Investigation of Stability and Power Consumption of an AlGaN/GaN Heterostructure Hydrogen Gas Sensor Using Different Bias Conditions
Abstract
:1. Introduction
2. Experiments
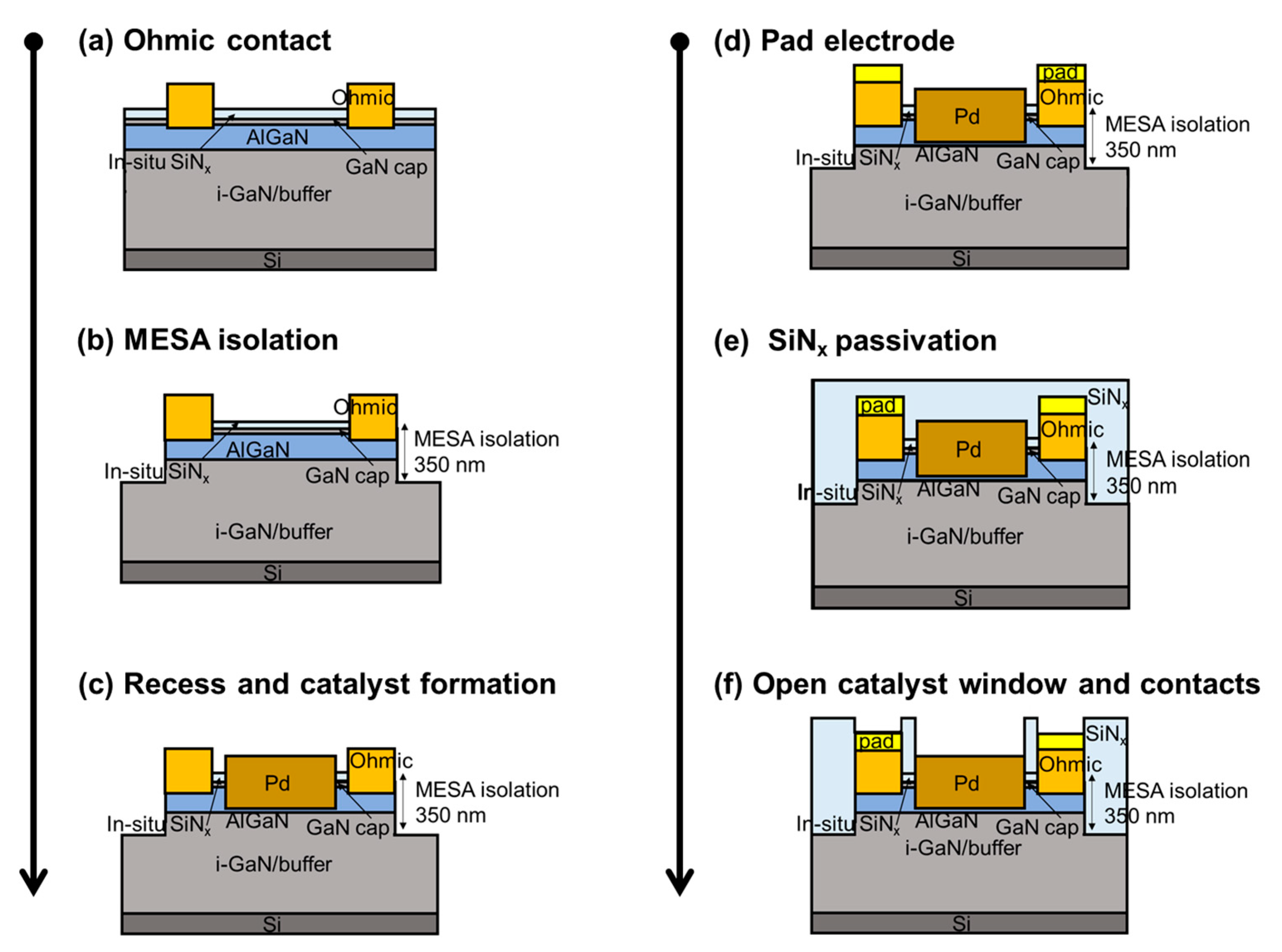
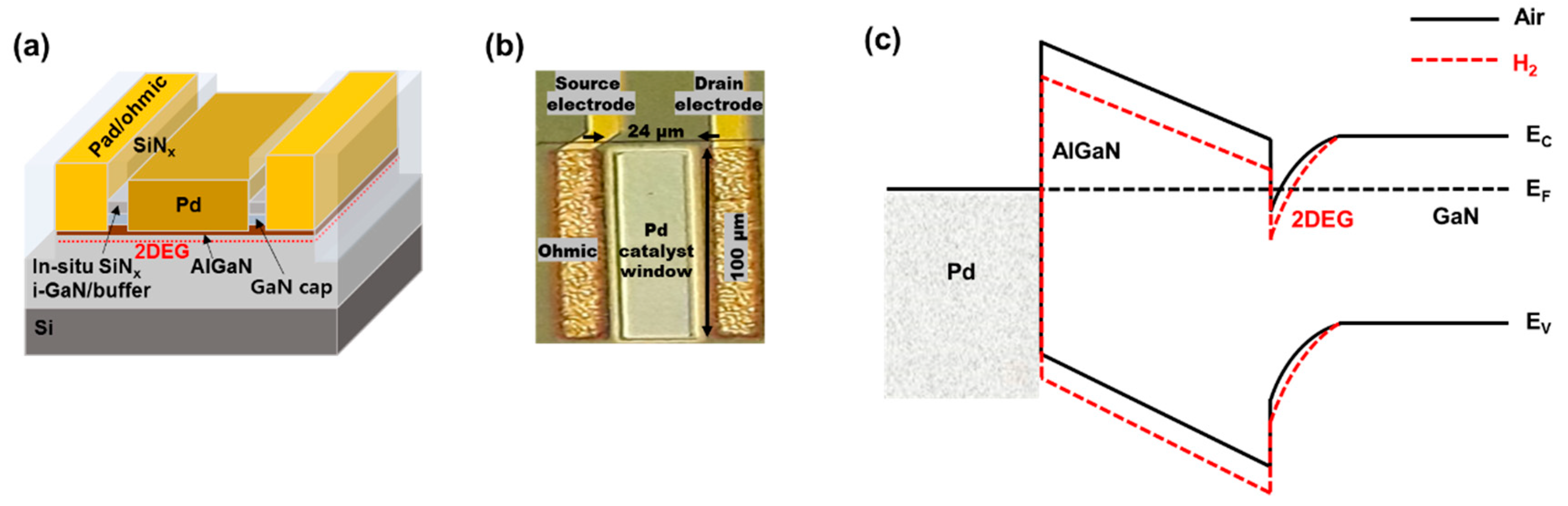
2.1. Sensor Fabrication
2.2. Sensor Characterization
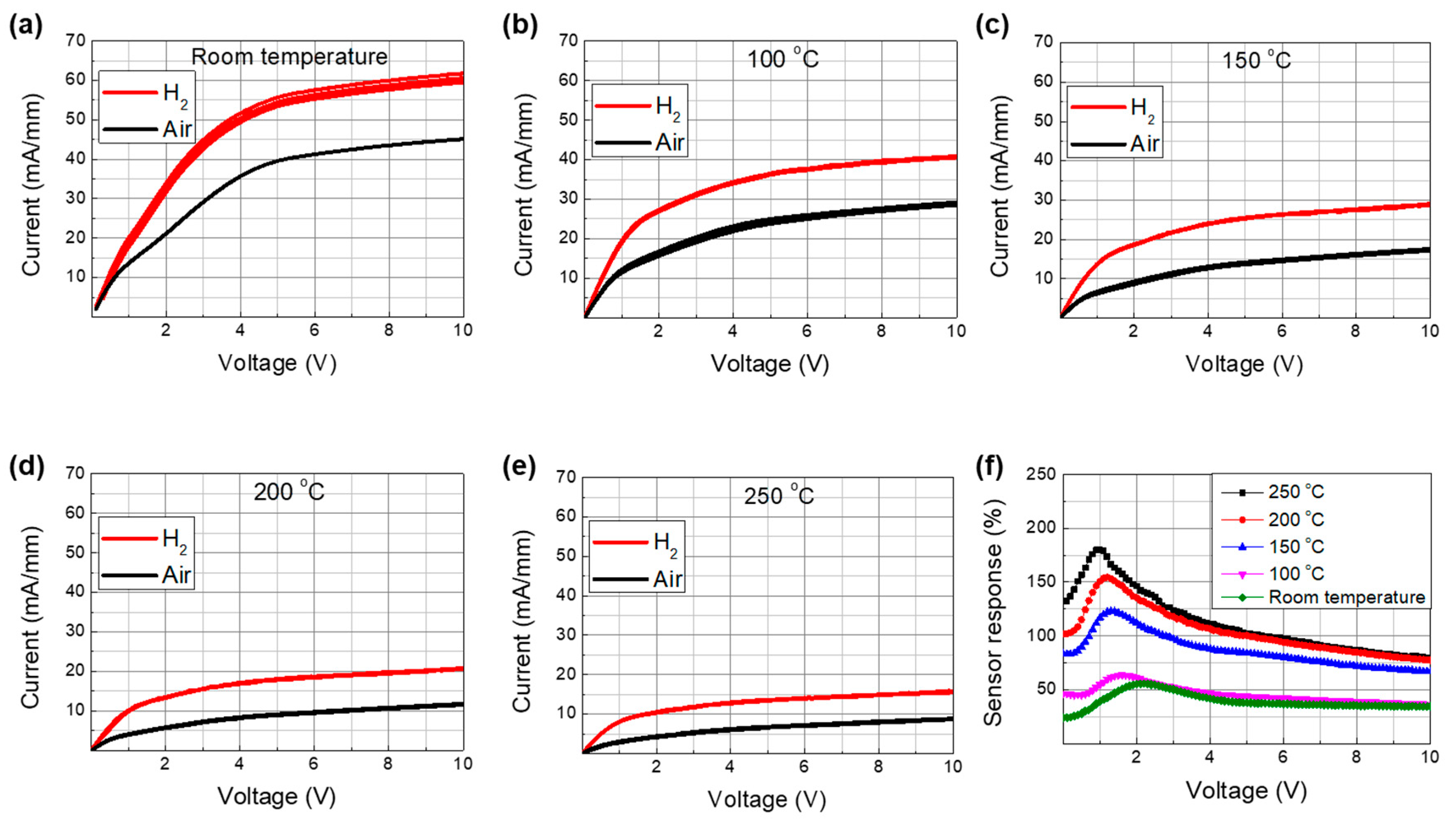
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Das, D. Advances in biohydrogen production process: An approach towards commercialization. Int. J. Hydrogen Energy 2009, 34, 7349–7357. [Google Scholar] [CrossRef]
- Ahluwalia, R.K.; Wang, X.; Rousseau, A.; Kumar, R. Fuel economy of hydrogen cell vehicles. J. Power Sources 2004, 130, 192–201. [Google Scholar] [CrossRef]
- Steinberg, M.; Cheng, H.C. Modern and prospective technologies for hydrogen production from fossil fuels. Int. J. Hydrogen Energy 1989, 14, 797–820. [Google Scholar] [CrossRef]
- Kanungo, J.; Saha, H.; Basu, S. Porous silicon hydrogen sensor at room temperature: The effect of surface modification and noble metal contacts. Sens. Transducers 2009, 103, 102–108. [Google Scholar]
- Salehi, A.; Nikfarjam, A.; Kalantari, D.J. Pd/porous-GaAs Schottky contact for hydrogen sensing application. Sens. Actuators B 2006, 113, 419–427. [Google Scholar] [CrossRef]
- Kim, S.; Choi, J.; Jung, M.; Joo, S.; Kim, S. Silicon carbide-based hydrogen gas sensors for high-temperature applications. Sensors 2013, 13, 13575–13583. [Google Scholar] [CrossRef] [PubMed]
- Hassan, J.J.; Mahdi, M.A.; Chin, C.W.; Abu-Hassan, H.; Hassan, Z. Room temperature hydrogen gas sensor based on ZnO nanorod arrays grown on a SiO2/Si substrate via microwave-assisted chemical solution method. J. Alloys Compd. 2013, 546, 107–111. [Google Scholar] [CrossRef]
- Shaposhnik, D.; Pavelko, R.; Llovet, E.; Gispert-Guirado, F.; Vilanova, X. Hydrogen sensors on the basis of SnO2-TiO2 systems. Procedia Eng. 2011, 25, 1133–1136. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Yao, Z.; Haidry, A.A.; Plecenik, T.; Xie, L.; Sun, L.; Fatima, Q. Resistive-type hydrogen gas sensor based on TiO2: A review. Int. J. Hydrogen Energy 2018, 43, 21114–21132. [Google Scholar] [CrossRef]
- Hao, L.; Liu, Y.; Du, Y.; Chen, Z.; Han, Z.; Xu, Z.; Zhu, J. Highly enhanced H2 sensing performance of few-layer MoS2/SiO2/Si heterojunctions by surface decoration of Pd nanoparticles. Nanoscale Res. Lett. 2017, 12, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Chung, G.; Cha, H.Y.; Kim, H. Enhanced hydrogen sensitivity of AlGaN/GaN heterojunction gas sensors by GaN-cap layer. Electron. Lett. 2018, 54, 896–897. [Google Scholar] [CrossRef]
- Burk, A.A., Jr.; O’Loughlin, M.J.; Siergiej, R.R.; Agarwal, A.K.; Sriram, S.; Clarke, R.C.; MacMillan, M.R.; Balakrishna, V.; Brandt, C.D. SiC and GaN wide bandgap semiconductor materials and devices. Solid-state Electron. 1999, 43, 1459–1464. [Google Scholar] [CrossRef]
- Chen, J.T.; Persson, I.; Nilsson, D.; Hsu, C.W.; Palisaitis, J.; Forsberg, U.; Persson, P.O.Å.; Janzen, E. Room temperature mobility above 2200 cm2/V·s of two-dimensional electron gas in a sharp-interface AlGaN/GaN heterostructure. Appl. Phys. Lett. 2015, 106, 251601. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Cheng, Y.T. Hydrogen diffusion and solubility in palladium thin films. Int. J. Hydrogen Energy 1996, 21, 281–291. [Google Scholar] [CrossRef]
- Safarik, D.K.; Schwarz, R.B.; Paglieri, S.N.; Quintana, R.L.; Tuggle, D.G.; Byler, D.D. Composition dependence of the elastic constants of β-phase and (α+β)-phase PdHx. Ultrasonics 2010, 50, 155–160. [Google Scholar] [CrossRef]
- Khanuja, M.; Shrestha, S.; Metha, B.R.; Kala, S.; Kruis, F.E. Magnitude and time response of electronic and topographical changes during hydrogen sensing in size selected palladium nanoparticles. J. Appl. Phys. 2011, 110, 014318. [Google Scholar] [CrossRef]
- Fisser, M.; Badcock, A.B.; Teal, P.D.; Hunze, A. Optimizing the sensitivity of palladium based hydrogen sensors. Sens. Actuators, B: Chem 2018, 259, 10–19. [Google Scholar] [CrossRef]
- Conde, J.J.; Marono, M.; Sanchez-Hervas, J.M. Pd-Based Membranes for Hydrogen Separation Review of Alloying Elements and Their Influence on Membrane Properties. Sep. Purif. Rev. 2017, 46, 152–177. [Google Scholar] [CrossRef]
- Baik, K.H.; Kim, J.; Jang, S. Highly sensitive nonpolar a-plane GaN based hydrogen diode sensor with textured active area using photo-chemical etching. Sens. Actuators B Chem. 2017, 238, 462–467. [Google Scholar] [CrossRef]
- Lee, I.H.; Kim, Y.H.; Chang, Y.J.; Shin, J.H.; Jang, T.; Jang, S.Y. Temperature-dependent Hall Measurement of AlGaN/GaN Heterostuctures on Si Substrates. J. Korean. Phys. Soc. 2015, 66, 61–64. [Google Scholar] [CrossRef]
- D’Amico, A.; Di Natale, C. A Contribution on Some Basic Definitions of Sensors Properties. IEEE. Sens. J. 2001, 1, 183–190. [Google Scholar] [CrossRef]
- Lin, Y.; Deng, P.; Nie, Y.; Hu, Y.; Xing, L.; Zhang, Y.; Xue, X. Room-temperature self-powered ethanol sensing of a Pd/ZnO nanoarray nanogenerator driven by human finger movement. Nanoscale 2014, 6, 4604–4610. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.Y.; Lin, K.W.; Lu, C.T.; Chen, H.I.; Chuang, H.M.; Chen, C.Y.; Cheng, C.C.; Liu, W.C. Investigation of Hydrogen-Sensing Properties of Pd/AlGaAs-Based Schottky Diodes. IEEE Trans. Electron. Devices 2003, 50, 2532–2539. [Google Scholar] [CrossRef]
- Liu, I.P.; Chang, C.H.; Ke, B.Y.; Lin, K.W. Study of a GaN Schottky diode based hydrogen sensor with a hydrogen peroxide oxidation approach and platinum catalytic metal. Int. J. Hydrogen Energy 2019, 44, 32351–32361. [Google Scholar] [CrossRef]
- Chen, G.; Choi, A.H.W.; Lai, P.T.; Tang, W.M. Schottky-diode hydrogen sensor based on InGaN/GaN multiple quantum well. J. Vac. Sci. Technol. B. 2014, 32, 011212. [Google Scholar] [CrossRef] [Green Version]
- Anderson, T.J.; Wang, H.T.; Kang, B.S.; Ren, F.; Pearton, S.J.; Osinsky, A.; Dabiran, A.; Chow, P.P. Effect of bias voltage polarity on hydrogen sensing with AlGaN/GaN Schottky diodes. Appl. Surf. Sci. 2008, 255, 2524–2526. [Google Scholar] [CrossRef]
- Jang, S.; Son, P.; Kim, J.; Lee, S.N.; Baik, K.H. Hydrogen sensitive Schottky diode using semipolar (112) AlGaN/GaN heterostructures. Sens. Actuators B 2016, 222, 43–47. [Google Scholar] [CrossRef]
- Kang, B.S.; Ren, F.; Gila, B.P.; Abernathy, C.R.; Pearton, S.J. AlGaN/GaN-based metal-oxide-semiconductor diode-based hydrogen sensor. Appl. Phys. Lett. 2004, 84, 1123–1125. [Google Scholar] [CrossRef]
- Kim, B.J.; Yoon, J.H.; Kim, J.S. Gas sensing characteristics of low-powered dual MOSFET hydrogen sensors. Mater. Chem. Phys. 2013, 142, 594–599. [Google Scholar] [CrossRef]
- Choi, J.H.; Jo, M.G.; Han, S.W.; Kim, H.; Jang, S.; Kim, S.H.; Kim, J.S.; Cha, H.Y. Hydrogen sensors Pd-functionalised AlGaN/GaN heterostructure with high sensitivity and low-power consumption. Electron. Lett. 2017, 53, 1200–1202. [Google Scholar] [CrossRef]






| Sensor Platform | Temp. | Hydrogen Concentration | Response Time | Recovery Time | Sensor Response | Power Consumption | Ref. |
|---|---|---|---|---|---|---|---|
| Diode (AlGaAs) | Room temp. | 1% | 58 s | - | 155.9% | - | [23] |
| Diode (GaN) | Room temp. | 1% | 15 s | 19 s | 1 × 105% | - | [24] |
| Diode (GaN) | 200 ℃ | 4% | - | - | 7 × 108% | - | [19] |
| Diode (GaN) | 300 ℃ | 0.081% | 25.1 s | 34.1 s | 0.11% | - | [25] |
| Diode (AlGaN/GaN) | Room temp. | 0.05% | - | - | 2.4% | - | [26] |
| Diode (AlGaN/GaN) | Room temp. | 4% | - | - | 3700% | 382 W/cm2 | [27] |
| MOS diode (AlGaN/GaN) | Room temp. | 10% | ~30 s | - | - | 5333 W/cm2 | [28] |
| FET (Si) | 150 ℃ | 0.5% | 18 s | 19 s | - | 35.8 mW (Sensor area N/A) | [29] |
| FET (AlGaN/GaN) | 200 ℃ | 4% | 3 s | - | 72% | 3.93 W/cm2 | [30] |
| FET (AlGaN/GaN) Constant voltage | 200 ℃ | 4% | < 0.4 s | 12.4 s | 80% | 347 W/cm2 | This work |
| FET (AlGaN/GaN) Constant current | 200 ℃ | 4% | < 0.4 s | 27.2 s | 120% | 0.54 W/cm2 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.-H.; Kim, H.; Sung, H.-K.; Cha, H.-Y. Investigation of Stability and Power Consumption of an AlGaN/GaN Heterostructure Hydrogen Gas Sensor Using Different Bias Conditions. Sensors 2019, 19, 5549. https://doi.org/10.3390/s19245549
Choi J-H, Kim H, Sung H-K, Cha H-Y. Investigation of Stability and Power Consumption of an AlGaN/GaN Heterostructure Hydrogen Gas Sensor Using Different Bias Conditions. Sensors. 2019; 19(24):5549. https://doi.org/10.3390/s19245549
Chicago/Turabian StyleChoi, June-Heang, Hyungtak Kim, Hyuk-Kee Sung, and Ho-Young Cha. 2019. "Investigation of Stability and Power Consumption of an AlGaN/GaN Heterostructure Hydrogen Gas Sensor Using Different Bias Conditions" Sensors 19, no. 24: 5549. https://doi.org/10.3390/s19245549






