Effect of Palladium Electrode Patterns on Hydrogen Response Characteristics from a Sensor Based on Ta2O5 Film on SiC at High Temperatures
Abstract
:1. Introduction
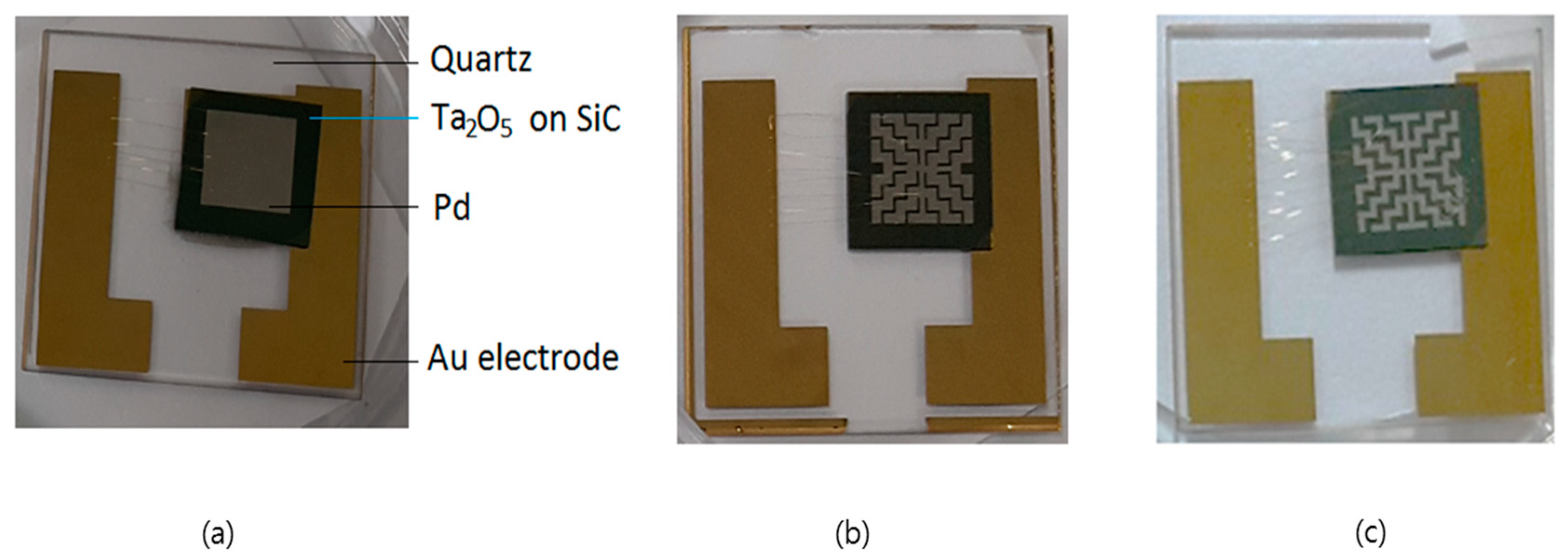
2. Experiments
3. Results and Discussion
3.1. Evaluation of Ta2O5 Properties in the MIS Structure
3.2. Response Characteristics for Hydrogen
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Eranna, G.; Joshi, B.C.; Runthala, D.P.; Gupta, R.P. Oxide materials for development of integrated gas sensors—A comprehensive review. Crit. Rev. Solid State Mater. Sci. 2004, 29, 111–188. [Google Scholar] [CrossRef]
- Kita, J.; Schubert, F.; Rettig, F.; Engelbrecht, A.; Grob, A.; Moos, R. Ceramic alumina substrates for high-temperature gas sensors-implications for applicability. Procedia Eng. 2014, 87, 1505–1508. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Ning, T.; Sun, P.; Yan, Y.; Zhang, D.; Li, Z. Effect of Al2O3-SiO2 substrate on gas-sensing properties of TiO2 based lambda sensor at high temperature. Ceram. Int. 2018, 44, 3000–3004. [Google Scholar] [CrossRef]
- Liu, X.; Peng, B.; Zhang, W.; Zhu, J.; Liu, X.; Wei, M. Improvement of high-temperature stability of Al2O3/Pt/ZnO/Al2O3 film electrode for SAW devices by using Al2O3 barrier layer. Materials 2017, 10, 1377. [Google Scholar] [CrossRef] [Green Version]
- Trinchi, A.; Kandasamy, S.; Wlodarski, W. High temperature field effect hydrogen and hydrocarbon gas sensors based on SiC MOS devices. Sens. Actuator B Chem. 2008, 133, 705–716. [Google Scholar] [CrossRef]
- Yu, J.; Chen, G.; Li, C.X.; Shafiei, M.; Ou, J.Z.; du Plessis, J.; Kalantar-zadeh, K.; Lai, P.T.; Wlodarski, W. Hydrogen gas sensing properties of Pt/Ta2O5 Schottky diodes based on Si and SiC substrates. Sens. Actuator B Chem. 2011, 172, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Spetz, A.L.; Baranzahi, A.; Tobias, P.; Lundstrom, I. High temperature sensors based on metal-insulator-silicon carbide devices. Phys. Status Solid A Appl. Res. 1997, 162, 493–551. [Google Scholar] [CrossRef]
- Wright, N.G.; Horsfall, A.B. SiC sensors: A review. J. Phys. D Appl. Phys. 2007, 40, 6345–6354. [Google Scholar] [CrossRef]
- Ghosh, R.N.; Tobias, P. SiC field-effect devices operating at high temperature. J. Electron. Mater. 2005, 34, 345–350. [Google Scholar] [CrossRef]
- Lu, C.; Chen, Z. High-temperature resistive hydrogen sensor based on thin nanoporous rutile TiO2 film on anodic aluminum oxide. Sens. Actuator B Chem. 2009, 140, 109–115. [Google Scholar] [CrossRef]
- Hunter, G.W.; Gary, W. A survey and analysis of commercially available hydrogen sensors. NASA Tech. Memo. 1992, 105878. [Google Scholar]
- Kim, S. Capacitance response characteristics of hydrogen sensor with tantalum oxide dielectric layer. Int. J Hydrogen Energy 2018, 43, 19810–19815. [Google Scholar] [CrossRef]
- Soo, M.T.; Cheong, K.Y.; Noor, A. Advances of SiC-based MOS capacitor hydrogen sensors for harsh environment applications. Sens. Actuators B Chem. 2010, 151, 39–55. [Google Scholar] [CrossRef]
- Gu, H.; Wang, Z.; Hu, Y. Hydrogen gas sensors based on semiconductor oxide nanostructures. Sensors 2012, 12, 5517–5550. [Google Scholar] [CrossRef] [Green Version]
- Xue, N.; Zhang, Q.; Zhang, S.; Zong, P.; Yang, F. Highly sensitive and selective hydrogen gas sensor using the mesoporous SnO2 modified layers. Sensors 2017, 17, 2351. [Google Scholar] [CrossRef] [Green Version]
- Joo, S.; Choi, J.; Kim, S.; Kim, S. Pd/Ta2O5/SiC Schottky-diode hydrogen sensors formed by using rapid thermal oxidation of a Ta thin films. J. Korean Phys. Soc. 2013, 63, 1794–1798. [Google Scholar] [CrossRef]
- Chaneliere, C.; Autran, J.L.; Devine, R.A.B.; Balland, B. Tantalum pentoxide thin films for advanced dielectric applications. Mater. Sci. Eng. B Rep. 1998, 22, 269–322. [Google Scholar] [CrossRef]
- Chandrasekharan, R.; Park, I.; Masel, R.; Shannon, M. Thermal oxidation of tantalum films at various oxidation states from 300 to 700 °C. J. Appl. Phys. 2005, 98, 114908. [Google Scholar] [CrossRef]
- Ezhilvalavan, S.; Tseng, T. Preparation and properties of tantalum pentoxide (Ta2O5) thin films for ultra large scale integrated circuits (ULSIs) application—A review. J. Mater. Sci. Mater. Elec. 1999, 10, 9–31. [Google Scholar] [CrossRef]
- Fisser, M.; Badcock, R.; Teal, T.; Hunze, A. Optimizing the sensitivity of palladium based hydrogen sensors. Sens. Actuators B Chem. 2018, 259, 10–18. [Google Scholar] [CrossRef]
- Gabrielli, C.; Grand, P.; Lasia, A.; Perrot, H. Investigation of hydrogen adsorption and absorption in palladium thin films i. theory. J. Electrochem. Soc. 2004, 151, A1925–A1936. [Google Scholar] [CrossRef]
- Gabrielli, C.; Grand, P.; Lasia, A.; Perrot, H. Investigation of hydrogen adsorption and absorption in palladium thin films iii. impedance spectroscopy. J. Electrochem. Soc. 2004, 151, A1943–A1949. [Google Scholar] [CrossRef]
- Jurczakowski, R.; Losiewicz, B.; Lasia, A. Kinetic and thermodynamic parameters of hydrogen sorption in Pd, Pd-Pt and on Pt. ECS Trans. 2007, 2, 11–19. [Google Scholar]
- Chang, S.; Kim, Y.; Lee, J.; Choi, K. Thermal stability study of Ni–Si silicide films on Ni/4H-SiC contact by in-situ temperature-dependent sheet resistance measurement. Jpn. J. Appl. Phys. 2019, 58, 075503. [Google Scholar] [CrossRef]









© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, K.-K.; Kim, S. Effect of Palladium Electrode Patterns on Hydrogen Response Characteristics from a Sensor Based on Ta2O5 Film on SiC at High Temperatures. Sensors 2019, 19, 5478. https://doi.org/10.3390/s19245478
Choi K-K, Kim S. Effect of Palladium Electrode Patterns on Hydrogen Response Characteristics from a Sensor Based on Ta2O5 Film on SiC at High Temperatures. Sensors. 2019; 19(24):5478. https://doi.org/10.3390/s19245478
Chicago/Turabian StyleChoi, Kyeong-Keun, and Seongjeen Kim. 2019. "Effect of Palladium Electrode Patterns on Hydrogen Response Characteristics from a Sensor Based on Ta2O5 Film on SiC at High Temperatures" Sensors 19, no. 24: 5478. https://doi.org/10.3390/s19245478
APA StyleChoi, K.-K., & Kim, S. (2019). Effect of Palladium Electrode Patterns on Hydrogen Response Characteristics from a Sensor Based on Ta2O5 Film on SiC at High Temperatures. Sensors, 19(24), 5478. https://doi.org/10.3390/s19245478




