Vital Sign Monitoring and Cardiac Triggering at 1.5 Tesla: A Practical Solution by an MR-Ballistocardiography Fiber-Optic Sensor
Abstract
:1. Introductionn
2. State-of-the-Art
3. Methods
3.1. Fiber Bragg Grating (FBG)
3.2. Encapsulation Process of the Sensor
3.3. Grating Ballistocardiography
3.4. Signal Processing
4. Experimental Setup
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Weckesser, M.; Posse, S.; Olthoff, U.; Kemna, L.; Dager, S.; Müller-Gärtner, H.W. Functional imaging of the visual cortex with bold-contrast MRI: Hyperventilation decreases signal response. Mag. Res. Med. 1999, 41, 213–216. [Google Scholar] [CrossRef] [Green Version]
- Giardino, N.D.; Friedman, S.D.; Dager, S.R. Anxiety, respiration, and cerebral blood flow: Implications for functional brain imaging. Compr. Psychiatry 2007, 48, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, S.; Yamada, T.; Okuyama, Y.; Morita, T.; Sanada, S.; Tsukamoto, Y. Cardiac iodine-123 metaiodobenzylguanidine imaging predicts sudden cardiac death independently of left ventricular ejection fraction in patients with chronic heart failure and left ventricular systolic dysfunction: results from a comparative study with signal-averaged electrocardiogram, heart rate variability, and QT dispersion. J. Am. Coll. Cardiol. 2009, 53, 426–435. [Google Scholar] [PubMed]
- Zabel, M.; Acar, B.; Klingenheben, T.; Franz, M.R.; Hohnloser, S.H.; Malik, M. Analysis of 12-lead T-wave morphology for risk stratification after myocardial infarction. Circulation 2000, 102, 1252–1257. [Google Scholar] [CrossRef] [PubMed]
- Ogura, R.; Hiasa, Y.; Takahashi, T.; Yamaguchi, K.; Fujiwara, K.; Ohara, Y.; Hosokawa, S. Specific findings of the standard 12-lead ECG in patients with takotsubo’ cardiomyopathy. Circ. J. 2003, 67, 687–690. [Google Scholar] [CrossRef] [PubMed]
- Chakeres, D.W.; Kanarlu, A.; Boudoulas, H.; Young, D.C. Effect of static magnetic field exposure of up to 8 Tesla on sequential human vital sign measurements. J. Magn. Reson. Imaging 2003, 18, 346–352. [Google Scholar] [CrossRef] [Green Version]
- Jekic, M.; Ding, Y.; Dzwonczyk, R. Magnetic field threshold for accurate electrocardiography in the MRI environment. Magn. Reson. Med. 2010, 64, 1586–1591. [Google Scholar] [CrossRef] [Green Version]
- Krug, J.W.; Rose, G. Magnetohydrodynamic distortions of the ECG in different MR scanner configurations. In Proceedings of the 2011 Computing in Cardiology, Hangzhou, China, 18–21 September 2011; pp. 769–772. [Google Scholar]
- Lanzer, P.; Barta, C.; Botvinick, E.H.; Wiesendanger, H.U.; Modin, G.; Higgins, C.B. ECG-synchronized cardiac MR imaging: Method and evaluation. Radiology 1985, 155, 681–686. [Google Scholar] [CrossRef]
- Weissler, A.M.; Harris, W.S.; Schoenfeld, C.D. Systolic Time Intervals in Heart Failure in Man. Circulation 1968, 37, 149–159. [Google Scholar] [CrossRef]
- Berne, R.M.; Levy, M.N. Cardiovascular Physiology; Mosby: St. Louis, MO, USA, 1997. [Google Scholar]
- Brau, A.C.; Wheeler, C.T.; Hedlund, L.W.; Johnson, G.A. Fiber-optic stethoscope: A cardiac monitoring and gating system for magnetic resonance microscopy. Magn. Reson. Med. 2002, 47, 314–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baliyan, V.; Das, C.J.; Sharma, R.; Gupta, A.K. Diffusion weighted imaging: Technique and applications. World J. Radiol. 2016, 8, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Biel, L.; Pettersson, O.; Philipson, L.; Wide, P. ECG analysis: A new approach in human identification. IEEE Trans. Ind. Meas. 2001, 50, 808–812. [Google Scholar] [CrossRef]
- Zhang, Y.; Hou, Z. An algorithm for evaluating the ECG signal quality in 12 lead ECG monitoring system. In Proceedings of the IEEE International Conference on Software Engineering and Service Sciences (ICSESS), Beijing, China, 23–25 September 2015; pp. 453–456. [Google Scholar]
- Bousseljot, R.; Kreiseler, D. ECG signal analysis by pattern comparison. Comput. Cardiol. 1998, 0, 349–352. [Google Scholar]
- Araoye, M.A.; Omotoso, A.B.; Opadijo, G.O. The orthogonal and 12 lead ECG in adult negroes with systemic hypertension: comparison with age-matched control. West Afr. J. med. 1998, 17, 157–164. [Google Scholar] [PubMed]
- Tse, Z.T.H.; Dumoulin, C.L.; Clifford, G.D.; Schweitzer, J.; Qin, L.; Oster, J.; Jerosch-Herold, M.; Kwong, R.Y.; Michaud, G.; Stevenson, W.G.; et al. 1.5 Tesla MRI-Conditional 12-lead ECG for MR Imaging and Intra-MR Intervention. Mag. Reson. Med. 2014, 71, 1336–1347. [Google Scholar] [CrossRef] [PubMed]
- Dabaghyan, M.; Zhang, S.H.; Ward, J.; Kwong, R.Y.; Stevenson, W.G.; Watkins, R.D.; Zion, T.T.; Schmidt, E.J. 3 T cardiac imaging with on-line 12-lead ECG monitoring. J. Cardiovasc. Mag. Reson. 2016, 18, 212. [Google Scholar] [CrossRef]
- Frauenrath, T.; Hezel, F.; Renz, W.; D’Orth, T.; Dieringer, M.; Von Knobelsdorff-Brenkenhoff, F.; Prothmann, M.; Schulz-Menger, J.; Niendorf, T. Acoustic cardiac triggering: A practical solution for synchronization and gating of cardiovascular magnetic resonance at 7 Tesla. J. Cardiovasc. Mag. Reson. 2010, 12. [Google Scholar] [CrossRef]
- Maderwald, S.; Orzada, S.; Lin, Z.; Schäfer, L.C.; Bitz, A.K.; Kraff, O.; Brote, I.; Häring, L.; Czylwik, A.; Zenge, M.O.; et al. 7 Tesla Cardiac Imaging with a Phonocardiogram Trigger Device. Proc. Int. Soc. Mag. Reson. Med. 2011, 19, 201. [Google Scholar]
- Frauenrath, T.; Hezel, F.; Heinrichs, U.; Kozerke, S.; Utting, J.F.; Kob, M.; Butenweg, C.; Boesiger, P.; Niendorf, T. Feasibility of cardiac gating free of interference with electro-magnetic fields at 1.5 Tesla, 3.0 Tesla and 7.0 Tesla using an MR-stethoscope. Investig. Radiol. 2009, 44. [Google Scholar] [CrossRef]
- Rotariu, C.; Cristea, C.; Arotaritei, D.; Bozomitu, R.G.; Pasarica, A. Continuous respiratory monitoring device for detection of sleep apnea episodes. In Proceedings of the 2016 IEEE 22nd International Symposium for Design and Technology in Electronic Packaging (SIITME), Oradea, Romania, 20–23 Octorber 2016; pp. 106–109. [Google Scholar]
- Vasanawala, S.S.; Jackson, E. A method of rapid robust respiratory synchronization for MRI. Pediatr. Radiol. 2010, 40, 1690–1692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, J.-W.; Noh, Y.-S.; Kwon, Y.-S.; Kim, W.-K.; Yoon, H.-R. Improvement of dynamic respiration monitoring through sensor fusion of accelerometer and gyro-sensor. J. Electr. Eng. Technol. 2014, 9, 334–343. [Google Scholar] [CrossRef]
- Favero, F.C.; Villatoro, J.; Pruneri, V. Microstructured optical fiber interferometric breathing sensor. J. Biomed. Opt. 2012, 17. [Google Scholar] [CrossRef] [PubMed]
- Sprager, S.; Donlagic, D.; Zazula, D. Monitoring of basic human vital functions using optical interferometer. In Proceedings of the IEEE 10th International Conference on Signal Processing (ICSP), Beijing, China, 24–28 October 2010; pp. 1738–1741. [Google Scholar]
- Sprager, S.; Donlagic, D.; Zazula, D. Estimation of heart rate, respiratory rate and motion by using optical interferometer as body sensor. In Proceedings of the IASTED International Conference on Signal and Image Processing, Honolulu, HI, USA, 14–16 December 2011; pp. 280–287. [Google Scholar]
- Sprager, S.; Zazula, D. Detection of heartbeat and respiration from optical interferometric signal by using wavelet transform. Comput. Methods Prog. Biomed. 2013, 111, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Will, C.; Shi, K.; Lurz, F.; Weigel, R.; Koelpin, A. Intelligent signal processing routine for instantaneous heart rate detection using a Six-Port microwave interferometer. In Proceedings of the 2015 International Symposium on Intelligent Signal Processing and Communication Systems (ISPACS), Nusa Dua, Bali, 9–12 November 2015; pp. 483–487. [Google Scholar]
- Zazula, D.; Sprager, S. Detection of the first heart sound using fibre-optic interferometric measurements and neural networks. In Proceedings of the Symposium on Neural Network Applications in Electrical Engineering, Belgrade, Serbia, 20–22 September 2012; pp. 171–176. [Google Scholar]
- Byeong, H.L.; Young, H.K.; Kwan, S.P.; Joo, B.E.; Myoung, J.K.; Byung, S.R.; Hae, Y.C. Interferometric Fiber Optic Sensors. Sensors 2012, 12, 2467–2486. [Google Scholar] [Green Version]
- Hsieh, Y.H.; Chen, N.K. Micro tapered Mach–Zehnder fiber interferometer for monitoring pressure fluctuation and its applications in pulse rate detection. In Proceedings of the 2013 6th IEEE/International Conference on Advanced Infocomm Technology (ICAIT), Taiwan, China, 6–9 July 2013; pp. 113–115. [Google Scholar]
- Roriz, P.; Carvalho, L.; Frazão, O.; Santos, J.L.; Simoes, A.J. From conventional sensors to fibre optic sensors for strain and force measurements in biomechanics applications: A review. J. Biomech. 2014, 47, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
- Dziuda, L. Fiber-optic sensors for monitoring patient physiological parameters: A review of applicable technologies and relevance to use during magnetic resonance imaging procedures. J. Biomech. 2015, 20, 010901. [Google Scholar] [CrossRef]
- Chethana, K.; Guru Prasad, A.S.; Omkar, S.N.; Asokan, S. Fiber bragg grating sensor-based device for simultaneous measurement of respiratory and cardiac activities. J. Biophotonics 2016, 10, 278–285. [Google Scholar] [CrossRef]
- Dzuida, L.; Skibniewski, F.W.; Krej, M.; Lewandowski, J. Monitoring respiration and cardiac activity using fiber Bragg grating-based sensor. IEEE Trans. Biomed. Eng. 2012, 59, 1934–1942. [Google Scholar] [CrossRef]
- Dziuda, L.; Krej, M.; Skibniewski, F.W. Fiber Bragg grating strain sensor incorporated to monitor patient vital signs during MRI. IEEE Sens. J. 2013, 13, 4986–4991. [Google Scholar] [CrossRef]
- Emamian, M.; Hasanian, S.M.; Tayefi, M.; Bijari, M.; Movahedian far, F.; Shafiee, M.; Avan, A.; Heidari-Bakavoli, A.; Moohebati, M.; Ebrahimi, M.; et al. Association of hematocrit with blood pressure and hypertension. J. Clin. Lab. Anal. 2017, 31. [Google Scholar] [CrossRef] [PubMed]
- Hang-yin, L. Experimental study of non-uniform strains in composites with embedded fiber bragg grating. Meas. Sci. Technol. 2005, 16. [Google Scholar] [CrossRef]
- Anoshkin, A.N.; Shipunov, G.S.; Voronkov, A.A.; Shardakov, I.N. Effect of temperature on the spectrum of fiber Bragg grating sensors embedded in polymer composite. AIP Conf. Proc. 1909. [Google Scholar] [CrossRef]
- Zhang, Y. The Packaging Technology Study on Smart Composite Structure Based on the Embedded FBG Sensor. IOP Conf. Ser. Mater. Sci. Eng. 2018, 322. [Google Scholar] [CrossRef]
- Fajkus, M.; Nedoma, J.; Martinek, R.; Vasinek, V.; Nazeran, H.; Siska, P. A Non-invasive Multichannel Hybrid Fiber-optic Sensor System for Vital Sign Monitoring. Sensors 2017, 17, 111. [Google Scholar] [CrossRef] [PubMed]
- Nedoma, J.; Kepak, S.; Fajkus, M.; Cubik, J.; Siska, P.; Martinek, R.; Krupa, P. Magnetic Resonance Imaging Compatible Non-Invasive Fibre-Optic Sensors Based on the Bragg Gratings and Interferometers in the Application of Monitoring Heart and Respiration Rate of the Human Body: A Comparative Study. Sensors 2018, 18, 3713. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Z.; Elvin, C.S.M.; Janice, L.H.Y.; Ng, S.H.; Teo, J.T.; Wu, R. Textile Fiber Optic Microbend Sensor Used for Heartbeat and Respiration Monitoring. IEEE Sens. J. 2015, 15, 757–761. [Google Scholar] [CrossRef]
- Grilett, A.; Kinet, D.; Witt, J.; Schukar, M.; Krebber, K.; Pirotte, F.; Depre, A. Optical fiber sensors embedded into medical textiles for healthcare monitoring. IEEE Sens. J. 2008, 8, 1215–1222. [Google Scholar] [CrossRef]
- Chen, D.; Lau, J.T.; Teo, S.H.; Ng, X.; Yang, P.; Kei, L. Simultaneous measurement of breathing rate and heart rate using a microbend multimode fiber optic sensor. J. Biomed. Opt. 2014, 19, 57001. [Google Scholar] [CrossRef]
- Krehel, M.; Schmid, M.; Rossi, R.M.; Boesel, F.L.; Bona, G.L.; Scherer, L.J. An optical fibre-based sensor for respiratory monitoring. Sensors 2014, 14, 13088–13101. [Google Scholar] [CrossRef] [PubMed]
- Leal-Junior, A.G.; Díaz, C.R.; Leitão, C.; Pontes, M.J.; Marques, C.; Frizera, A. Polymer optical fiber-based sensor for simultaneous measurement of breath and heart rate under dynamic movements. Opt. Laser Technol. 2018, 109, 429–436. [Google Scholar] [CrossRef]
- Taffoni, F.; Formica, D.; Saccomandi, P.; Di Pino, G.; Schena, E. Optical fiber-based MR-compatible sensors for medical applications: An overview. Sensors 2013, 13, 14105–14120. [Google Scholar] [CrossRef] [PubMed]
- Grillet, A.; Kinet, D.; Witt, J.; Schukar, M.; Krebber, K.; Pirotte, F.; Depré, A. Optical fibre sensors embedded into medical textiles for monitoring of respiratory movements in MRI environment. In Proceedings of the Third European Workshop on Optical Fibre Sensors, Napoli, Italy, 4–6 July 2007. [Google Scholar]
- Moerman, K.M.; Sprengers, A.M.J.; Nederveen, A.J.; Simms, C.K. A novel MRI compatible soft tissue indentor and fibre Bragg grating force sensor. Med. Eng. Phys. 2013, 35, 486–499. [Google Scholar] [CrossRef] [Green Version]
- Tan, U.-X.; Yang, B.; Gullapalli, R.; Desai, J.P. Triaxial MRI-compatible fiber-optic force sensor IEEE. Trans. Robot. 2011, 27, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Yoo, W.J.; Jang, K.W.; Seo, J.K.; Heo, J.Y.; Moon, J.S.; Park, J.Y.; Lee, B. Development of respiration sensors using plastic optical fiber for respiratory monitoring inside MRI system. J. Opt. Soc. Korea 2010, 14, 235–239. [Google Scholar] [CrossRef]
- Kam, W.; Mohammed, W.S.; Leen, G.; O’Sullivan, K.; O’Keeffe, M.; O’Keeffe, S.; Lewis, E. All plastic optical fiber-based respiration monitoring sensor. In Proceedings of the IEEE Sensors, New Delhi, India, 29–31 December 2017; pp. 1–3. [Google Scholar]
- Filograno, M.L.; Pisco, M.; Catalano, A.; Forte, E.; Aiello, M.; Cavaliere, C.; Soricelli, A.; Davino, D.; Visone, C.; Cutolo, A.; et al. Triaxial Fiber Optic Magnetic Field Sensor for Magnetic Resonance Imaging. J. Lightw. Technol. 2017, 35, 3924–3933. [Google Scholar] [CrossRef]
- Nedoma, J.; Fajkus, M.; Novak, M.; Strbikova, N.; Vasinek, V.; Nazeran, H.; Vanus, J.; Perecar, F.; Martinek, R. Validation of a novel fiber-optic sensor system for monitoring cardiorespiratory activities during mri examinations. Adv. Electr. Electron. Eng. 2017, 15, 536–543. [Google Scholar] [CrossRef]
- Su, H.; Shang, W.; Li, G.; Patel, N.; Fischer, G.S. An MRI-Guided Telesurgery System Using a Fabry-Perot Interferometry Force Sensor and a Pneumatic Haptic Device. Ann. Biomed. Eng. 2017, 45, 1917–1928. [Google Scholar] [CrossRef]
- Dziuda, L.; Skibniewski, F.W. A new approach to ballistocardiographic measurements using fibre Bragg grating-based sensors. Biocybern. Biomed. Eng. 2014, 34, 101–106. [Google Scholar] [CrossRef]
- Agarwal, R.P.; O’Regan, D. Two-Dimensional Wave Equation. In Ordinary and Partial Differential Equations; Springer: New York, NY, USA, 2009. [Google Scholar]
- Kersay, A.D.; Davis, M.A.; Patrick, H.J.; LeBlanc, M.; Koo, K.P.; Askins, C.G.; Putnam, M.A.; Friebele, E.J. Fiber grating sensors. J. Lightw. Technol. 1997, 15, 1442–1463. [Google Scholar] [CrossRef]
- Pinheiro, E.; Postolache, O.; Girao, P. Theory and Developments in an Unobtrusive Cardiovascular System Representation: Ballistocardiography. Open Biomed. Eng. J. 2010, 4. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, A.; Pihlajamäki, K.; Jalonen, J.; Laaksonen, V.; Alihanka, J. Static-charge-sensitive bed ballistocardiography in cardiovascular monitoring. Clin. Physiol. 1996, 16, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Inan, O.T. Novel Technologies for Cardiovascular Monitoring Using Ballistocardiography and Electrocardiography; Proquest: Ann Arbor, MI, USA, 2011. [Google Scholar]
- Wiard, R.M.; Inan, O.T.; Giovangrandi, L.; Cuttino, C.M.; Kovacs, G.T.A. Preliminary results from standing ballistocardiography measurements in microgravity. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013. [Google Scholar]
- Zhu, Y.; Zhang, H.; Jayachandran, M.; Ng, A.K.; Biswas, J.; Chen, Z. Ballistocardiography with fiber optic sensor in headrest position: A feasibility study and a new processing algorithm. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013. [Google Scholar]
- Jiao, C.; Lyons, P.; Zare, A.; Rosales, L.; Skubic, M. Heart beat characterization from ballistocardiogram signals using extended functions of multiple instances. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society EMBS, Lake Buena Vista, FL, USA, 16–20 August 2016. [Google Scholar]
- Product FBGuard. Available online: http://www.safibra.cz/en/fbguard-interrogation-unit (accessed on 6 December 2018).
- Product TSD221-MRI. Available online: https://www.biopac.com/wp-content/uploads/TSD221-MRI.pdf (accessed on 6 December 2018).
- Bland, J.M.; Altman, D.G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xu, L.; Schoepf, U.J.; Wichmann, J.L.; Fox, M.A.; Yan, J.; Fan, Z.; Zhang, Z. Prospectively ECG-triggered sequential dual-source coronary CT angiography in patients with atrial fibrillation: Influence of heart rate on image quality and evaluation of diagnostic accuracy. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Stäb, D.; Roessler, J.; O’Brien, K.; Hamilton-Craig, C.; Barth, M. ECG Triggering in Ultra-High Field Cardiovascular MRI. Tomography 2016, 2, 167–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dziuda, L.; Skibniewski, F.W.; Krej, M.; Baran, P.M. Fiber Bragg grating-based sensor for monitoring respiration and heart activity during magnetic resonance imaging examinations. J. Biomed. Opt. 2013, 18, 057006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
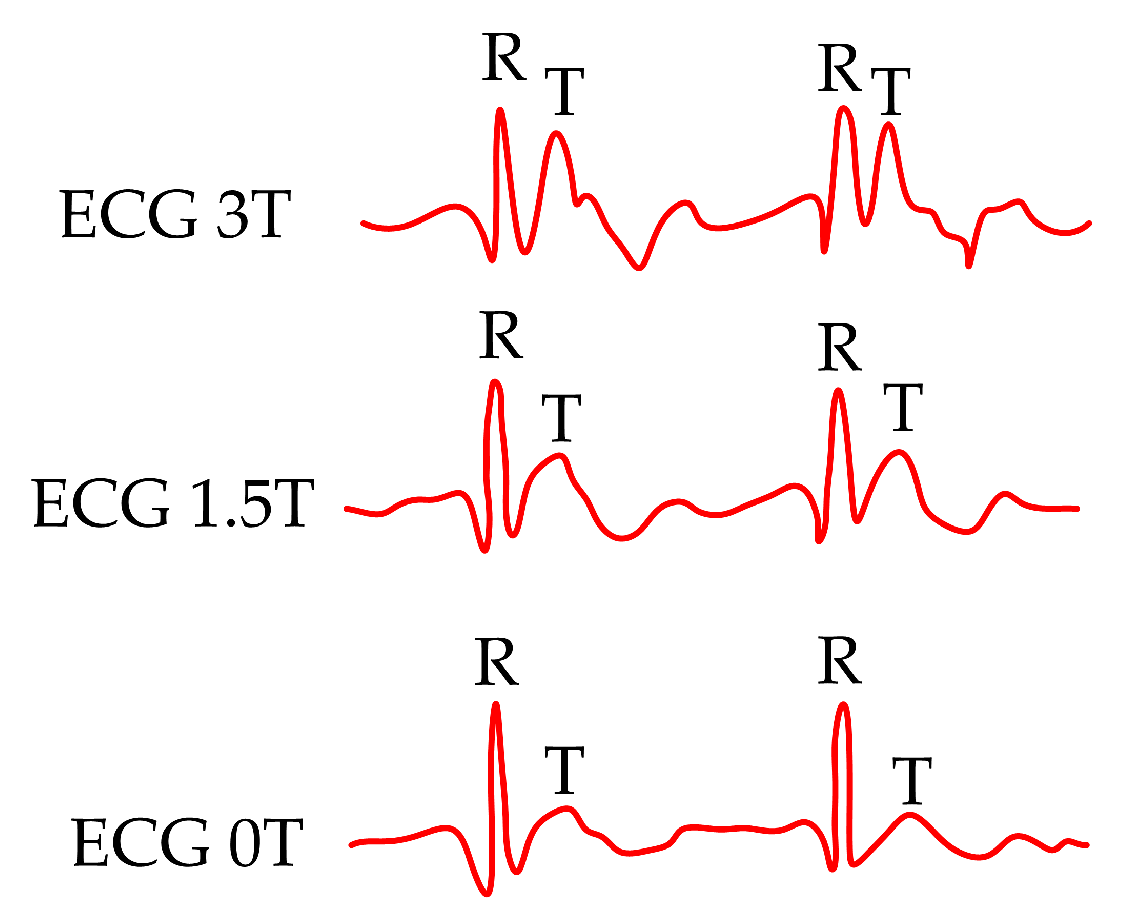
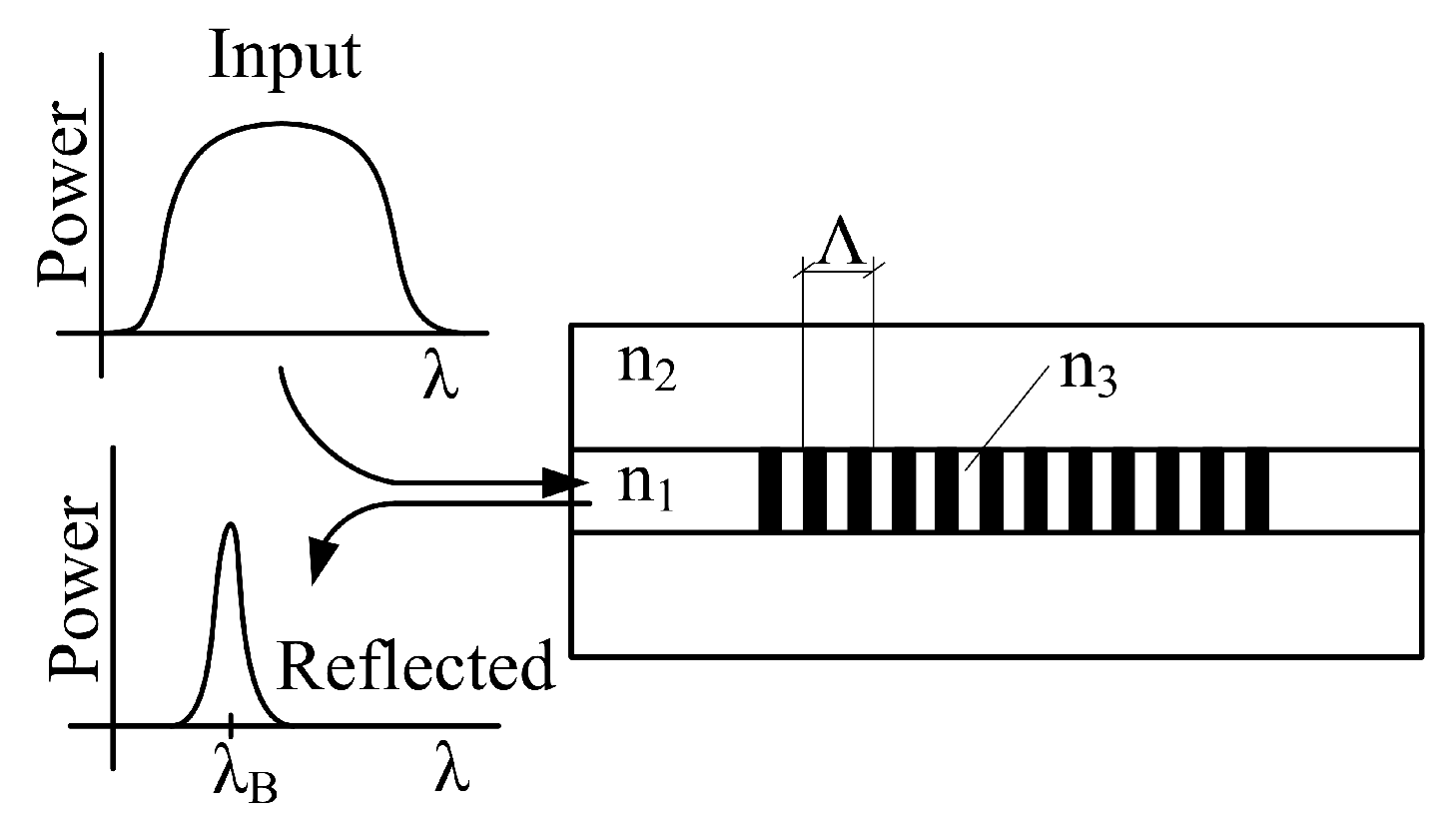
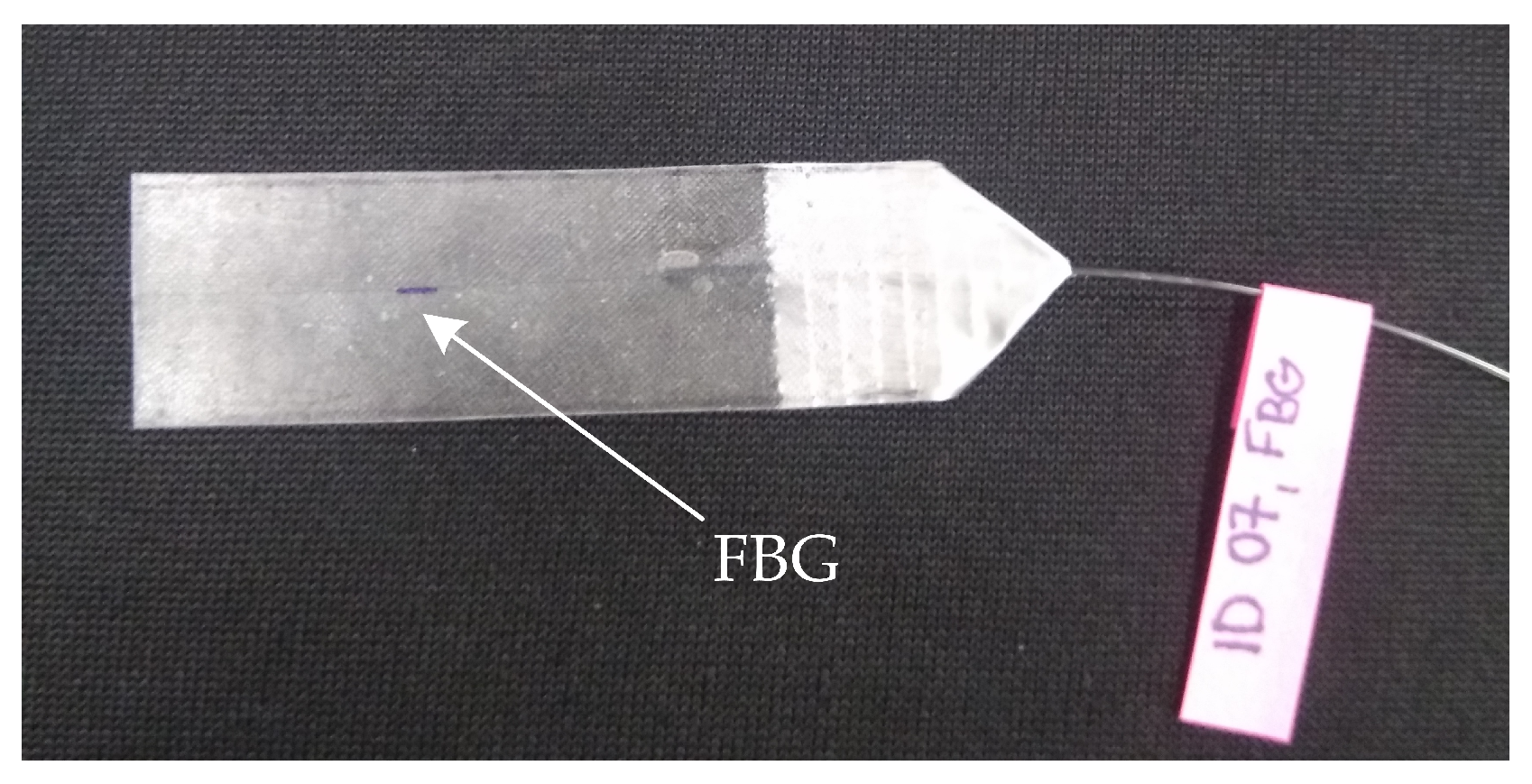
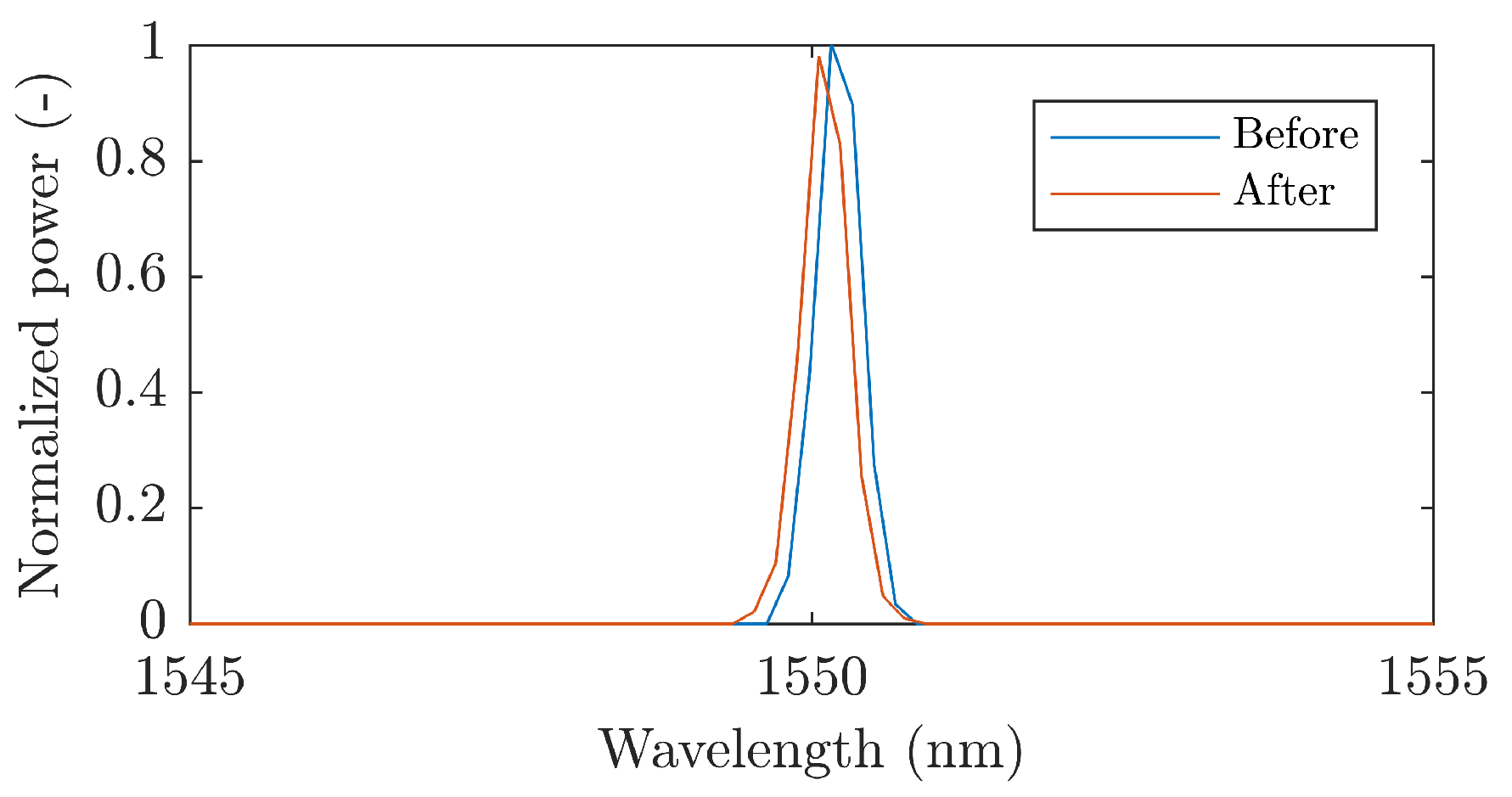
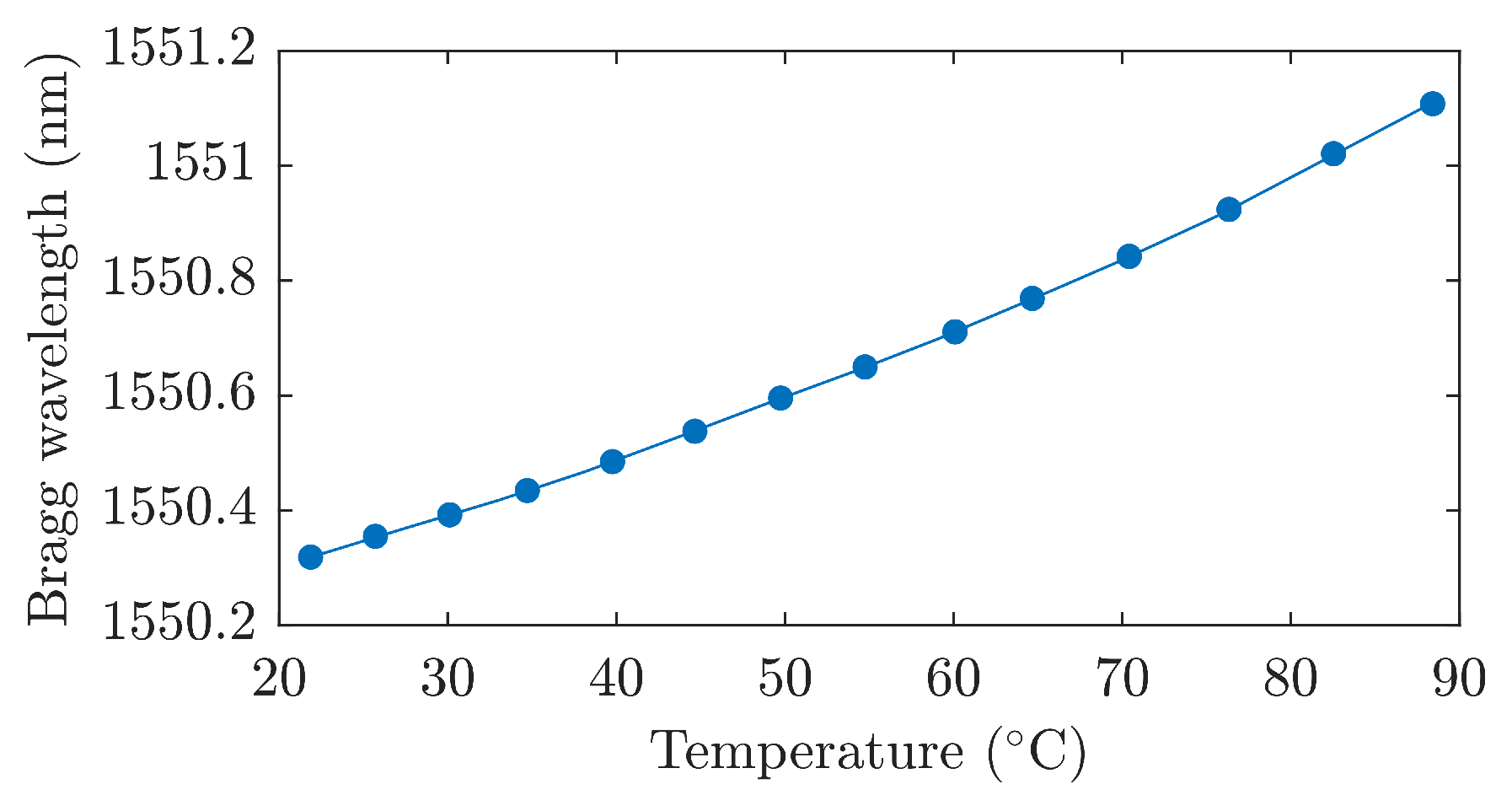
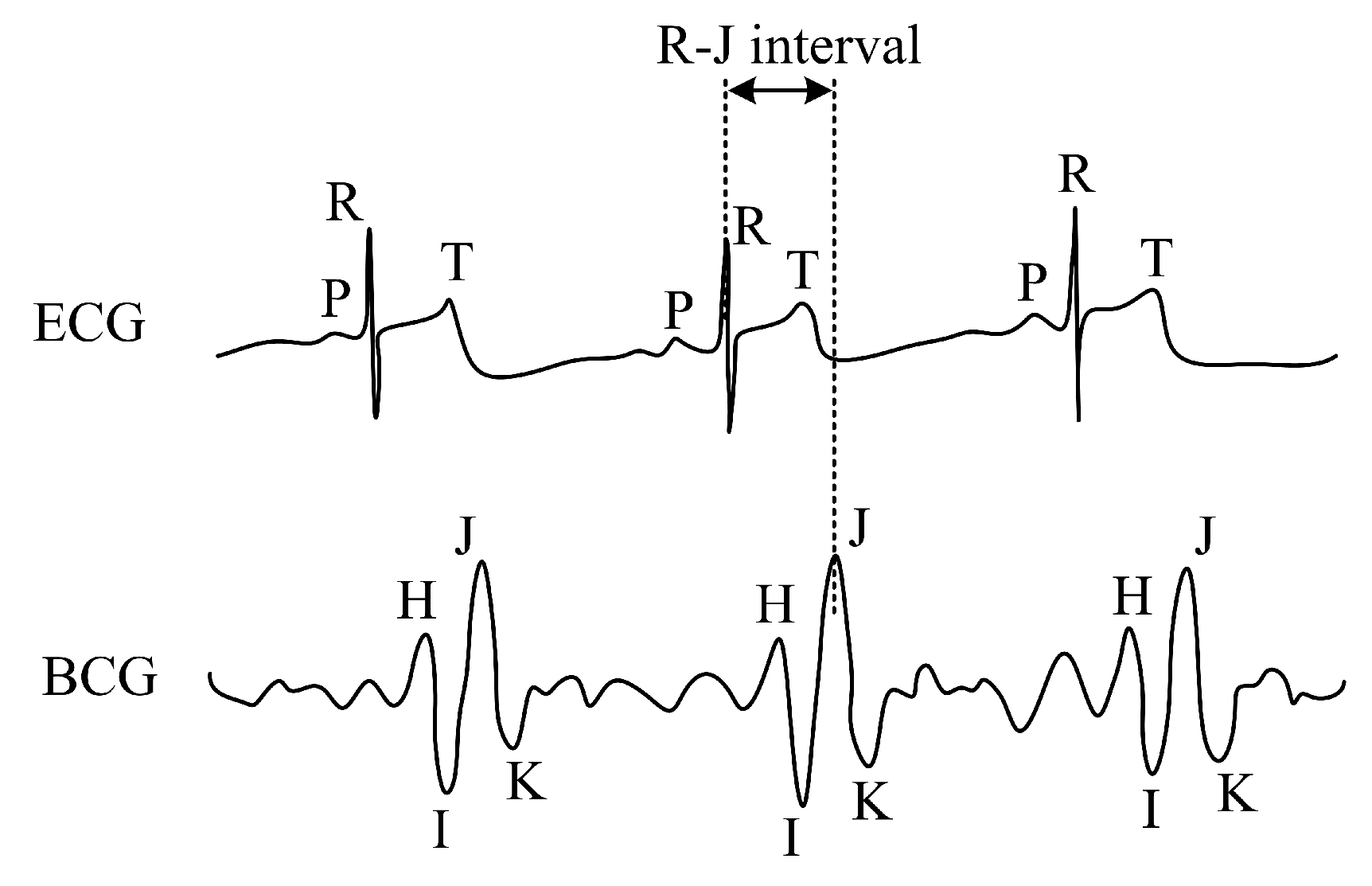

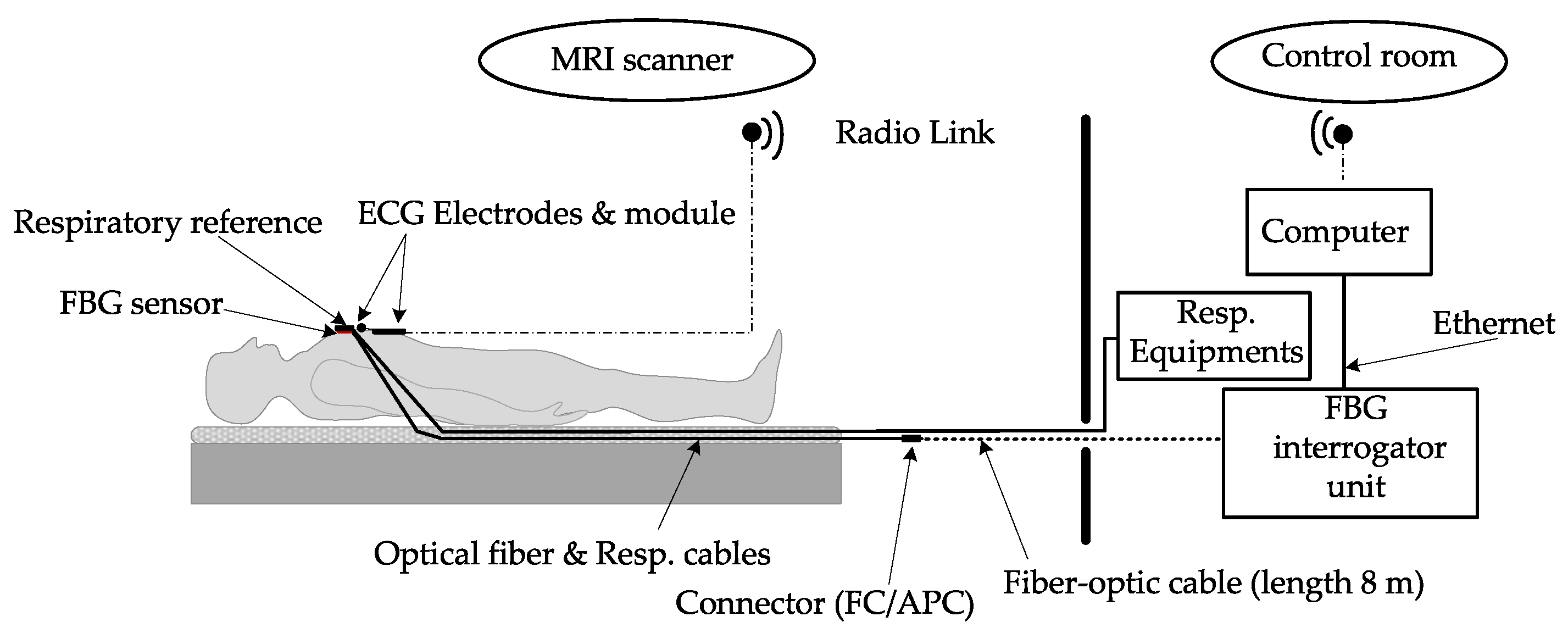

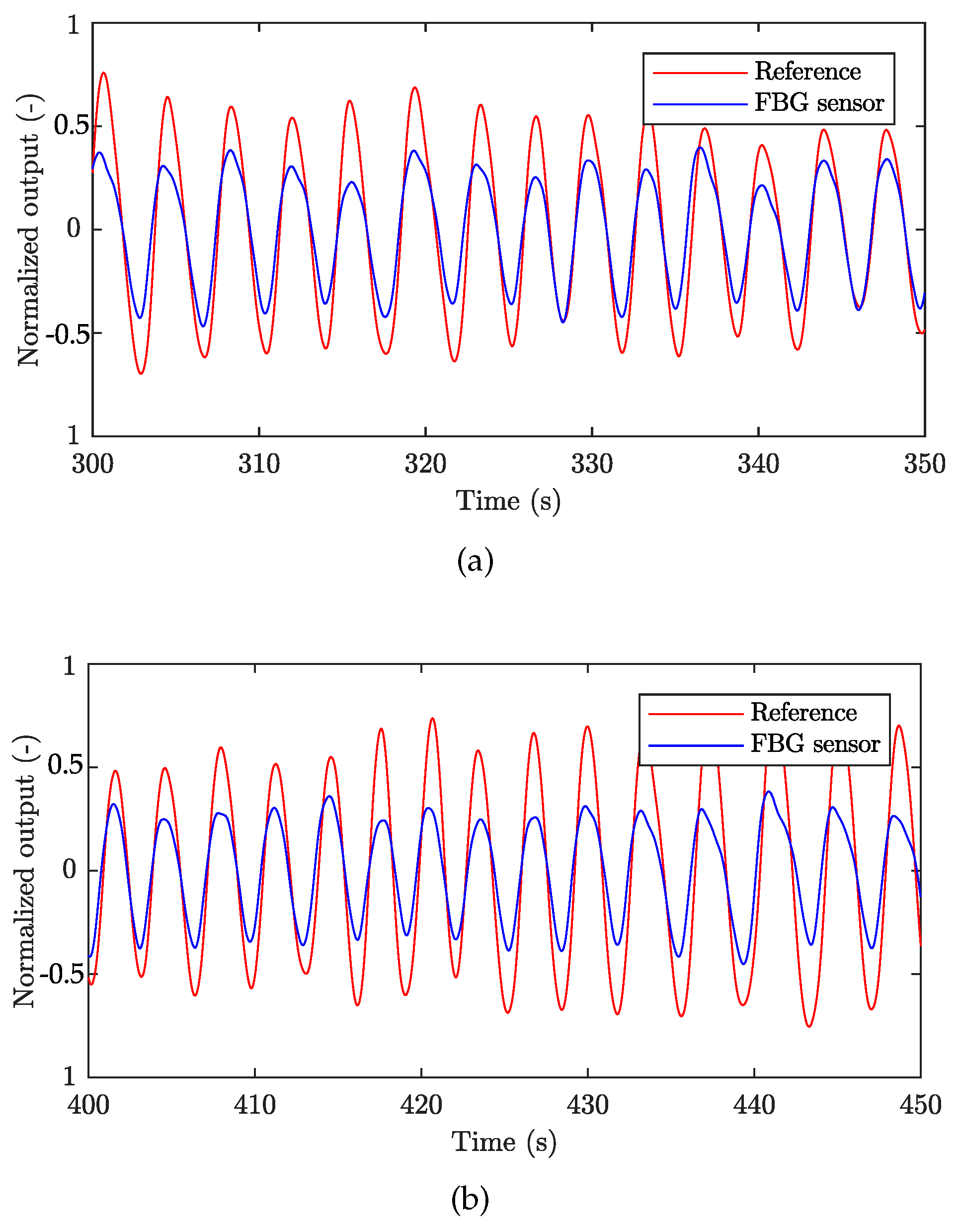
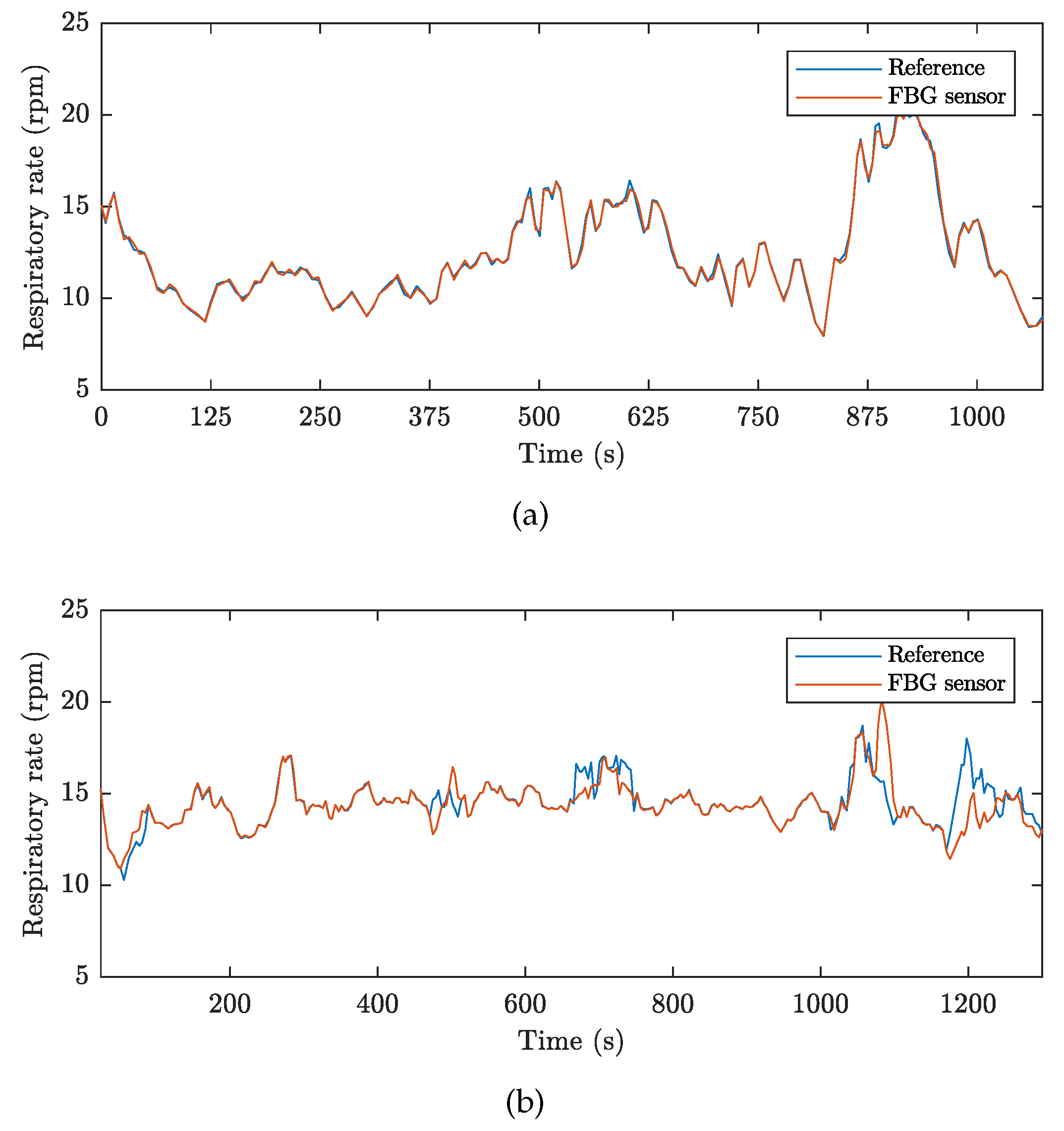
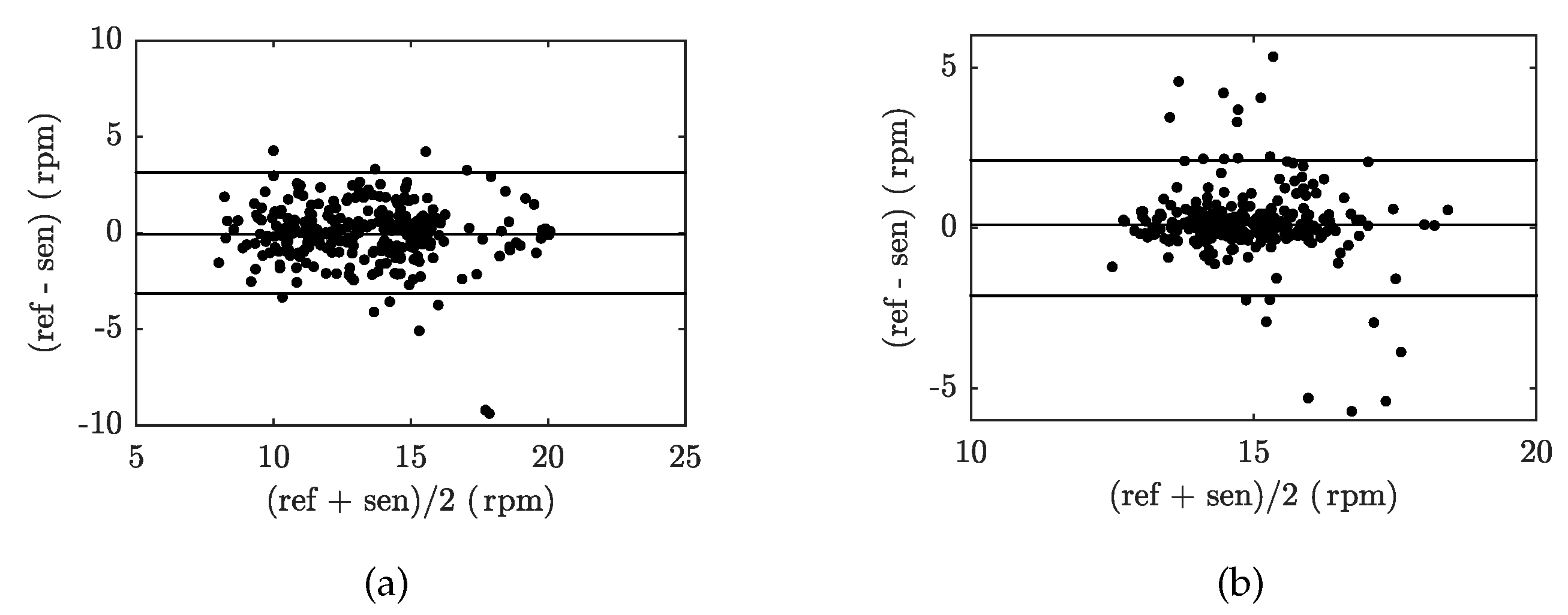

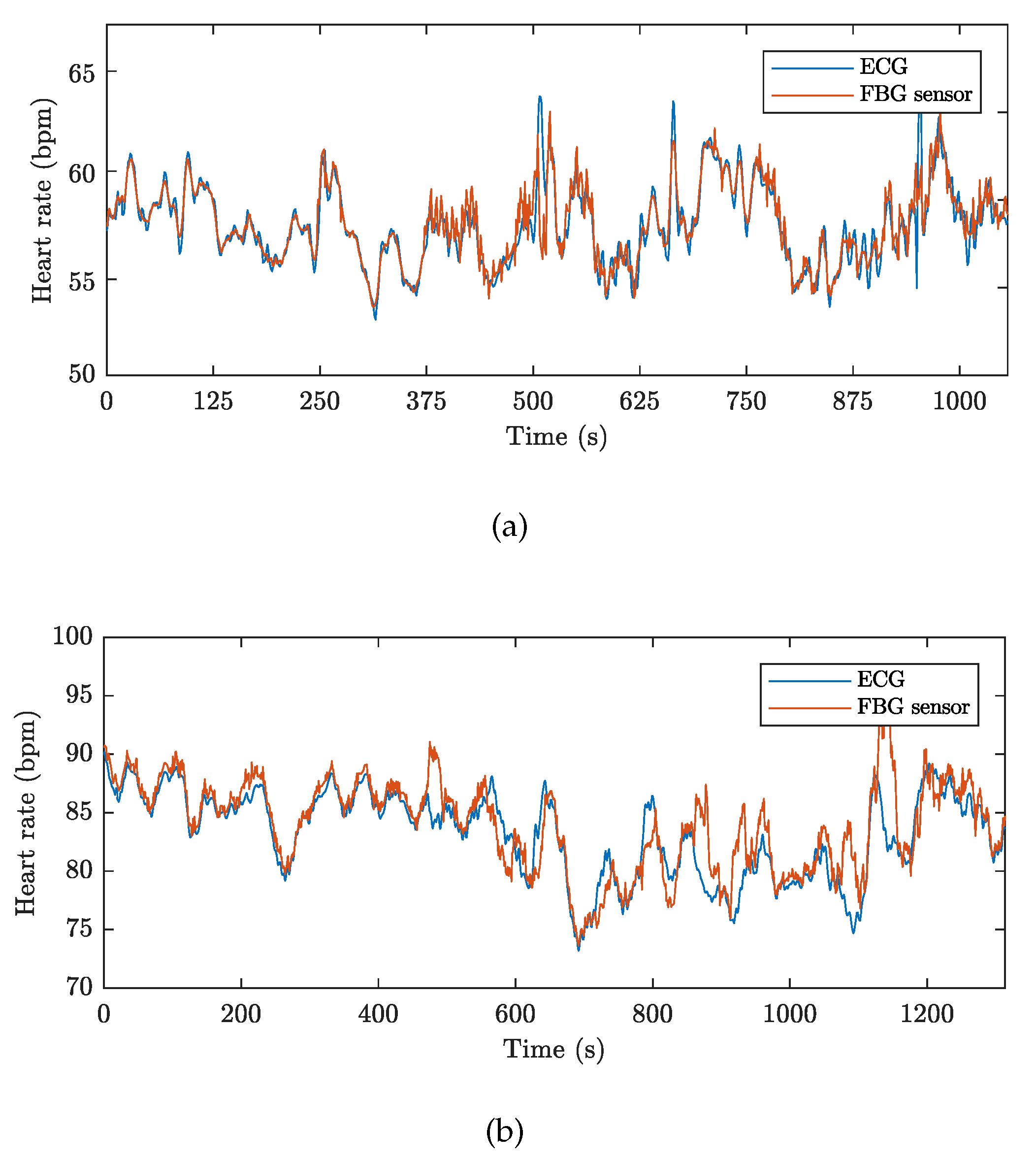
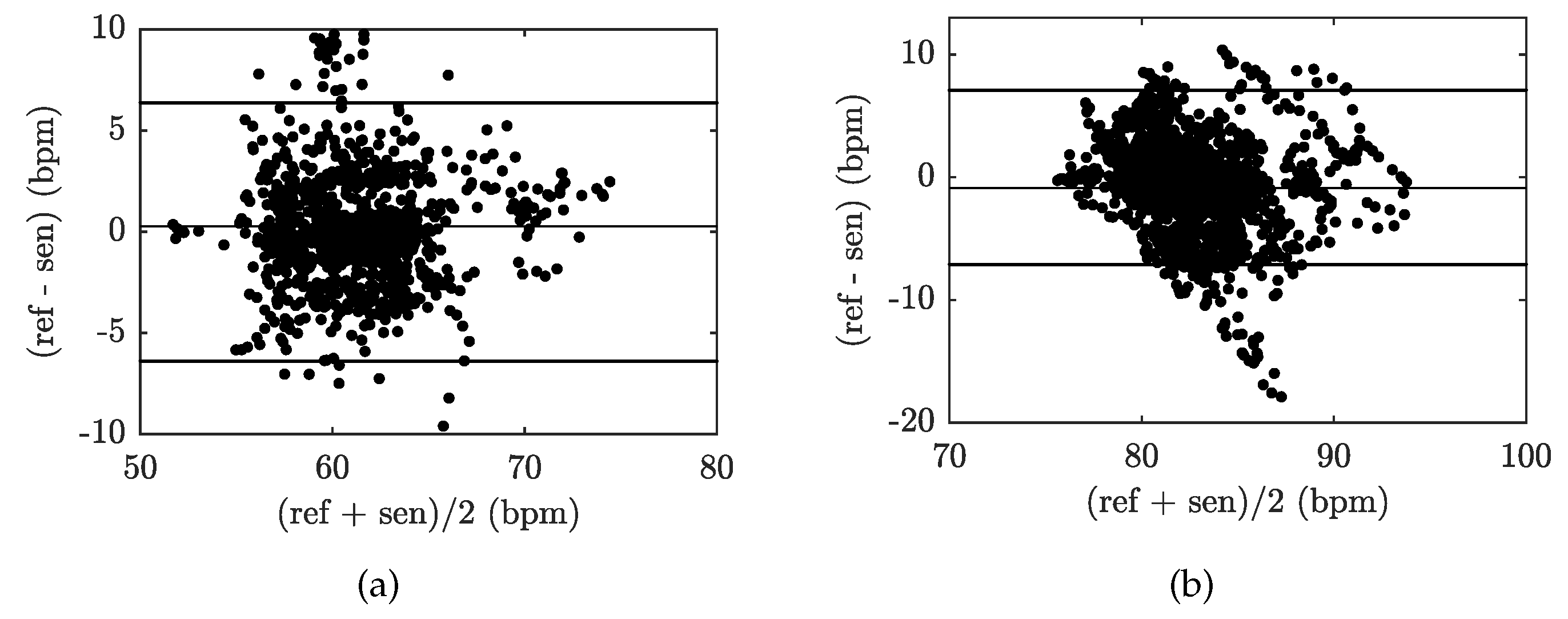



| Reference | Sensor Size and Weight | Encapsulation Material | Quantitative Data on Sensor Efficiency |
|---|---|---|---|
| 73 | board 220 × 95 mm; thickness 1.5 mm; weight: No data presented | polymethyl methacrylate (PMMA) | Twelve test subjects: maximum errors of RR and HR was within 7% (±1.2 rpm) and 6.5%(±3 bpm) of the average values |
| 38 | board 220 × 95 × 1.5 mm; weight: No data presented | polymethyl methacrylate (PMMA/Plexiglass) | Three test subjects: maximum relative errors of RR and HR was below 8% (7.67% and 6.61%) |
| 59 | PMMA board with dimmensions of 370 mm × 500 mm and thickness 3 mm; board 220 × 95 × 1.5 mm; weight: No data presented | (PMMA/Plexiglass) | Three test subjects: fractional error of determining HR was well below 7% (sitting and supine positions); maximum error was not more than 7.5% (standing position) |
| 44 | 60 × 30 × 3 mm; weight: No data presented | Polydimethylsiloxane (PDMS) | Four test subjects: No data presented |
| 57 | 50 × 50 × 5 mm; weight: No data presented | Polydimethylsiloxane (PDMS) | Four test subjects: maximum relative errors of RR and HR was 4.86% and 4.41% |
| Respiratory Rate | ||||||
|---|---|---|---|---|---|---|
| Subject | Time (s) | ARR (rpm) | NoS | Error | Rel. Error | Samples in ±1.96 SD (%) |
| 1041 | 14 | 272 | 11 | 4.04 | 95.96 | |
| 1126 | 15 | 281 | 12 | 4.27 | 95.73 | |
| 1312 | 17 | 372 | 15 | 4.03 | 95.97 | |
| 1085 | 18 | 324 | 12 | 3.70 | 96.30 | |
| 1419 | 15 | 356 | 14 | 3.93 | 96.07 | |
| 1358 | 14 | 317 | 15 | 4.73 | 95.27 | |
| 1276 | 16 | 307 | 18 | 5.86 | 94.14 | |
| 1412 | 18 | 425 | 21 | 4.94 | 95.06 | |
| 1095 | 16 | 296 | 17 | 5.74 | 94.26 | |
| 1183 | 17 | 332 | 17 | 5.12 | 94.88 | |
| Sum | 12,307 | 3282 | 152 | 4.64 | 95.36 | |
| Heart Rate | ||||||
|---|---|---|---|---|---|---|
| Subject | Time (s) | AHR (bpm) | NoS | Error | Rel. Error | Samples in ±1.96 SD (%) |
| 1041 | 61 | 1045 | 54 | 5.17 | 94.83 | |
| 1126 | 68 | 1281 | 57 | 4.45 | 95.55 | |
| 1312 | 74 | 1614 | 69 | 4.28 | 95.72 | |
| 1085 | 65 | 1179 | 54 | 4.58 | 95.42 | |
| 1419 | 71 | 1683 | 74 | 4.40 | 95.60 | |
| 1358 | 70 | 1588 | 76 | 4.79 | 95.21 | |
| 1276 | 84 | 1771 | 97 | 5.48 | 94.52 | |
| 1412 | 82 | 1925 | 98 | 5.09 | 94.91 | |
| 1095 | 86 | 1568 | 76 | 4.85 | 95.15 | |
| 1183 | 82 | 1621 | 92 | 5.68 | 94.32 | |
| Sum | 12,307 | 15,275 | 747 | 4.87 | 95.13 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nedoma, J.; Fajkus, M.; Martinek, R.; Nazeran, H. Vital Sign Monitoring and Cardiac Triggering at 1.5 Tesla: A Practical Solution by an MR-Ballistocardiography Fiber-Optic Sensor. Sensors 2019, 19, 470. https://doi.org/10.3390/s19030470
Nedoma J, Fajkus M, Martinek R, Nazeran H. Vital Sign Monitoring and Cardiac Triggering at 1.5 Tesla: A Practical Solution by an MR-Ballistocardiography Fiber-Optic Sensor. Sensors. 2019; 19(3):470. https://doi.org/10.3390/s19030470
Chicago/Turabian StyleNedoma, Jan, Marcel Fajkus, Radek Martinek, and Homer Nazeran. 2019. "Vital Sign Monitoring and Cardiac Triggering at 1.5 Tesla: A Practical Solution by an MR-Ballistocardiography Fiber-Optic Sensor" Sensors 19, no. 3: 470. https://doi.org/10.3390/s19030470
APA StyleNedoma, J., Fajkus, M., Martinek, R., & Nazeran, H. (2019). Vital Sign Monitoring and Cardiac Triggering at 1.5 Tesla: A Practical Solution by an MR-Ballistocardiography Fiber-Optic Sensor. Sensors, 19(3), 470. https://doi.org/10.3390/s19030470






