Electro-Oxidation and Simultaneous Determination of Indole-3-Acetic Acid and Salicylic Acid on Graphene Hydrogel Modified Electrode
Abstract
1. Introduction
2. Experiment
2.1. Materials and Reagents
2.2. Apparatus
2.3. Procedures
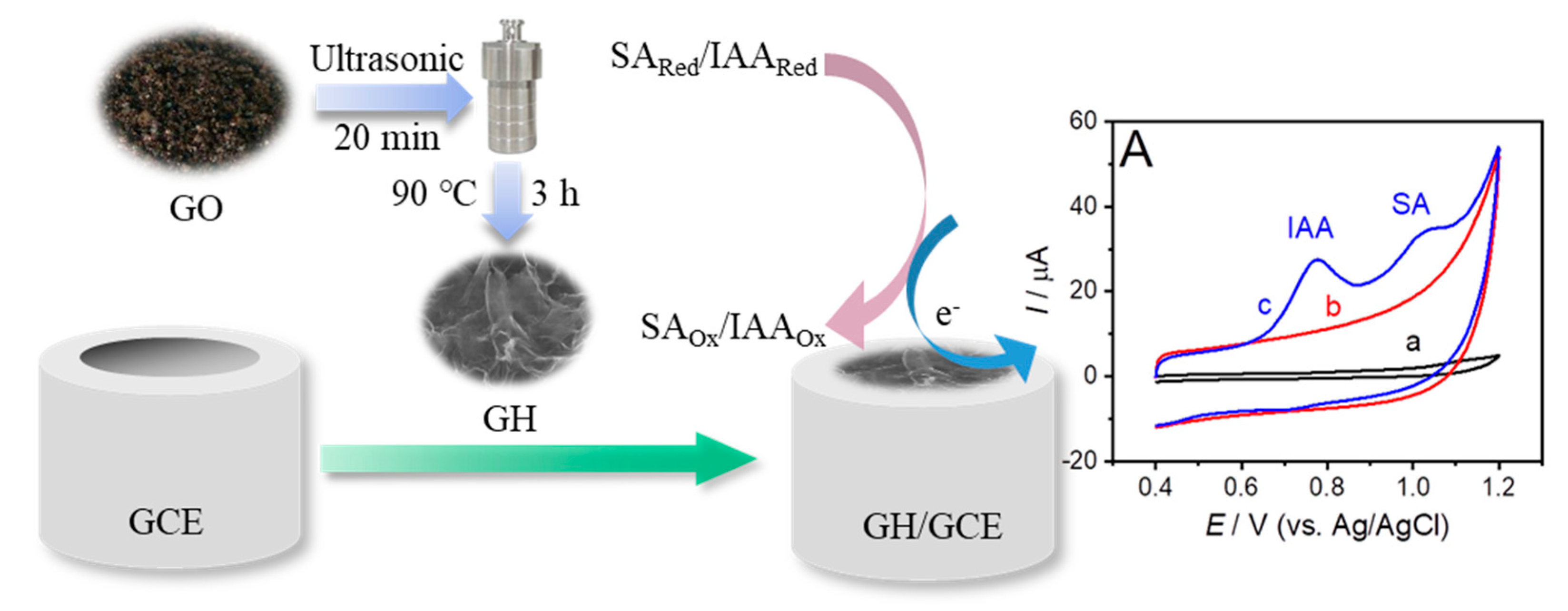
2.3.1. Synthesis of GH
2.3.2. Preparation of GH Modified GCE
2.4. Sample Analysis
3. Results and Discussion
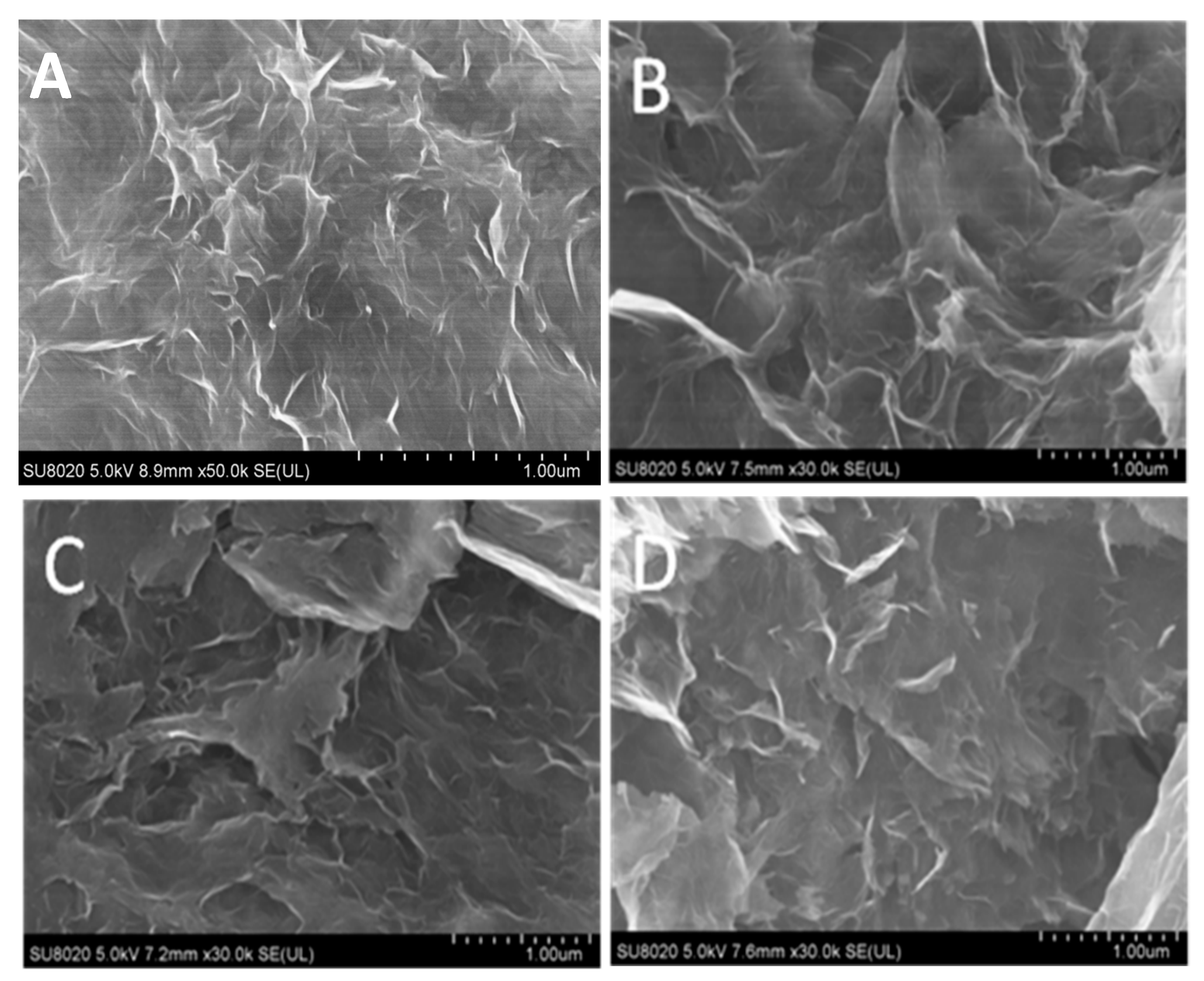
3.1. Characterization of GH
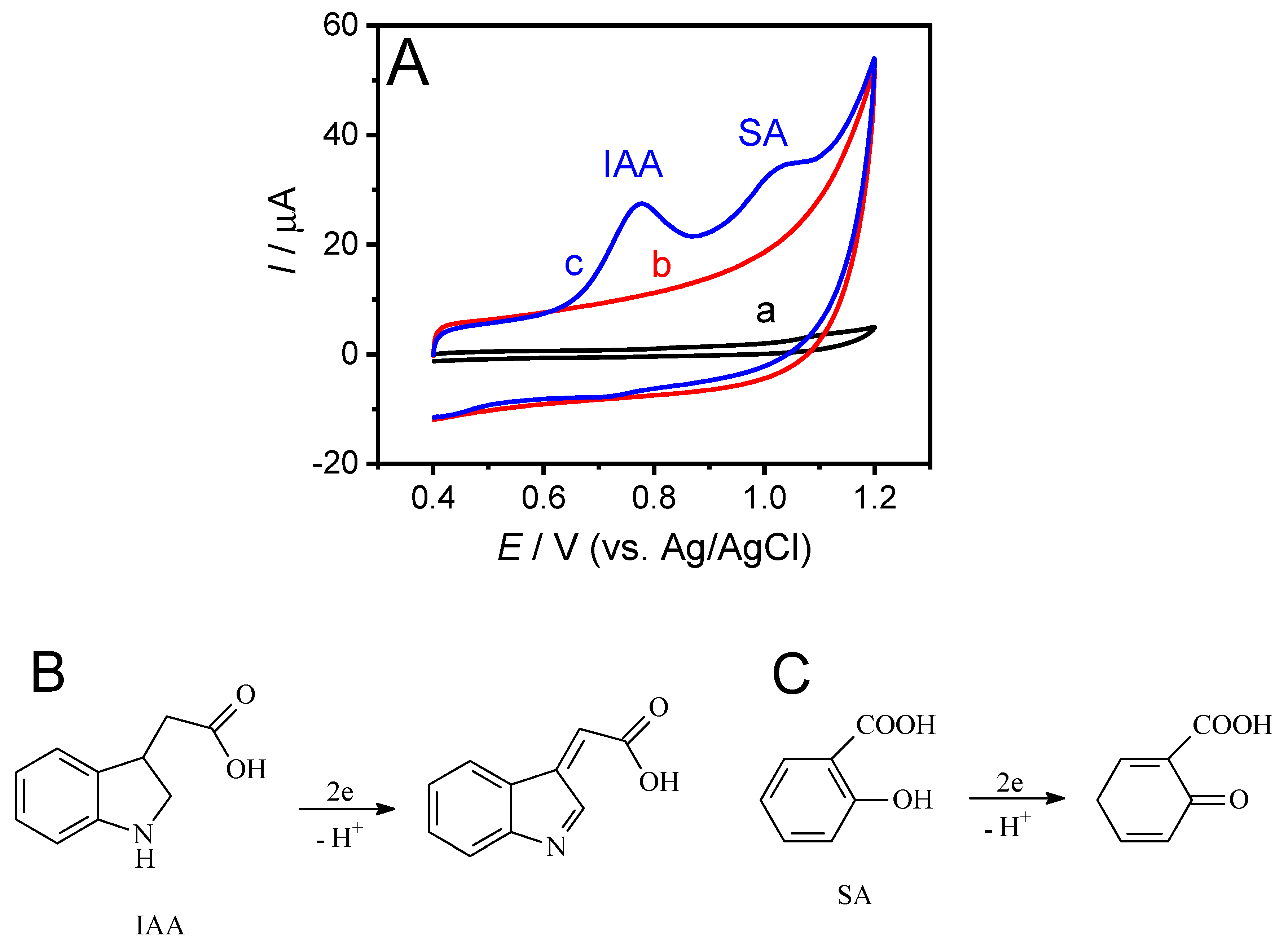
3.2. Electrochemical Oxidation of IAA and SA on GH/GCE
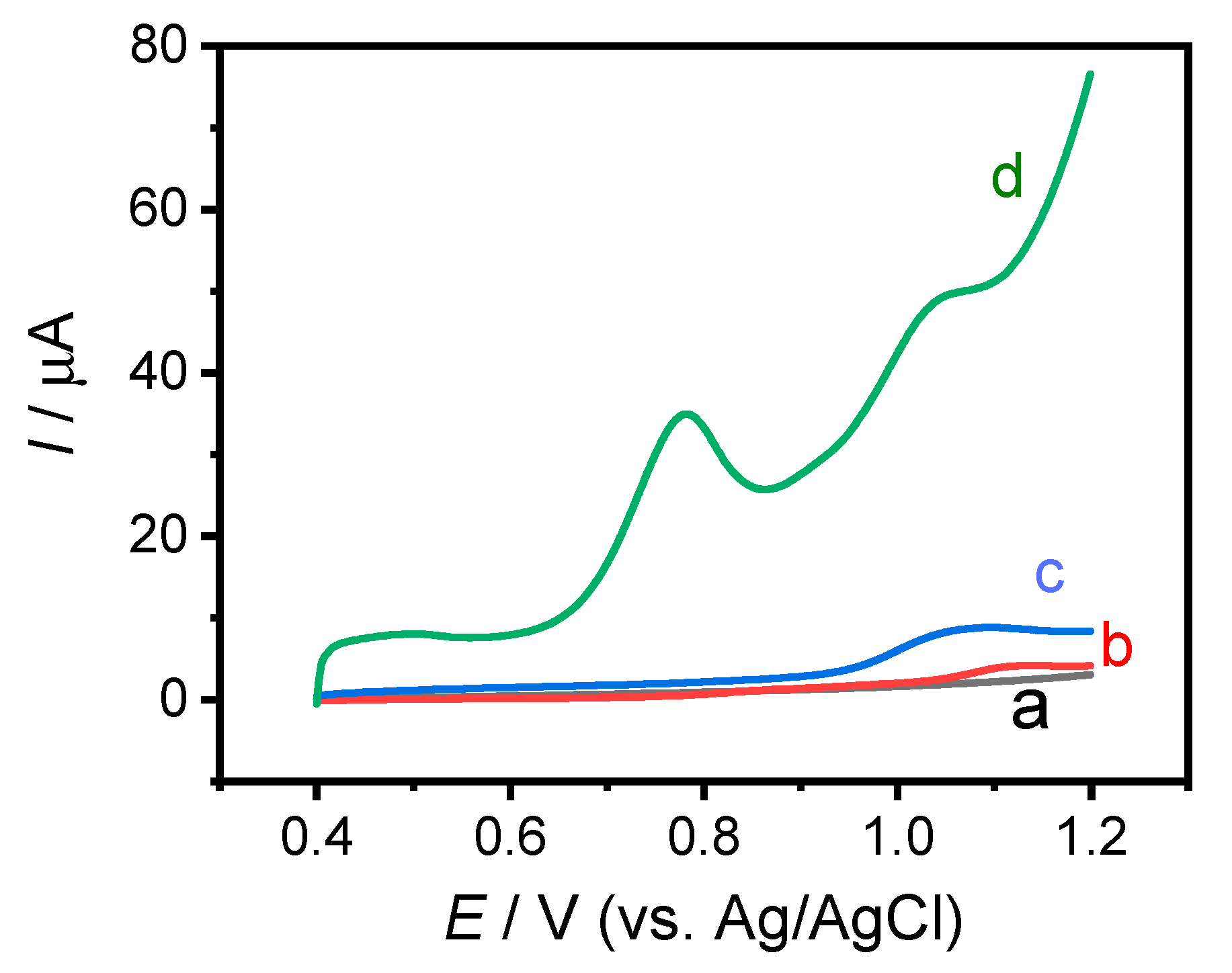
3.3. Electrochemical Detection of IAA and SA
3.4. The Selectivity, Reproducibility, and Stability of the GH/GCE
3.5. Detection of IAA and SA in Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Su, C.; Liu, C.; Chen, J.; Chen, Z.; He, Z. Simultaneous determination of zeatin and systemin by coupling graphene oxide-protected aptamers with catalytic recycling of DNase i. Sens. Actuators B Chem. 2016, 230, 442–448. [Google Scholar] [CrossRef]
- Zhang, X.; Niu, J.; Zhang, X.; Xiao, R.; Lu, M.; Cai, Z. Graphene oxide-SiO2 nanocomposite as the adsorbent for extraction and preconcentration of plant hormones for HPLC analysis. J. Chromatogr. B 2017, 1046, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Kumar, M.; Brahmbhatt, H.; Reddy, C.R.K.; Seth, A.; Jha, B. Simultaneous determination of different endogenetic plant growth regulators in common green seaweeds using dispersive liquid-liquid microextraction method. Plant Physiol. Biochem. 2011, 49, 1259–1263. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.J.; Xie, Y.; Yan, Y.F.; Yang, H.; Gu, H.Y.; Bao, N. Paper-based analytical devices for direct electrochemical detection of free IAA and SA in plant samples with the weight of several milligrams. Sens. Actuators B Chem. 2017, 247, 336–342. [Google Scholar] [CrossRef]
- Seo, H.; Kriechbaumer, V.; Park, W.J. Modern quantitative analytical tools and biosensors for functional studies of auxin. J. Plant Biol. 2016, 59, 93–104. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Y.; Zhao, S.; Zhang, W.; Du, H.; Deng, Z.; Zhang, S. Simultaneous determination of indole-3-acetic acid and indole-3-butyric acid in plant by field-amplified sample stacking open-tubular capillary electrochromatography based on solid-phase extraction with calixarene sorbent. Chromatographia 2016, 79, 243–254. [Google Scholar] [CrossRef]
- Muhammad, N.; Subhani, Q.; Wang, F.; Lou, C.; Liu, J.; Zhu, Y. Simultaneous determination of two plant growth regulators in ten food samples using ion chromatography combined with QuEChERS extraction method (IC-QuEChERS) and coupled with fluorescence detector. Food Chem. 2018, 241, 308–316. [Google Scholar] [CrossRef]
- Wang, W.; He, M.; Chen, B.; Hu, B. Simultaneous determination of acidic phytohormones in cucumbers and green bean sprouts by ion-pair stir bar sorptive extraction-high performance liquid chromatography. Talanta 2017, 170, 128–136. [Google Scholar] [CrossRef]
- Wu, Y.; Hu, B. Simultaneous determination of several phytohormones in natural coconut juice by hollow fiber-based liquid-liquid-liquid microextraction-high performance liquid chromatography. J. Chromatogr. A 2009, 1216, 7657–7663. [Google Scholar] [CrossRef]
- Li, G.; Liu, S.; Sun, Z.; Xia, L.; Chen, G.; You, J. A simple and sensitive HPLC method based on pre-column fluorescence labelling for multiple classes of plant growth regulator determination in food samples. Food Chem. 2015, 170, 123–130. [Google Scholar] [CrossRef]
- Perin, E.C.; Crizel, R.L.; Galli, V.; da Silva Messias, R.; Rombaldi, C.V.; Chaves, F.C. Extraction and quantification of abscisic acid and derivatives in strawberry by LC-MS. Food Anal. Methods 2018, 11, 2547–2552. [Google Scholar] [CrossRef]
- Suárez-Pantaleón, C.; Esteve-Turrillas, F.A.; Mercader, J.V.; Agulló, C.; Abad-Somovilla, A.; Abad-Fuentes, A. Development and validation of a direct competitive monoclonal antibody-based immunoassay for the sensitive and selective analysis of the phytoregulator forchlorfenuron. Anal. Bioanal. Chem. 2012, 403, 2019–2026. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wen, Y.; Bai, L.; Liu, G.; Chen, Y.; Du, H.; Wang, X. pH-controlled voltammetric behaviors and detection of phytohormone 6-benzylaminopurine using MWCNT/GCE. J. Electroanal. Chem. 2015, 750, 89–99. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, Z.; Wang, M.; Meng, X.; Yin, H. Electrochemical immunoassay platform for high sensitivity detection of indole-3-Acetic acid. Electrochim. Acta 2013, 96, 66–73. [Google Scholar] [CrossRef]
- Liu, F.; Tang, J.; Xu, J.; Shu, Y.; Xu, Q.; Wang, H.; Hu, X. Low potential detection of indole-3-acetic acid based on the peroxidase-like activity of hemin/reduced graphene oxide nanocomposite. Biosens. Bioelectron. 2016, 86, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Tvorynska, S.; Josypčuk, B.; Barek, J.; Dubenska, L. Electrochemical behavior and sensitive methods of the voltammetric determination of food azo dyes amaranth and allura red AC on amalgam electrodes. Food Anal. Methods 2019, 12, 409–421. [Google Scholar] [CrossRef]
- Gan, T.; Hu, C.; Chen, Z.; Hu, S. A disposable electrochemical sensor for the determination of indole-3-acetic acid based on poly(safranine T)-reduced graphene oxide nanocomposite. Talanta 2011, 85, 310–316. [Google Scholar] [CrossRef]
- Chandler, J.W. Auxin as compère in plant hormone crosstalk. Planta 2009, 231, 1–12. [Google Scholar] [CrossRef]
- Rivas-San Vicente, M.; Plasencia, J. Salicylic acid beyond defence: Its role in plant growth and development. J. Exp. Bot. 2011, 62, 3321–3338. [Google Scholar] [CrossRef]
- Shakirova, F.M.; Sakhabutdinova, A.R.; Bezrukova, M.V.; Fatkhutdinova, R.A.; Fatkhutdinova, D.R. Changes in the hormonal status of wheat seedlings induced by salicylic acid and salinity. Plant Sci. 2003, 164, 317–322. [Google Scholar] [CrossRef]
- Park, J.E.; Park, J.Y.; Kim, Y.S.; Staswick, P.E.; Jeon, J.; Yun, J.; Kim, S.Y.; Kim, J.; Lee, Y.H.; Park, C.M. GH3-mediated auxin homeostasis links growth regulation with stress adaptation response in Arabidopsis. J. Biol. Chem. 2007, 282, 10036–10046. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liu, X.; Gao, L.; Lu, Y.; Li, Y.; Pan, Z.; Bao, N.; Gu, H. Simultaneous electrochemical determination of indole-3-acetic acid and salicylic acid in pea roots using a multiwalled carbon nanotube modified electrode. Anal. Lett. 2015, 48, 1578–1592. [Google Scholar] [CrossRef]
- Lu, S.; Bai, L.; Wen, Y.; Li, M.; Yan, D.; Zhang, R.; Chen, K. Water-dispersed carboxymethyl cellulose-montmorillonite-single walled carbon nanotube composite with enhanced sensing performance for simultaneous voltammetric determination of two trace phytohormones. J. Solid State Electrochem. 2015, 19, 2023–2037. [Google Scholar] [CrossRef]
- Xu, Y.; Sheng, K.; Li, C.; Shi, G. Self-assembled graphene hydrogel. ACS Nano 2010, 4, 4324–4330. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Li, W.; Bao, Y.; Sun, Z.; Gao, L.; Nawaz, M.H.; Han, D.; Niu, L. Enhanced pseudocapacitance and electrolyte-wettability of graphene hydrogels to tailor high mass loading all-solid-state supercapacitor with ultra-high volumetric energy density. Carbon 2018, 136, 46–53. [Google Scholar] [CrossRef]
- Song, H.; Zhang, X.; Liu, Y.; Su, Z. Developing graphene-based nanohybrids for electrochemical sensing. Chem. Rec. 2019, 19, 534–549. [Google Scholar] [CrossRef]
- Zhang, J.; Li, R.; Li, Z.; Liu, J.; Gu, Z.; Wang, G. Synthesis of nitrogen-doped activated graphene aerogel/gold nanoparticles and its application for electrochemical detection of hydroquinone and o-dihydroxybenzene. Nanoscale 2014, 6, 5458–5466. [Google Scholar]
- Kokulnathan, T.; Ramaraj, S.; Chen, S.-M.; Yang, H.-Y. Eco-friendly synthesis of biocompatible pectin stabilized graphene nanosheets hydrogel and their application for the simultaneous electrochemical determination of dopamine and paracetamol in real samples. J. Electrochem. Soc. 2018, 165, B240–B249. [Google Scholar] [CrossRef]
- Rahmani, T.; Bagheri, H.; Behbahani, M.; Hajian, A.; Afkhami, A. Modified 3D graphene-Au as a novel sensing layer for direct and sensitive electrochemical determination of carbaryl pesticide in fruit, vegetable, and water samples. Food Anal. Methods 2018, 11, 3005–3014. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, Z.; Guo, X.; Jin, K.; Wang, Y.X.; Li, L.; Zhang, Y.; Wang, Z.; Sun, L.; Zhang, T. N/S co-doped three-dimensional graphene hydrogel for high performance supercapacitor. Electrochim. Acta 2018, 278, 51–60. [Google Scholar] [CrossRef]
- Zhu, Q.; Bao, J.; Huo, D.; Yang, M.; Hou, C.; Guo, J.; Chen, M.; Fa, H.; Luo, X.; Ma, Y. 3D Graphene hydrogel—Gold nanoparticles nanocomposite modified glassy carbon electrode for the simultaneous determination of ascorbic acid, dopamine and uric acid. Sens. Actuators B Chem. 2016, 238, 1316–1323. [Google Scholar] [CrossRef]
- Zhu, Q.; Bao, J.; Huo, D.; Yang, M.; Wu, H.; Hou, C.; Zhao, Y.; Luo, X.; Fa, H. 3DGH-Fc based electrochemical sensor for the simultaneous determination of ascorbic acid, dopamine and uric acid. J. Electroanal. Chem. 2017, 799, 459–467. [Google Scholar] [CrossRef]
- Xie, Y.; Sheng, X.; Xie, D.; Liu, Z.; Zhang, X.; Zhong, L. Fabricating graphene hydrogels with controllable pore structure via one-step chemical reduction process. Carbon 2016, 109, 637–680. [Google Scholar] [CrossRef]
- Meng, X.; Lu, L.; Sun, C. Green synthesis of three-dimensional MnO2/graphene hydrogel composites as a high-performance electrode material for supercapacitors. ACS Appl. Mater. Interfaces 2018, 10, 16474–16481. [Google Scholar] [CrossRef]
- Wu, Q.; Sun, Y.; Bai, H.; Shi, G. High-performance supercapacitor electrodes based on graphene hydrogels modified with 2-aminoanthraquinone moieties. Phys. Chem. Chem. Phys. 2011, 13, 11193–11198. [Google Scholar] [CrossRef]
- Cao, X.; Zhu, X.; He, S.; Xu, X.; Ye, Y.; Gunasekaran, S. Gold nanoparticle-doped three-dimensional reduced graphene hydrogel modified electrodes for amperometric determination of indole-3-acetic acid and salicylic acid. Nanoscale 2019, 11, 10247–10256. [Google Scholar] [CrossRef]
- Hao, N.; Hua, R.; Chen, S.; Zhang, Y.; Zhou, Z.; Qian, J.; Liu, Q.; Wang, K. Multiple signal-amplification via Ag and TiO2 decorated 3D nitrogen doped graphene hydrogel for fabricating sensitive label-free photoelectrochemical thrombin aptasensor. Biosens. Bioelectron. 2018, 101, 14–20. [Google Scholar] [CrossRef]
- Hao, N.; Zhang, X.; Zhou, Z.; Hua, R.; Zhang, Y.; Liu, Q.; Qian, J.; Li, H.; Wang, K. AgBr nanoparticles/3D nitrogen-doped graphene hydrogel for fabricating all-solid-state luminol-electrochemiluminescence Escherichia coli aptasensors. Biosens. Bioelectron. 2017, 97, 377–383. [Google Scholar] [CrossRef]
- Li, J.; Xie, J.; Gao, L.; Li, C.M. Au nanoparticles-3D graphene hydrogel nanocomposite to boost synergistically in situ detection sensitivity toward cell-released nitric oxide. ACS Appl. Mater. Interfaces 2015, 7, 2726–2734. [Google Scholar] [CrossRef]
- Cao, X.; Xu, H.; Ding, S.; Ye, Y.; Ge, X.; Yu, L. Electrochemical determination of sulfide in fruits using alizarin-reduced graphene oxide nanosheets modified electrode. Food Chem. 2016, 194, 1224–1229. [Google Scholar] [CrossRef]
- Ye, Y.; Ding, S.; Ye, Y.; Xu, H.; Cao, X.; Liu, S.; Sun, H. Enzyme-based sensing of glucose using a glassy carbon electrode modified with a one-pot synthesized nanocomposite consisting of chitosan, reduced graphene oxide and gold nanoparticles. Microchim. Acta 2015, 182, 1783–1789. [Google Scholar] [CrossRef]
- Cançado, L.G.; Jorio, A.; Ferreira, E.H.M.; Stavale, F.; Achete, C.A.; Capaz, R.B.; Moutinho, M.V.O.; Lombardo, A.; Kulmala, T.S.; Ferrari, A.C. Quantifying defects in graphene via Raman spectroscopy at different excitation energies. Nano Lett. 2011, 11, 3190–3196. [Google Scholar] [CrossRef] [PubMed]
- Bhaskaram, D.S.; Govindaraj, G. Carrier transport in reduced graphene oxide probed using Raman spectroscopy. J. Phys. Chem. C 2018, 122, 10303–10308. [Google Scholar] [CrossRef]
- Li, X.; Wang, Q.; Zhao, Y.; Wu, W.; Chen, J.; Meng, H. Green synthesis and photo-catalytic performances for ZnO-reduced graphene oxide nanocomposites. J. Colloid Interface Sci. 2013, 411, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Yoon, Y.; Ahn, J.; Lim, H.; Kim, G.; Kim, J.H.; Choi, K.B.; Lee, J.J. Facile laser fabrication of high quality graphene-based microsupercapacitors with large capacitance. Carbon 2018, 137, 136–145. [Google Scholar] [CrossRef]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. Interfacial Electrochem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Yardim, Y.; Erez, M.E. Electrochemical behavior and electroanalytical determination of indole-3-acetic acid phytohormone on a boron-doped diamond electrode. Electroanalysis 2011, 23, 667–673. [Google Scholar] [CrossRef]
- Rawlinson, S.; McLister, A.; Kanyong, P.; Davis, J. Rapid determination of salicylic acid at screen printed electrodes. Microchem. J. 2018, 137, 71–77. [Google Scholar] [CrossRef]
- de Ribeiro, C.L.; Santos, J.G.M.; de Souza, J.R.; Pereira-da-Silva, M.A.; Paterno, L.G. Electrochemical oxidation of salicylic acid at ITO substrates modified with layer-by-layer films of carbon nanotubes and iron oxide nanoparticles. J. Electroanal. Chem. 2017, 805, 53–59. [Google Scholar] [CrossRef]
- Sun, L.J.; Pan, Z.Q.; Xie, J.; Liu, X.J.; Sun, F.T.; Song, F.M.; Bao, N.; Gu, H.Y. Electrocatalytic activity of salicylic acid on Au@Fe3O4 nanocomposites modified electrode and its detection in tomato leaves infected with Botrytis cinerea. J. Electroanal. Chem. 2013, 706, 127–132. [Google Scholar] [CrossRef]
- Park, J.; Eun, C. Electrochemical behavior and determination of salicylic acid at carbon-fiber electrodes. Electrochim. Acta 2016, 194, 346–356. [Google Scholar] [CrossRef]
- Wang, Z.; Ai, F.; Xu, Q.; Yang, Q.; Yu, J.H.; Huang, W.H.; Zhao, Y.D. Electrocatalytic activity of salicylic acid on the platinum nanoparticles modified electrode by electrochemical deposition. Colloids Surf. B Biointerfaces 2010, 76, 370–374. [Google Scholar] [CrossRef] [PubMed]


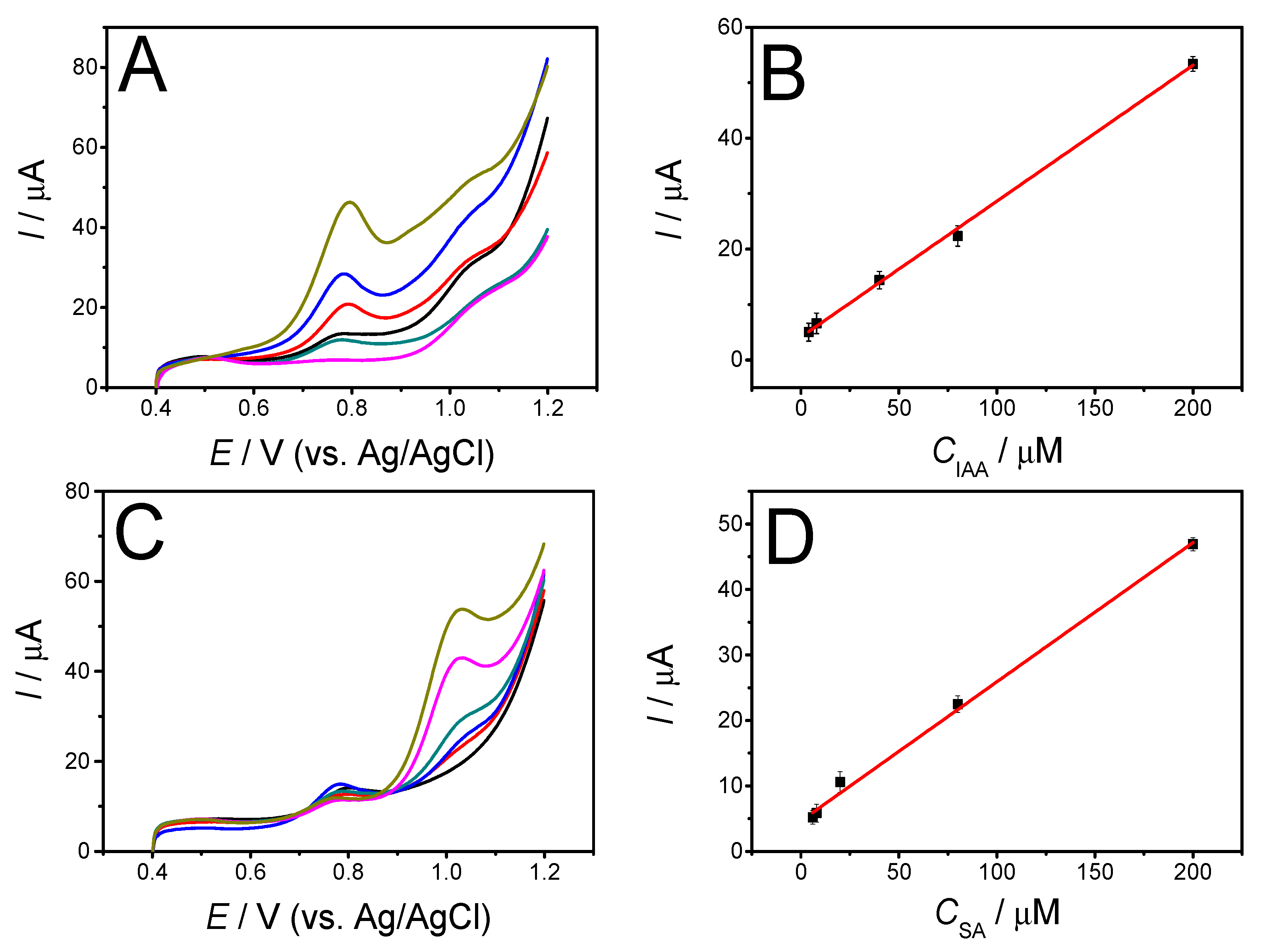
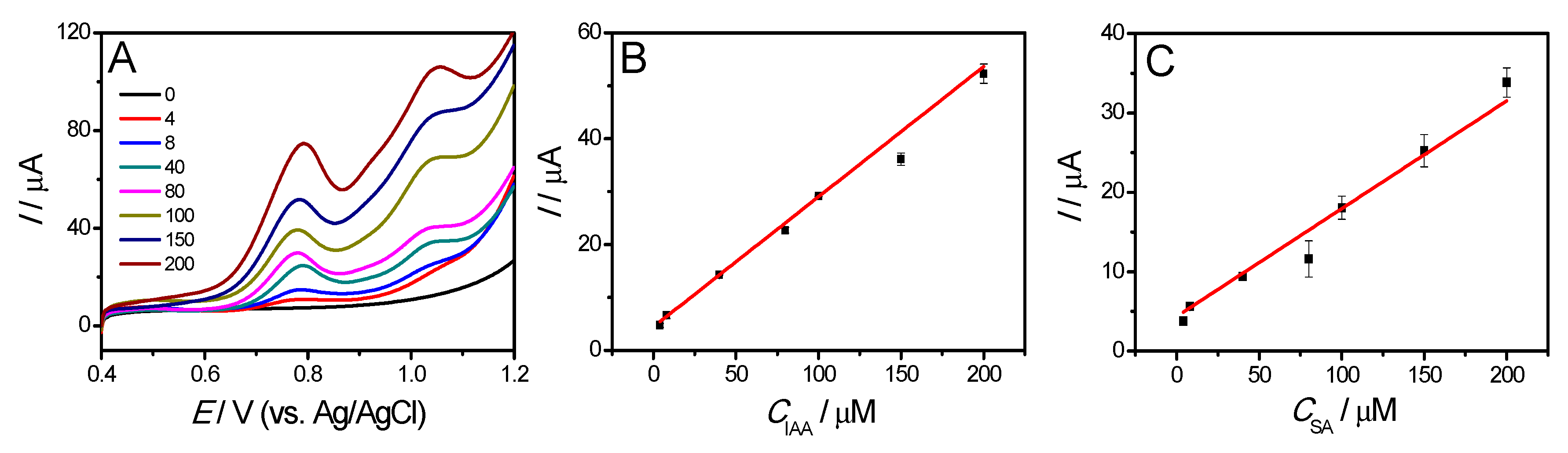
| Electrode * | Substance | Potential/V | Method | Linear Range/μM | LOD/μM | Ref. |
|---|---|---|---|---|---|---|
| CMC-MMT-SWCNT/GCE | IAA SA | 0.89 (vs. SCE) 1.18 (vs. SCE) | LSV | 0.005–0.30, 0.30–70.0 (with 80 μM SA) 0.01–300 (with 40 μM IAA) | 0.002 0.0063 | [23] |
| MWCNT-CS/GCE | IAA SA | 0.72 (vs. SCE) 0.88 (vs. SCE) | DPV | 0.67–48.82 0.67–48.82 | 0.10 0.10 | [22] |
| BDD | IAA | 0.93 (vs. Ag/AgCl) | SWV | 5–50 | 1.22 | [47] |
| SPE | SA | -- | SWV | 16–300 | 5.60 | [48] |
| CNT-PABS/MAGNP/PADC/ITO | SA | 1.14 (vs. Ag/AgCl) | CV | 6–100 | 0.105 | [49] |
| Au@Fe3O4/GCE | SA | -- | DPV | 1.0–1200.0 | 0.10 | [50] |
| CFE | SA | -- | DPV | 2.0–3000.0 | 1.68 | [51] |
| PNP/Pt | SA | -- | i-t | 20–500 | 6.40 | [52] |
| GH/GCE | IAA SA | 0.78 (vs. Ag/AgCl) 1.00 (vs. Ag/AgCl) | LSV | 4–200 4–200 | 1.42 2.80 | This work |
| Sample | IAA Added (μM) | SA Added (μM) | IAA Detected (μM) | SA Detected (μM) | Recovery of IAA (%) | Recovery of SA (%) |
|---|---|---|---|---|---|---|
| Celery | -- | -- | 5.02 | 3.98 | ||
| 40.00 | 40.00 | 45.30 ± 0.07 | 42.50 ± 0.23 | 100.6 ± 0.16 | 96.6 ± 0.52 | |
| Tomato leaves | -- | -- | 4.00 | 4.12 | ||
| 4.00 | 4.00 | 7.59 ± 0.04 | 8.54 ± 0.11 | 94.9 ± 0.50 | 105.2 ± 1.35 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, X.; Zhu, X.; He, S.; Xu, X.; Ye, Y. Electro-Oxidation and Simultaneous Determination of Indole-3-Acetic Acid and Salicylic Acid on Graphene Hydrogel Modified Electrode. Sensors 2019, 19, 5483. https://doi.org/10.3390/s19245483
Cao X, Zhu X, He S, Xu X, Ye Y. Electro-Oxidation and Simultaneous Determination of Indole-3-Acetic Acid and Salicylic Acid on Graphene Hydrogel Modified Electrode. Sensors. 2019; 19(24):5483. https://doi.org/10.3390/s19245483
Chicago/Turabian StyleCao, Xiaodong, Xueting Zhu, Shudong He, Xuan Xu, and Yongkang Ye. 2019. "Electro-Oxidation and Simultaneous Determination of Indole-3-Acetic Acid and Salicylic Acid on Graphene Hydrogel Modified Electrode" Sensors 19, no. 24: 5483. https://doi.org/10.3390/s19245483
APA StyleCao, X., Zhu, X., He, S., Xu, X., & Ye, Y. (2019). Electro-Oxidation and Simultaneous Determination of Indole-3-Acetic Acid and Salicylic Acid on Graphene Hydrogel Modified Electrode. Sensors, 19(24), 5483. https://doi.org/10.3390/s19245483






