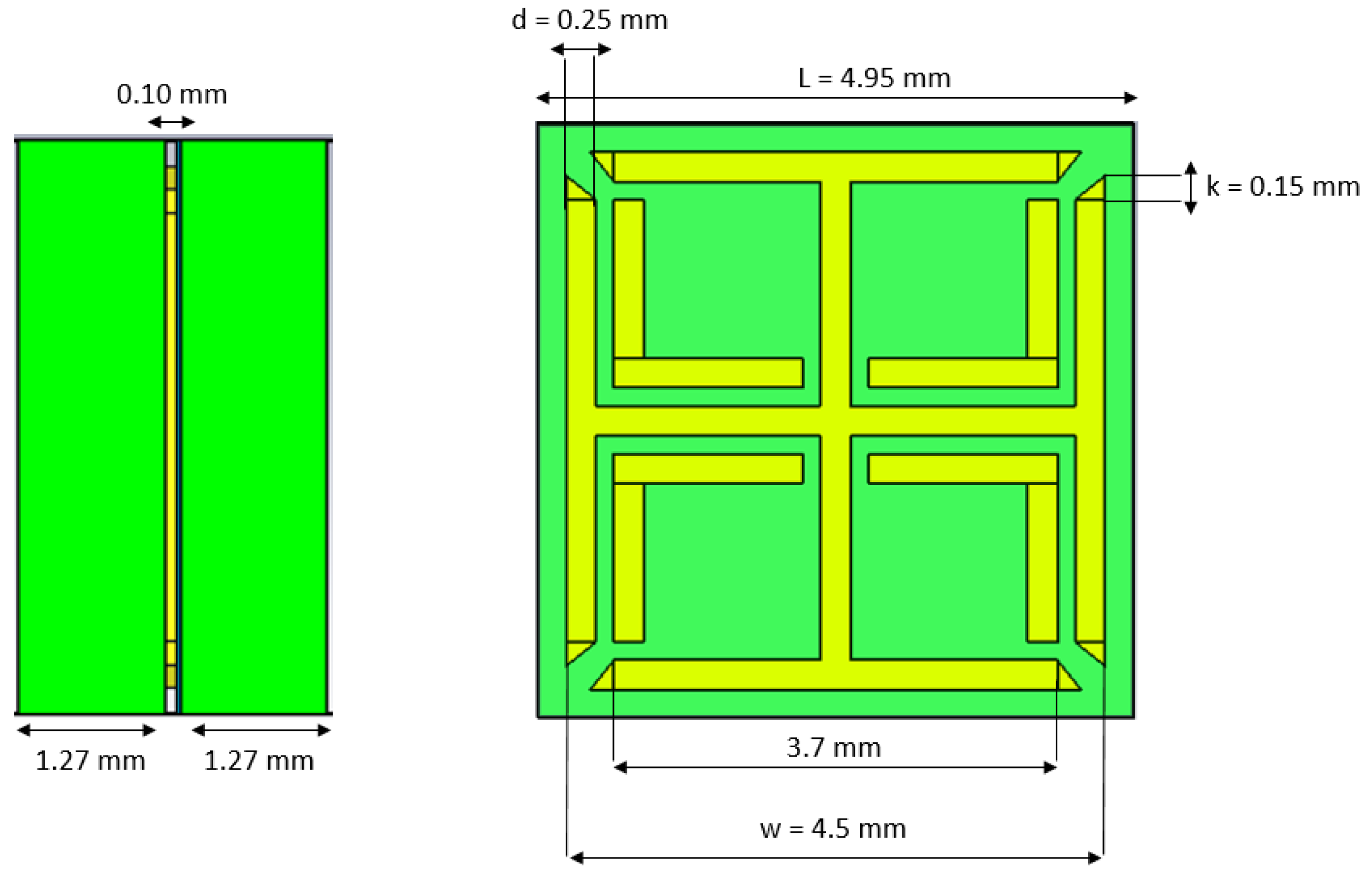
2.1. MM Design and Simulations for Impedance Matching
2.2. Experimental Validation
2.3. Simulations with a Brain Imaging Scanner
3. Results
3.1. Experimental and Simulation Results for the Two-Port Planar Setup
3.2. Simulation Results with the Headband Scanner
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MM | Metamaterial |
| SRR-CS | Cross-Shaped Split-Ring Resonator |
| MWT | Microwave Tomography |
| RF | Radio Frequency |
| MWI | Microwave Imaging |
| VNA | Vector Network Analyzer |
| EDTA | Ethylenediaminetetraacetic Acid |
| ABS | Acrylonitrile Butadiene Styrene |
| NC | Negative Control |
References
- The Kavli Foundation. Available online: https://www.kavlifoundation.org/tags/brain-diseases-disorders (accessed on 5 December 2019).
- Crocco, L.; Karanasiou, I.; James, M.; Conceição, R. Emerging Electromagnetic Technologies for Brain Diseases Disagnostics, Monitoring and Theraphy; Springer International Publishing AG: Cham, Switzerland, 2018; p. V. [Google Scholar]
- Uchiyama, T.; Mohri, K.; Honkura, Y.; Panina, L.V. Recent advances of pico-Tesla resolution magneto-impedance sensor based on amorphous wire CMOS IC MI sensor. IEEE Trans. Magn. 2012, 48, 3833–3839. [Google Scholar] [CrossRef]
- Nikolova, N.K. Microwave Imaging for Breast Cancer. IEEE Microw. Mag. 2011, 12, 78–94. [Google Scholar] [CrossRef]
- Chandra, R.; Zhou, H.; Balasingham, I.; Narayanan, R.M. On the Opportunities and Challenges in Microwave Medical Sensing and Imaging. IEEE Trans. Biomed. Eng. 2015, 62, 1667–1682. [Google Scholar] [CrossRef] [PubMed]
- Kosmas, P.; Crocco, L. Introduction to special issue on Electromagnetic technologies for medical diagnostics: Fundamental issues, clinical applications and perspectives. Diagnostics 2019, 9, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semenov, S.Y.; Bulyshev, A.E.; Abubakar, A.; Posukh, V.G.; Sizov, Y.E.; Souvorov, A.E.; Berg, P.M.V.D.; Williams, T.C. Microwave-Tomographic Imaging of the High Dielectric-Contrast Objects Using Different Image-Reconstruction Approaches. IEEE Trans. Microw. Theory Techn. 2005, 53, 2284–2294. [Google Scholar] [CrossRef]
- Winters, D.W.; Member, S.; Shea, J.D.; Member, S.; Kosmas, P.; Veen, B.D.V.; Hagness, S.C.; Member, S. Three-Dimensional Microwave Breast Imaging: Dispersive Dielectric Properties Estimation Using Patient-Specific Basis Functions. IEEE Trans. Med. Imag. 2009, 28, 969–981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, G.; Ahsan, S.; Kosmas, P. Portable Microwave Imaging Head Scanners for Intracranial Haemorrhagic Detection. In Proceedings of the 2019 Asia-Pacific Microwave Conference (APMC), Singapore, 10–13 December 2019. [Google Scholar]
- Scapaticci, R.; Tobon, J.; Bellizzi, G.; Vipiana, F.; Crocco, L. Design and Numerical Characterization of a Low-Complexity Microwave Device for Brain Stroke Monitoring. IEEE Trans. Antennas Propag. 2018, 66, 7328–7338. [Google Scholar] [CrossRef]
- Persson, M.; Fhager, A.; Trefna, H.D.; Yu, Y.; McKelvey, T.; Pegenius, G.; Karlsson, J.E.; Elam, M. Microwave-based stroke diagnosis making global prehospital thrombolytic treatment possible. IEEE Trans. Biomed. Eng. 2014, 61, 2806–2817. [Google Scholar] [CrossRef] [Green Version]
- Hopfer, M.; Planas, R.; Hamidipour, A.; Henriksson, T.; Semenov, S. Electromagnetic tomography for detection, differentiation, and monitoring of brain stroke: A virtual data and human head phantom study. IEEE Trans. Antennas Propag. 2017, 59, 86–97. [Google Scholar] [CrossRef]
- Mobashsher, A.T.; Abbosh, A.M. On-site Rapid Diagnosis of Intracranial Hematoma using Portable Multi-slice Microwave Imaging System. Sci. Rep. 2016, 6, 1–17. [Google Scholar] [CrossRef]
- Candefjord, S.; Winges, J.; Malik, A.A.; Yu, Y.; Rylander, T.; McKelvey, T.; Fhager, A.; Elam, M.; Persson, M. Microwave technology for detecting traumatic intracranial bleedings: Tests on phantom of subdural hematoma and numerical simulations. Med. Biol. Eng. Comput. 2017, 55, 1177–1188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mustafa, S.; Mohammed, B.; Abbosh, A. Novel preprocessing techniques for accurate microwave imaging of human brain. IEEE Antennas Wirel. Propag. Lett. 2013, 12, 460–463. [Google Scholar] [CrossRef]
- Semenov, S.Y.; Corfield, D.R. Microwave Tomography for Brain Imaging: Feasibility Assessment for Stroke Detection. Int. J. Antennas Propag. 2008, 2008, 254830. [Google Scholar] [CrossRef] [Green Version]
- Scapaticci, R.; Di Donato, L.; Catapano, I.; Crocco, L. A Feasibility Study on Microwave Imaging for Brain Stroke Monitoring. Prog. Electromagn. Res. 2014, 40, 305–324. [Google Scholar] [CrossRef] [Green Version]
- Semenov, S.; Planas, R.; Hopfer, M.; Hamidipour, A.; Vasilenko, A.; Stoegmann, E.; Auff, E. Electromagnetic tomography for brain imaging: Initial assessment for stroke detection. In Proceedings of the IEEE Biomedical Circuits and Systems Conference: Engineering for Healthy Minds and Able Bodies, BioCAS, Atlanta, GA, USA, 22–24 October 2015. [Google Scholar]
- Meaney, P.M.; Shubitidze, F.; Fanning, M.W.; Kmiec, M.; Epstein, N.R.; Paulsen, K.D. Surface Wave Multipath Signals in Near-Field Microwave Imaging. Int. J. Biomed. Imag. 2012, 2012, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Fox, C.J.; Meaney, P.M.; Shubitidze, F.; Potwin, L.; Paulsen, K.D. Characterization of an Implicitly Resistively-Loaded Monopole Antenna in Lossy Liquid Media. Int. J. Antennas Propag. 2008, 2008, 580782. [Google Scholar] [CrossRef] [Green Version]
- Cano-Garcia, H.; Kosmas, P.; Kallos, E. Enhancing electromagnetic transmission through biological tissues at millimeter waves using subwavelength metamaterial antireflection coatings. In Proceedings of the 9th International Congress on Advanced Electromagnetic Materials in Microwaves and Optics, METAMATERIALS 2015, Oxford, UK, 7–12 September 2015. [Google Scholar]
- Cano-Garcia, H.; Kosmas, P.; Kallos, E. Demonstration of enhancing the transmission of 60 GHz waves through biological tissue using thin metamaterial antireflection coatings. In Proceedings of the 10th International Congress on Advanced Electromagnetic Materials in Microwaves and Optics, METAMATERIALS 2016, Heraklion, Greece, 19–22 September 2016. [Google Scholar]
- Chen, H.T.; Zhou, J.; O’Hara, J.F.; Chen, F.; Azad, A.K.; Taylor, A.J. Antireflection coating using metamaterials and identification of its mechanism. Phys. Rev. Lett. 2010, 105, 073901. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Zhou, J.; O’Hara, J.; Taylor, A. A Numerical Investigation of Metamaterial Antireflection Coatings. Terahertz Sci. Technol. 2010, 3, 66–73. [Google Scholar]
- Pendry, J.B.; Holden, A.J.; Robbins, D.J.; Stewart, W.J. Magnetism from Conductors and Enhanced Nonlinear Phenomena. IEEE Trans. Microw. Theory Technol. 1999, 47, 2075–2084. [Google Scholar] [CrossRef] [Green Version]
- Smith, D.R.; Pendry, J.B.; Wiltshire, M.C. Metamaterials and negative refractive index. Science 2004, 305, 788–792. [Google Scholar] [CrossRef] [Green Version]
- Puentes Vargas, M. Planar Metamaterial Based Microwave Sensor Arrays for Biomedical Analysis and Treatment; Springer: New York, NY, USA, 2014; Chapter 2; pp. 7–31. [Google Scholar]
- Ziolkowski, R.W. Metamaterial-based antennas: Research and developments. IEICE Trans. Electron. 2006, E89-C, 1267–1275. [Google Scholar] [CrossRef]
- Spada, L.L.; Bilotti, F.; Vegni, L. Metamaterial biosensor for cancer detection. In Proceedings of the IEEE Sensors Proceedings, Limerik, Ireland, 28–31 October 2011. [Google Scholar]
- Sugumaran, S.; Faizal, M.; Noor, M.; Shekar, C.; Schreurs, D. Biosensors and Bioelectronics Nanostructured materials with plasmonic nanobiosensors for early cancer detection: A past and future prospect. Biosens. Bioelectron. 2018, 100, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Aydin, K.; Bulu, I.; Guven, K.; Kafesaki, M.; Soukoulis, C.M.; Ozbay, E. Investigation of magnetic resonances for different split-ring resonator parameters and designs. New J. Phys. 2005, 7, 168. [Google Scholar] [CrossRef] [Green Version]
- Lopez, M.A.; Freire, M.J.; Algarin, J.M.; Behr, V.C.; Jakob, P.M.; Marqús, R. Nonlinear split-ring metamaterial slabs for magnetic resonance imaging. Appl. Phys. Lett. 2011, 98, 133508. [Google Scholar] [CrossRef] [Green Version]
- Isakov, D.; Stevens, C.J.; Castles, F.; Grant, P.S. A Split Ring Resonator Dielectric Probe for Near-Field Dielectric Imaging. Sci. Rep. 2017, 7, 2038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, S.; Shi, X.; Udpa, L.; Udpa, S.; Deng, Y.; Chahal, P. Design of a Split-Ring Resonator Sensor for Near-Field Microwave Imaging. IEEE Sens. J. 2018, 18, 7066–7076. [Google Scholar] [CrossRef]
- Mukherjee, S.; Su, Z.; Udpa, L.; Udpa, S.; Tamburrino, A. Enhancement of microwave imaging using a metamaterial lens. IEEE Sens. J. 2019, 20, 4962–4971. [Google Scholar] [CrossRef]
- Islam, M.; Islam, M.T.; Rashed, M.; Faruque, I.; Misran, N.; Mansor, M.F. A Miniaturized Antenna with Negative Index Metamaterial Based on Modified SRR and CLS Unit Cell for UWB Microwave Imaging Applications. Materials 2015, 8, 392–407. [Google Scholar] [CrossRef]
- Mahmud, Z.; Islam, M.T. A Negative Index Metamaterial to Enhance the Performance of Miniaturized UWB Antenna for Microwave Imaging Applications. Appl. Sci. 2017, 7, 1149. [Google Scholar] [CrossRef] [Green Version]
- Koutsoupidou, M.; Karanasiou, I.S.; Uzunoglu, N.; Member, F. Rectangular Patch Antenna on Split-ring Resonators Substrate for THz Brain Imaging: Modeling and Testing. In Proceedings of the 13th IEEE International Conference on BioInformatics and BioEngineering, Chania, Greece, 10–13 November 2013. [Google Scholar]
- Koutsoupidou, M.; Karanasiou, I.S.; Uzunoglu, N. Substrate constructed by an array of split ring resonators for a THz planar antenna. J. Comput. Electron. 2014, 13, 593–598. [Google Scholar] [CrossRef]
- Yurduseven, O.; Gowda, V.R.; Gollub, J.N.; Smith, D.R. Printed Aperiodic Cavity for Computational and Microwave Imaging. IEEE Microw. Wirel. Compon. Lett. 2016, 26, 367–369. [Google Scholar] [CrossRef]
- Gollub, J.N.; Yurduseven, O.; Trofatter, K.P.; Arnitz, D.; Imani, M.F.; Sleasman, T.; Boyarsky, M.; Rose, A.; Odabasi, H.; Zvolensky, T.; et al. Large Metasurface Aperture for Millimeter Wave Computational Imaging at the Human-Scale. Sci. Rep. 2017, 7, 42650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yurduseven, O.; Smith, D. A reconfigurable millimeter-wave spotlight metasurface aperture integrated with a frequency-diverse microwave imager for security screening. Proc. SPIE 2018, 10634. [Google Scholar] [CrossRef]
- Razzicchia, E.; Koutsoupidou, M.; Cano-garcia, H.; Sotiriou, I.; Kallos, E.; Palikaras, G. Metamaterial Designs to Enhance Microwave Imaging Applications. In Proceedings of the ICEAA 2019 International Conference on Electromagnetics in Advanced Applications, Granada, Spain, 9–13 September 2019. [Google Scholar]
- Ahsan, S.; Guo, Z.; Miao, Z.; Sotiriou, I.; Koutsoupidou, M.; Kallos, E.; Palikaras, G.; Kosmas, P. Multiple-Frequency Microwave Tomography System. Sensors 2018, 18, 3491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chopra, K.; Calva, D.; Sosin, M.; Tadisina, K.K.; Banda, A.; De La Cruz, C.; Chaudhry, M.R.; Legesse, T.; Drachenberg, C.B.; Manson, P.N.; et al. A comprehensive examination of topographic thickness of skin in the human face. Aesthetic Surg. J. 2015, 35, 1007–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynnerup, N.; Astrup, J.G.; Sejrsen, B. Thickness of the human cranial diploe in relation to age, sex and general body build. Head Face Med. 2005, 1, 13. [Google Scholar] [CrossRef] [Green Version]
- Lillie, E.M.; Urban, J.E.; Weaver, A.A.; Powers, A.K.; Stitzel, J.D. Estimation of skull table thickness with clinical CT and validation with microCT. J. Anat. 2015, 226, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Fischl, B.; Dale, A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. USA 2000, 97, 11050–11055. [Google Scholar] [CrossRef] [Green Version]
- Andreuccetti, D. An Internet Resource for the Calculation of the Dielectric Properties of Body Tissues in the Frequency Range 10 Hz–100 GHz. Available online: http://niremf.ifac.cnr.it/tissprop/ (accessed on 10 October 2018).
- Ahsan, S.; Koutsoupidou, M.; Razzicchia, E.; Sotiriou, I.; Kosmas, P. Advances towards the Development of a Brain Microwave Imaging Scanner. In Proceedings of the 13th European Conference on Antennas and Propagation, EuCAP, Krakow, Poland, 31 March–5 April 2019. [Google Scholar]
- Miao, Z.; Kosmas, P. Multiple-Frequency DBIM-TwIST Algorithm for Microwave Breast Imaging. IEEE Trans. Antennas Propag. 2017, 65, 2507–2516. [Google Scholar] [CrossRef] [Green Version]
- Miao, Z.; Ahsan, S.; Kosmas, P.; Vasquez, J.A.T.; Vipiana, F.; Casu, M.R.; Vacca, M. Application of the DBIM-TwIST Algorithm to Experimental Microwave Imaging Data. In Proceedings of the 11th European Conference on Antennas and Propagation (EUCAP), Paris, France, 19–24 March 2017. [Google Scholar]















| Type of Phantom | Water (%) | Glycerol (%) | ’ |
|---|---|---|---|
| Skin | 30 | 70 | 38.2 |
| Bone | 10 | 90 | 13.2 |
| Brain | 35 | 65 | 42 |
| Blood | 50 | 50 | 59.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razzicchia, E.; Sotiriou, I.; Cano-Garcia, H.; Kallos, E.; Palikaras, G.; Kosmas, P. Feasibility Study of Enhancing Microwave Brain Imaging Using Metamaterials. Sensors 2019, 19, 5472. https://doi.org/10.3390/s19245472
Razzicchia E, Sotiriou I, Cano-Garcia H, Kallos E, Palikaras G, Kosmas P. Feasibility Study of Enhancing Microwave Brain Imaging Using Metamaterials. Sensors. 2019; 19(24):5472. https://doi.org/10.3390/s19245472
Chicago/Turabian StyleRazzicchia, Eleonora, Ioannis Sotiriou, Helena Cano-Garcia, Efthymios Kallos, George Palikaras, and Panagiotis Kosmas. 2019. "Feasibility Study of Enhancing Microwave Brain Imaging Using Metamaterials" Sensors 19, no. 24: 5472. https://doi.org/10.3390/s19245472






