Multi-Site Photoplethysmographic and Electrocardiographic System for Arterial Stiffness and Cardiovascular Status Assessment
Abstract
:1. Introduction
2. Materials and Methods
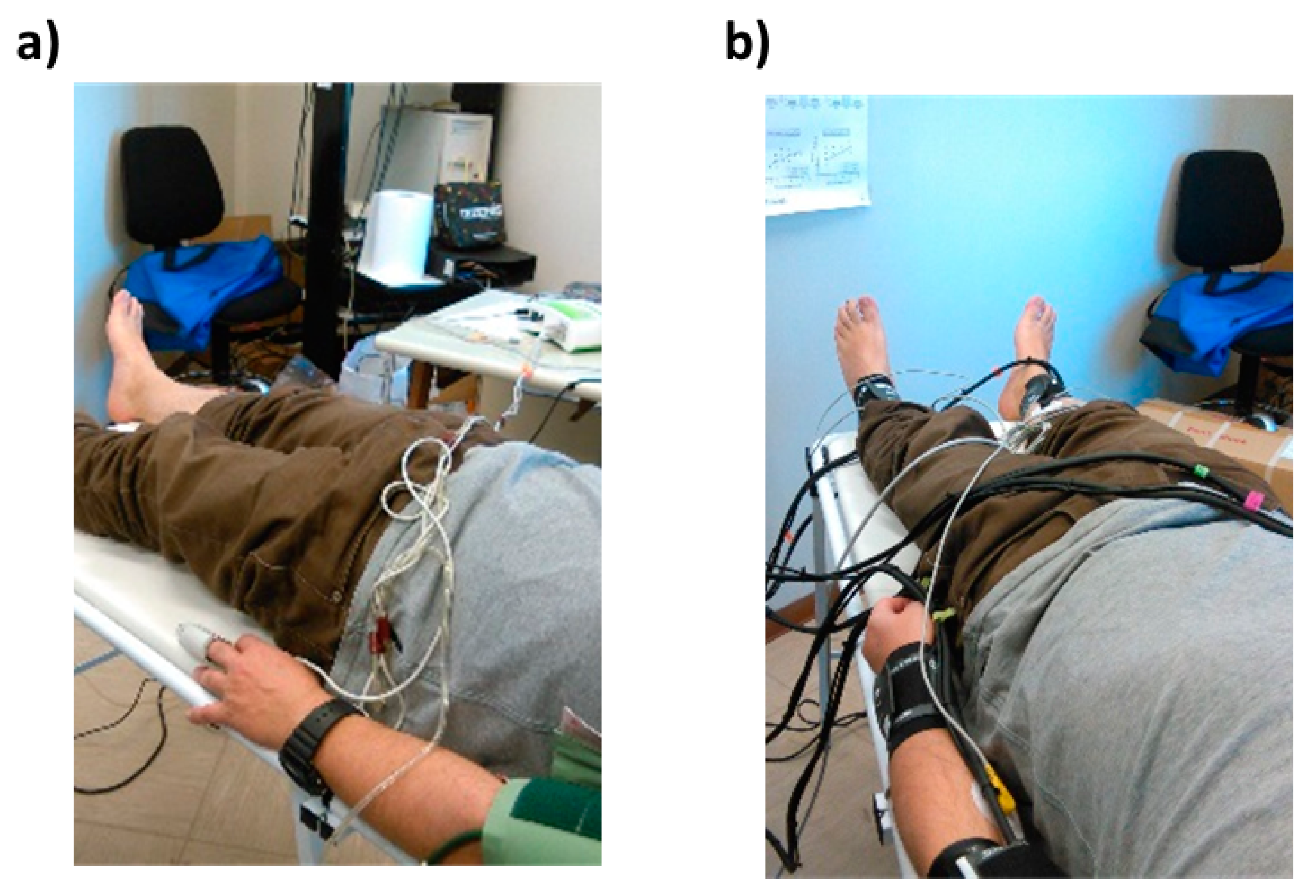
2.1. Optical Probes and Electrodes
2.2. System Architecture
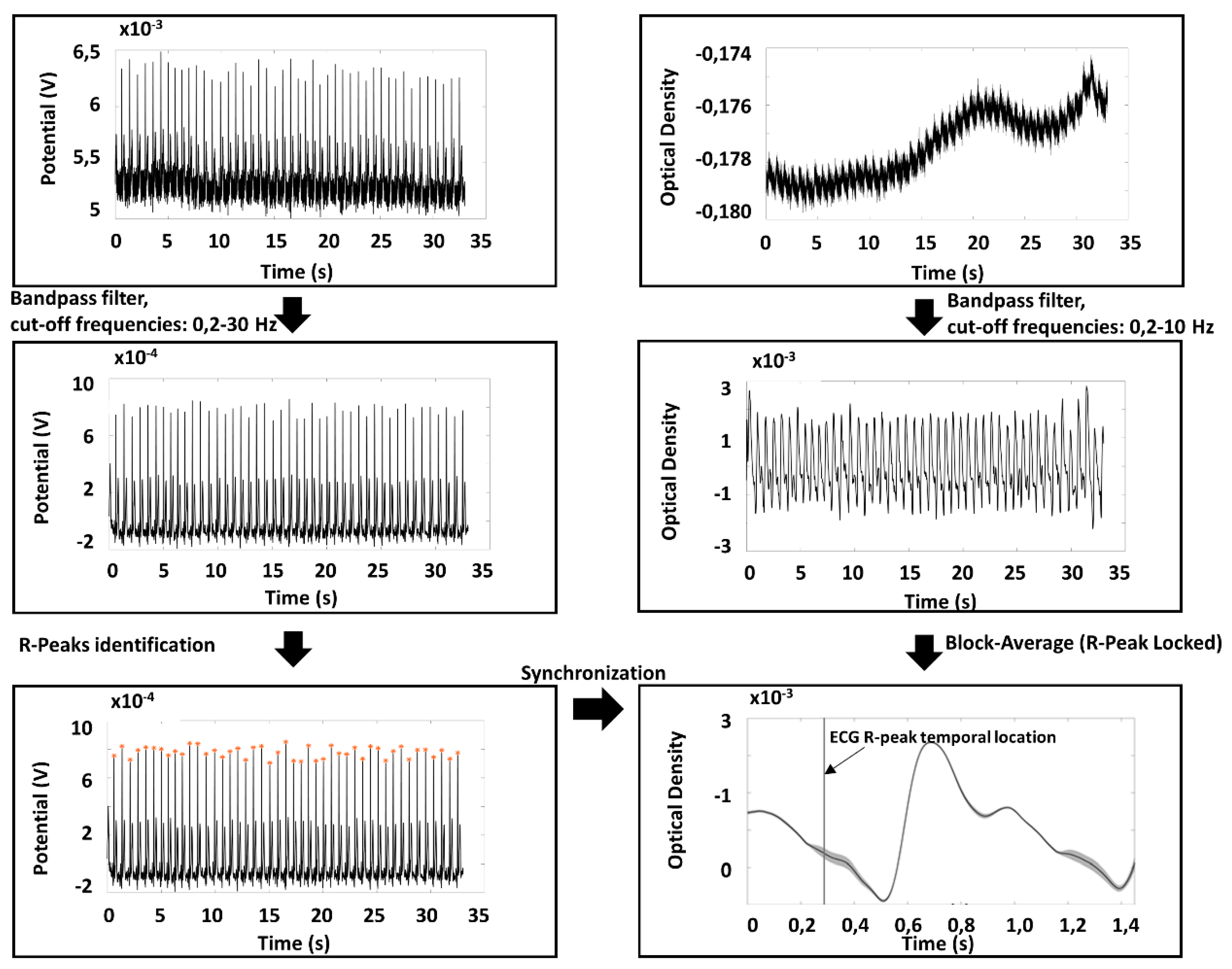
2.3. Preprocessing of PPG and ECG Signals
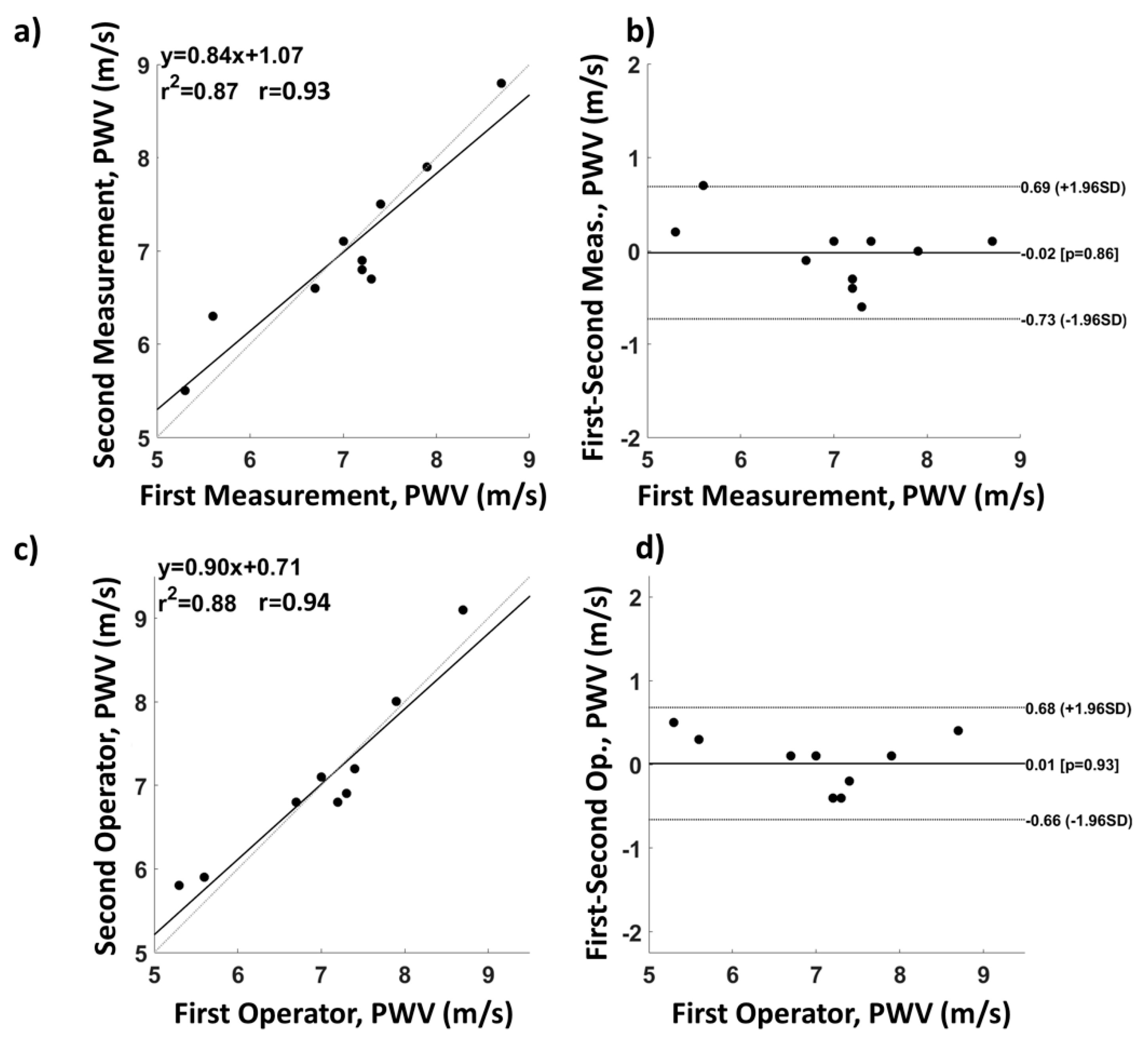
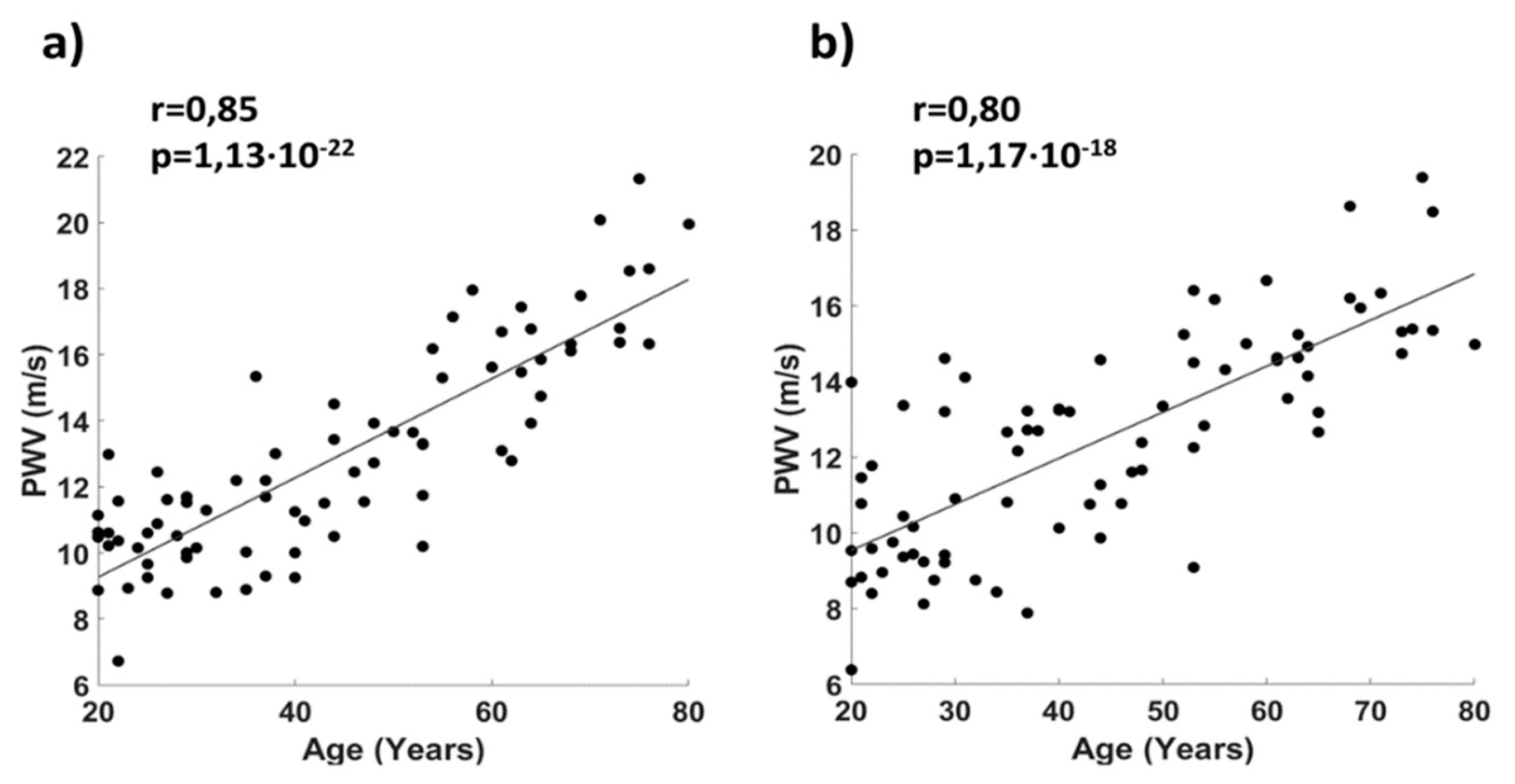
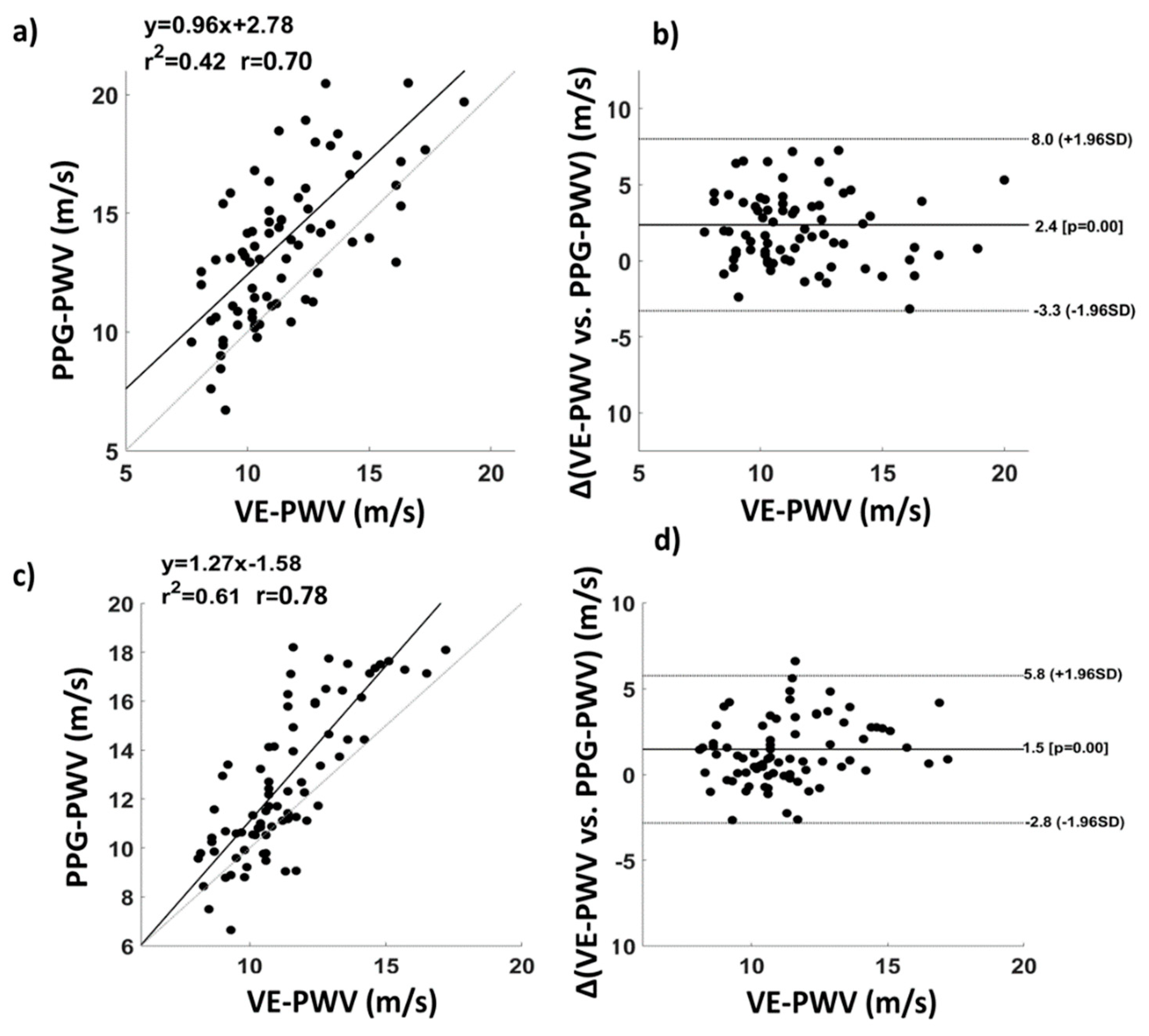
2.4. System Characterization and Validation
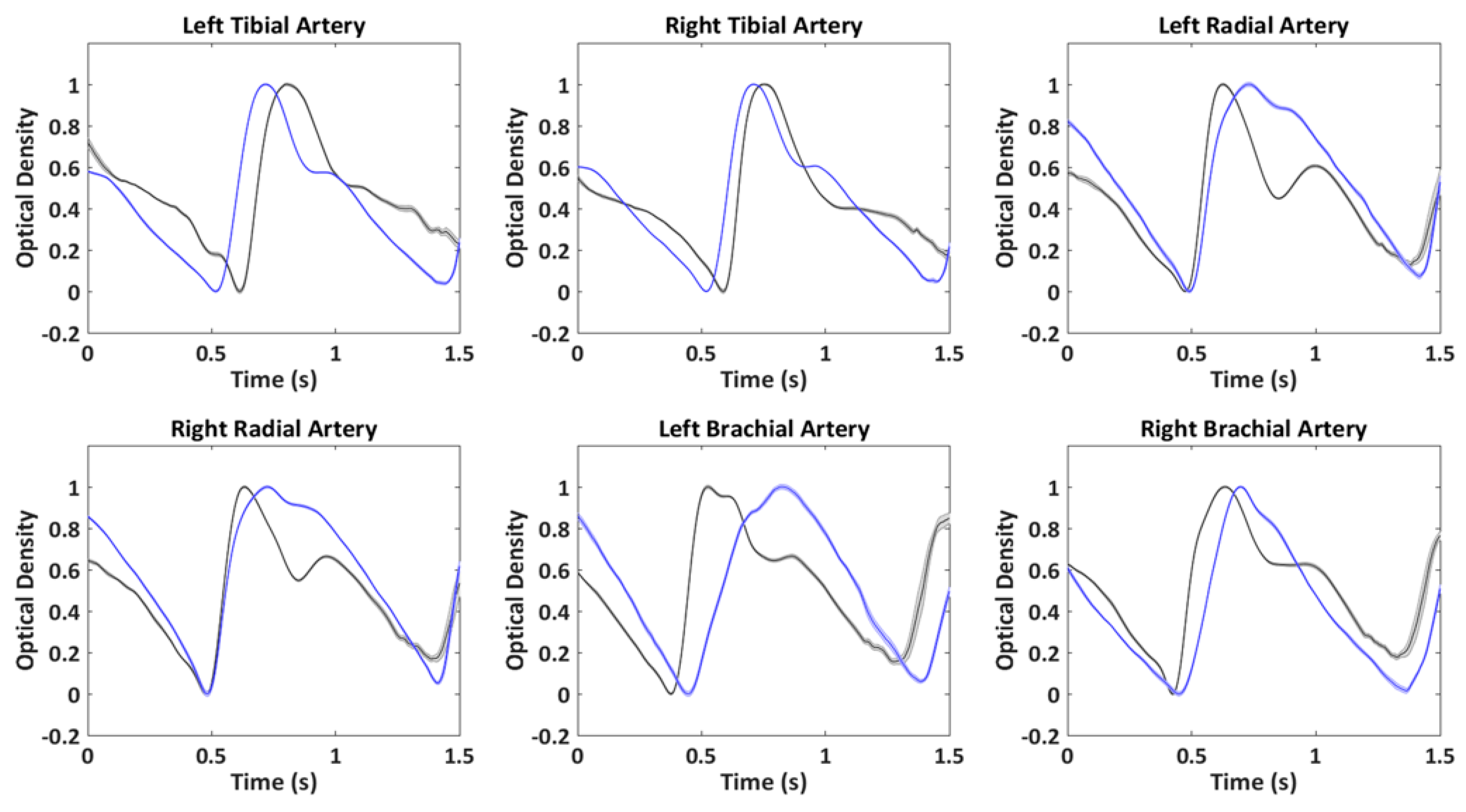
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Allen, J. Photoplethysmography and its application in clinical physiological measurement. Physiol. Meas. 2007, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancini, D.M.; Bolinger, L.; Li, H.; Kendrick, K.; Chance, B.; Wilson, J.R. Validation of near-infrared spectroscopy in humans. J. Appl. Physiol. 1994, 77, 2740–2747. [Google Scholar] [CrossRef] [PubMed]
- Rusch, T.L.; Sankar, R.; Scharf, J.E. Signal processing methods for pulse oximetry. Comput. Biol. Med. 1996, 26, 143–159. [Google Scholar] [CrossRef]
- Chiarelli, A.M.; Maclin, E.L.; Low, K.A.; Mathewson, K.E.; Fabiani, M.; Gratton, G. Combining energy and Laplacian regularization to accurately retrieve the depth of brain activity of diffuse optical tomographic data. J. Biomed. Opt. 2016, 21, 36008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.; Song, Z.; Yimin, Y. Study of pulse wave velocity noninvasive detecting instrument based on radial artery and finger photoplethysmography pulse wave. In Proceedings of the 2008 International Symposium on Intelligent Information Technology Application Workshops, Shanghai, China, 21–22 December 2008; pp. 705–708. [Google Scholar]
- Pilt, K.; Ferenets, R.; Meigas, K.; Lindberg, L.-G.; Temitski, K.; Viigimaa, M. New Photoplethysmographic Signal Analysis Algorithm for Arterial Stiffness Estimation. Available online: https://www.hindawi.com/journals/tswj/2013/169035/abs/ (accessed on 2 July 2018).
- Pilt, K.; Meigas, K.; Ferenets, R.; Temitski, K.; Viigimaa, M. Photoplethysmographic signal waveform index for detection of increased arterial stiffness. Physiol. Meas. 2014, 35, 2027. [Google Scholar] [CrossRef] [Green Version]
- Chiarelli, A.M.; Bianco, F.; Perpetuini, D.; Bucciarelli, V.; Filippini, C.; Cardone, D.; Zappasodi, F.; Gallina, S.; Merla, A. Data-driven assessment of cardiovascular ageing through multisite photoplethysmography and electrocardiography. Med. Eng. Phys. 2019, 73, 39–50. [Google Scholar] [CrossRef]
- Yousef, Q.; Reaz, M.B.I.; Ali, M.A.M. The analysis of PPG morphology: Investigating the effects of aging on arterial compliance. Meas. Sci. Rev. 2012, 12, 266–271. [Google Scholar] [CrossRef] [Green Version]
- Hansen, T.W.; Staessen, J.A.; Torp-Pedersen, C.; Rasmussen, S.; Thijs, L.; Ibsen, H.; Jeppesen, J. Prognostic Value of Aortic Pulse Wave Velocity as Index of Arterial Stiffness in the General Population. Circulation 2006, 113, 664–670. [Google Scholar] [CrossRef]
- Anliker, M.; Moritz, W.E.; Ogden, E. Transmission characteristics of axial waves in blood vessels. J. Biomech. 1968, 1, 235–246. [Google Scholar] [CrossRef] [Green Version]
- Nitzan, M.; Khanokh, B.; Slovik, Y. The difference in pulse transit time to the toe and finger measured by photoplethysmography. Physiol. Meas. 2002, 23, 85. [Google Scholar] [CrossRef]
- Perpetuini, D.; Chiarelli, A.M.; Vinciguerra, V.; Vitulli, P.; Rinella, S.; Cardone, D.; Bianco, F.; Perciavalle, V.; Gallina, S.; Fallica, G. Integrated Multi-channel PPG and ECG System for Cardiovascular Risk Assessment. In Proceedings of the 15th International Workshop on Advanced Infrared Technology and Applications (AITA 2019), Florence, Italy, 17–19 September 2019; Volume 27, p. 8. [Google Scholar]
- Sondej, T.; Sieczkowski, K.; Olszewski, R.; Dobrowolski, A. Simultaneous multi-site measurement system for the assessment of pulse wave delays. Biocybern. Biomed. Eng. 2019, 39, 488–502. [Google Scholar] [CrossRef]
- Chiarelli, A.M.; Libertino, S.; Zappasodi, F.; Mazzillo, M.C.; Di Pompeo, F.; Merla, A.; Lombardo, S.A.; Fallica, G.P. Characterization of a fiber-less, multichannel optical probe for continuous wave functional near-infrared spectroscopy based on silicon photomultipliers detectors: in-vivo assessment of primary sensorimotor response. Neurophotonics 2017, 4, 035002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sciacca, E.; Lombardo, S.; Mazzillo, M.; Condorelli, G.; Sanfilippo, D.; Contissa, A.; Belluso, M.; Torrisi, F.; Billotta, S.; Campisi, A. Arrays of Geiger mode avalanche photodiodes. IEEE Photonics Technol. Lett. 2006, 18, 1633–1635. [Google Scholar] [CrossRef]
- Buzhan, P.; Dolgoshein, B.; Filatov, L.; Ilyin, A.; Kantzerov, V.; Kaplin, V.; Karakash, A.; Kayumov, F.; Klemin, S.; Popova, E. Silicon photomultiplier and its possible applications. Nucl. Instrum. Methods Phys. Res. Sect. A 2003, 504, 48–52. [Google Scholar] [CrossRef]
- Pagano, R.; Libertino, S.; Sanfilippo, D.; Fallica, G.; Lombardo, S. Improvement of sensitivity in continuous wave near infrared spectroscopy systems by using silicon photomultipliers. Biomed. Opt. Express 2016, 7, 1183–1192. [Google Scholar] [CrossRef] [Green Version]
- Adamo, G.; Agrò, D.; Stivala, S.; Parisi, A.; Giaconia, C.; Busacca, A.C.; Fallica, G. SNR measurements of silicon photomultipliers in the continuous wave regime. Proc. SPIE OPTO 2014, 8990. [Google Scholar] [CrossRef]
- Mazzillo, M.; Ronzhin, A.; Los, S.; Abbisso, S.; Sanfilippo, D.; Valvo, G.; Carbone, B.; Piana, A.; Fallica, G.; Albrow, M.; et al. Electro-Optical Performances of p-on-n and n-on-p Silicon Photomultipliers. IEEE Trans. Electron Devices 2012, 59, 3419–3425. [Google Scholar] [CrossRef]
- Vinciguerra, V.; Ambra, E.; Maddiona, L.; Oliveri, S.; Romeo, M.F.; Mazzillo, M.; Rundo, F.; Fallica, G. Progresses towards a processing pipeline in photoplethysmogram (PPG) based on SiPMs. In Proceedings of the 2017 European Conference on Circuit Theory and Design (ECCTD), Catania, Italy, 4–6 September 2017; pp. 1–5. [Google Scholar]
- Vinciguerra, V.; Ambra, E.; Maddiona, L.; Romeo, M.; Mazzillo, M.; Rundo, F.; Fallica, G.; di Pompeo, F.; Chiarelli, A.M.; Zappasodi, F. PPG/ECG Multisite Combo System Based on SiPM Technology. In Proceedings of the Convegno Nazionale Sensori, Catania, Italy, 21–23 February 2018; Springer: Berlin, Germany, 2018; pp. 353–360. [Google Scholar]
- Barold, S.S. Willem Einthoven and the birth of clinical electrocardiography a hundred years ago. Card. Electrophysiol. Rev. 2003, 7, 99–104. [Google Scholar] [CrossRef]
- Chiarelli, A.M.; Perpetuini, D.; Filippini, C.; Cardone, D.; Merla, A. Differential pathlength factor in continuous wave functional near-infrared spectroscopy: Reducing hemoglobin’s cross talk in high-density recordings. Neurophotonics 2019, 6, 035005. [Google Scholar] [CrossRef] [Green Version]
- Bland, J.M.; Altman, D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 327, 307–310. [Google Scholar] [CrossRef]
- Dewitte, K.; Fierens, C.; Stöckl, D.; Thienpont, L.M. Application of the Bland–Altman plot for interpretation of method-comparison studies: A critical investigation of its practice. Clin. Chem. 2002, 48, 799–801. [Google Scholar] [PubMed]
- Krouwer, J.S. Why Bland–Altman plots should use X, not (Y+ X)/2 when X is a reference method. Stat. Med. 2008, 27, 778–780. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.; Lachenbruch, P.A. Paired t test. In Wiley Encyclopedia of Clinical Trials; Wiley: Hoboken, NJ, USA, 2007; pp. 1–3. [Google Scholar]
- Cohen, P.; West, S.G.; Aiken, L.S. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences; Psychology Press: Hove, UK, 2014; ISBN 1-135-46825-7. [Google Scholar]
- Yamashina, A.; Tomiyama, H.; Takeda, K.; Tsuda, H.; Arai, T.; Hirose, K.; Koji, Y.; Hori, S.; Yamamoto, Y. Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens. Res. 2002, 25, 359–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betge, S.; Kretzschmar, D.; Figulla, H.-R.; Lichtenauer, M.; Jung, C. Predictive value of the augmentation index derived vascular age in patients with newly diagnosed atherosclerosis. Heart Vessel. 2017, 32, 252–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McEniery, C.M.; Hall, I.R.; Qasem, A.; Wilkinson, I.B.; Cockcroft, J.R.; Investigators, A. Normal vascular aging: Differential effects on wave reflection and aortic pulse wave velocity: The Anglo-Cardiff Collaborative Trial (ACCT). J. Am. Coll. Cardiol. 2005, 46, 1753–1760. [Google Scholar] [CrossRef] [Green Version]
- Echt, M.; Duweling, J.; Gauer, O.H.; Lange, L. Effective compliance of the total vascular bed and the intrathoracic compartment derived from changes in central venous pressure induced by volume changes in man. Circ. Res. 1974, 34, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Westerhof, N. Snapshots of Hemodynamics; Springer: Berlin, Germany, 2018; ISBN 3-319-91932-6. [Google Scholar]
- Tsai, W.-C.; Chen, J.-Y.; Wang, M.-C.; Wu, H.-T.; Chi, C.-K.; Chen, Y.-K.; Chen, J.-H.; Lin, L.-J. Association of risk factors with increased pulse wave velocity detected by a novel method using dual-channel photoplethysmography. Am. J. Hypertens. 2005, 18, 1118–1122. [Google Scholar] [CrossRef] [Green Version]
- Madsen, H.; Thyregod, P. Introduction to General and Generalized Linear Models; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Chiarelli, A.M.; Romani, G.L.; Merla, A. Fast optical signals in the sensorimotor cortex: General Linear Convolution Model applied to multiple source–detector distance-based data. NeuroImage 2014, 85, 245–254. [Google Scholar] [CrossRef]
- Perpetuini, D.; Cardone, D.; Filippini, C.; Chiarelli, A.M.; Merla, A. Modelling Impulse Response Function of Functional Infrared Imaging for General Linear Model Analysis of Autonomic Activity. Sensors 2019, 19, 849. [Google Scholar] [CrossRef] [Green Version]
- Frontiers|Sudden Cardiac Risk Stratification with Electrocardiographic Indices—A Review on Computational Processing, Technology Transfer, and Scientific Evidence|Physiology. Available online: https://www.frontiersin.org/articles/10.3389/fphys.2016.00082/full (accessed on 10 December 2019).




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perpetuini, D.; Chiarelli, A.M.; Maddiona, L.; Rinella, S.; Bianco, F.; Bucciarelli, V.; Gallina, S.; Perciavalle, V.; Vinciguerra, V.; Merla, A.; et al. Multi-Site Photoplethysmographic and Electrocardiographic System for Arterial Stiffness and Cardiovascular Status Assessment. Sensors 2019, 19, 5570. https://doi.org/10.3390/s19245570
Perpetuini D, Chiarelli AM, Maddiona L, Rinella S, Bianco F, Bucciarelli V, Gallina S, Perciavalle V, Vinciguerra V, Merla A, et al. Multi-Site Photoplethysmographic and Electrocardiographic System for Arterial Stiffness and Cardiovascular Status Assessment. Sensors. 2019; 19(24):5570. https://doi.org/10.3390/s19245570
Chicago/Turabian StylePerpetuini, David, Antonio Maria Chiarelli, Lidia Maddiona, Sergio Rinella, Francesco Bianco, Valentina Bucciarelli, Sabina Gallina, Vincenzo Perciavalle, Vincenzo Vinciguerra, Arcangelo Merla, and et al. 2019. "Multi-Site Photoplethysmographic and Electrocardiographic System for Arterial Stiffness and Cardiovascular Status Assessment" Sensors 19, no. 24: 5570. https://doi.org/10.3390/s19245570





