Abstract
Due to the risks that water contamination implies for human health and environmental protection, monitoring the quality of water is a major concern of the present era. Therefore, in recent years several efforts have been dedicated to the development of fast, sensitive, and selective sensors for the detection of heavy metal ions. In particular, fluorescent sensors have gained in popularity due to their interesting features, such as high specificity, sensitivity, and reversibility. Thus, this review is devoted to the recent advances in fluorescent sensors for the monitoring of these contaminants, and special focus is placed on those devices based on fluorescent aptasensors, quantum dots, and organic dyes.
1. Introduction
Monitoring the presence of contaminants in water is of general interest in order to ensure the quality of surface, ground, and drinking water [1,2]. Among the several water pollutants, such as plastic or waste [3], chemical fertilizers or pesticides [4], and pathogens [5], heavy metal ions are known for their high toxicity [6]. Although some of them are essential nutrients (for instance, iron, zinc, or cobalt), they can be toxic at higher concentrations [7]. For their part, cadmium, lead, and mercury are highly poisonous even at trace levels [8,9], showing a close association to cancer or neurodegenerative diseases [10,11]. Furthermore, heavy metal ions are non-biodegradable substances [12] and they have an accumulative effect in human body [13], where they enter, typically, through the air [14], beverages [15], and the food chain [16], in which water plays a key role. There, metal ions can be found as a result of vehicle emissions [17], batteries [18], or industrial activities [19]. Thus, their detection at low concentrations is a matter of priority for environmental protection and disease prevention as well [20].
This issue requires highly sensitive and selective devices [21,22], which can be based on different technologies; for instance, electronics [23], electrochemistry [24], or optics [25]. In particular, optical sensors present numerous attractive features: the ease of integration in microfluidic platforms [26] and the capability of monitoring hazardous environments [27] are just two of them. Among the optical sensors, fluorescent ones have gained popularity in recent years since they provide high specificity as well as low detection limits, fast response time, and technical simplicity [28,29]. Their working principle consists of the emission of light by a material (fluorophore) after being excited at lower wavelengths [30]. The intensity (or lifetime) of that emission varies with the concentration of the target analyte [31]. So far, several materials, such as porphyrins [32], metal-organic frameworks [33], DNAzymes [34], fluorescent aptamers [35], quantum dots [36], or organic dyes [37] have been developed for the monitoring of heavy metal ions in water. This review is focused on the recent advances in sensors that employ the last three kinds of materials: the first section is devoted to the different techniques based on fluorescent aptamers, the second one is dedicated to the sensors fabricated with quantum dots and, finally, the third one analyzes the devices developed using organic dyes.
2. Heavy Metal Ion Sensors Based on Fluorescent Aptamers
Aptamers are a type of artificial oligonucleotide (ON) sequences with the ability of specifically binding to a target molecule [38]. Among their several attractive properties for the design of sensors are good thermal stability [39], the ease of synthesis and modification [40], or their simple immobilization procedure [41]. Their high affinity and specificity toward each of their target analytes [42,43] is the most remarkable property. Consequently, aptamer-based detection techniques have emerged as very selective recognition tools [44,45,46].
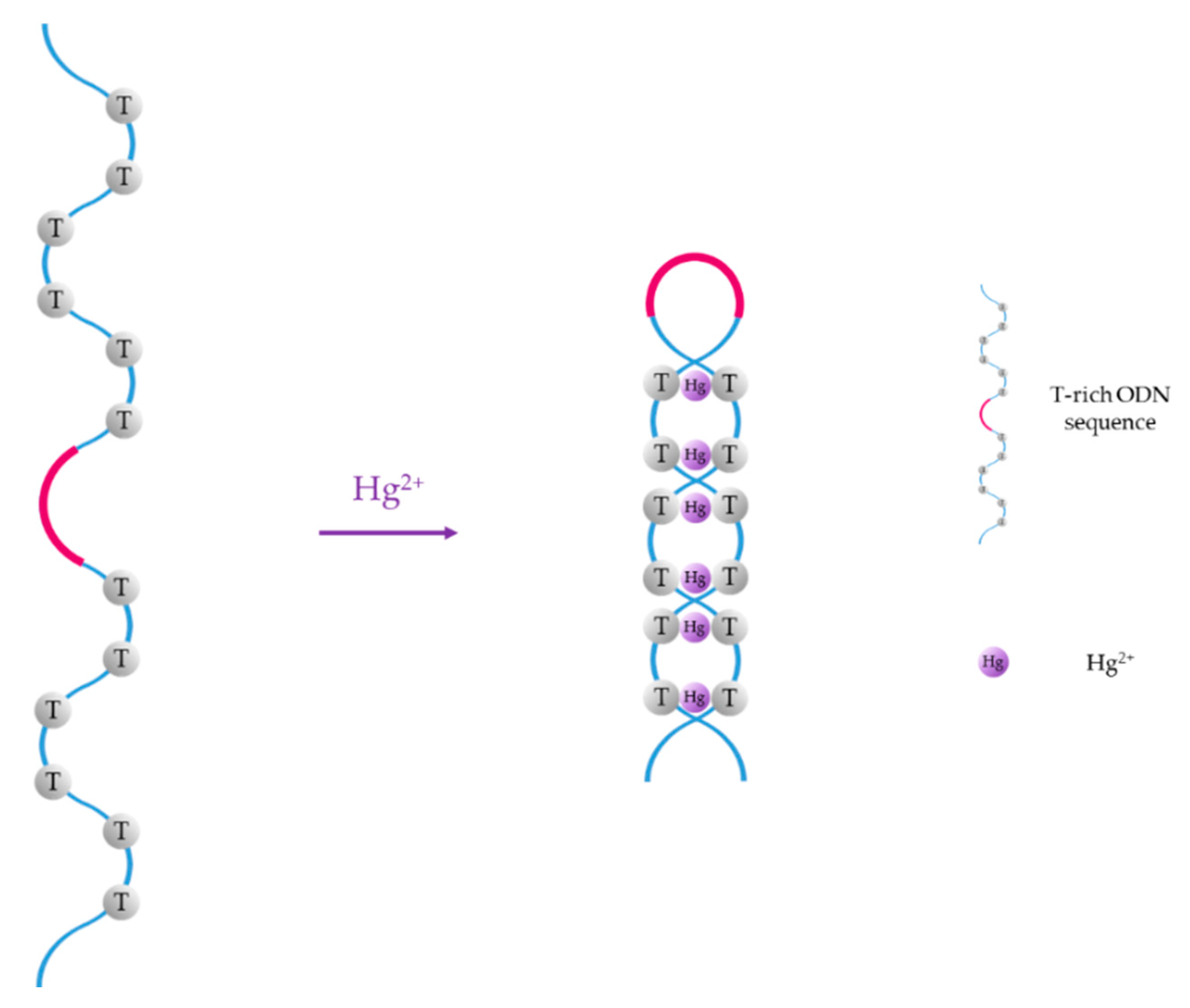
For instance, thymine (T) exhibits great affinity towards mercury (II) ions, forming T-Hg2+-T base pairs in DNA duplexes [47], and cytosine (C) forms C-Ag+-C mismatches when it interacts with Ag+ ions [48]. Thus, since the first ON-based Hg2+ sensor was reported by Ono et al. [49], T-rich ON sequences have been widely employed for the selective detection of Hg2+ in water samples [50,51,52]. Furthermore, several selective sensors for Ag+ have also been reported in the literature [53,54,55]. The basis of Hg2+ sensors based on aptamers is the conformational change of the T-rich ON sequence, which acquires a hairpin structure due to T-Hg2+-T mismatches, as shown in Figure 1. As Ag+ ions and cytosine form C-Ag+-C base pairs, Ag+ monitoring is carried out with similar sequences than Hg2+, but substituting the thymine groups with cytosine ones.
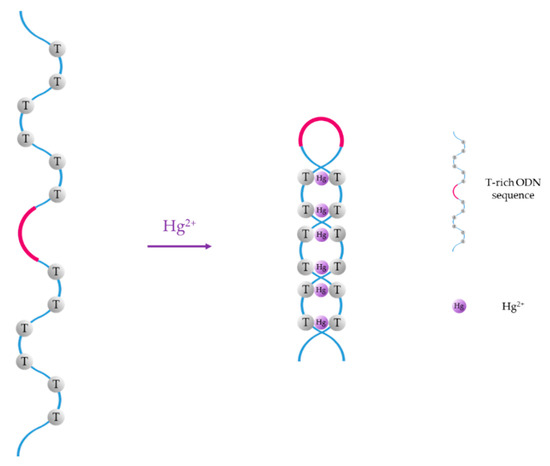
Figure 1.
Hg2+-induced formation of T-Hg2+-T mismatches.
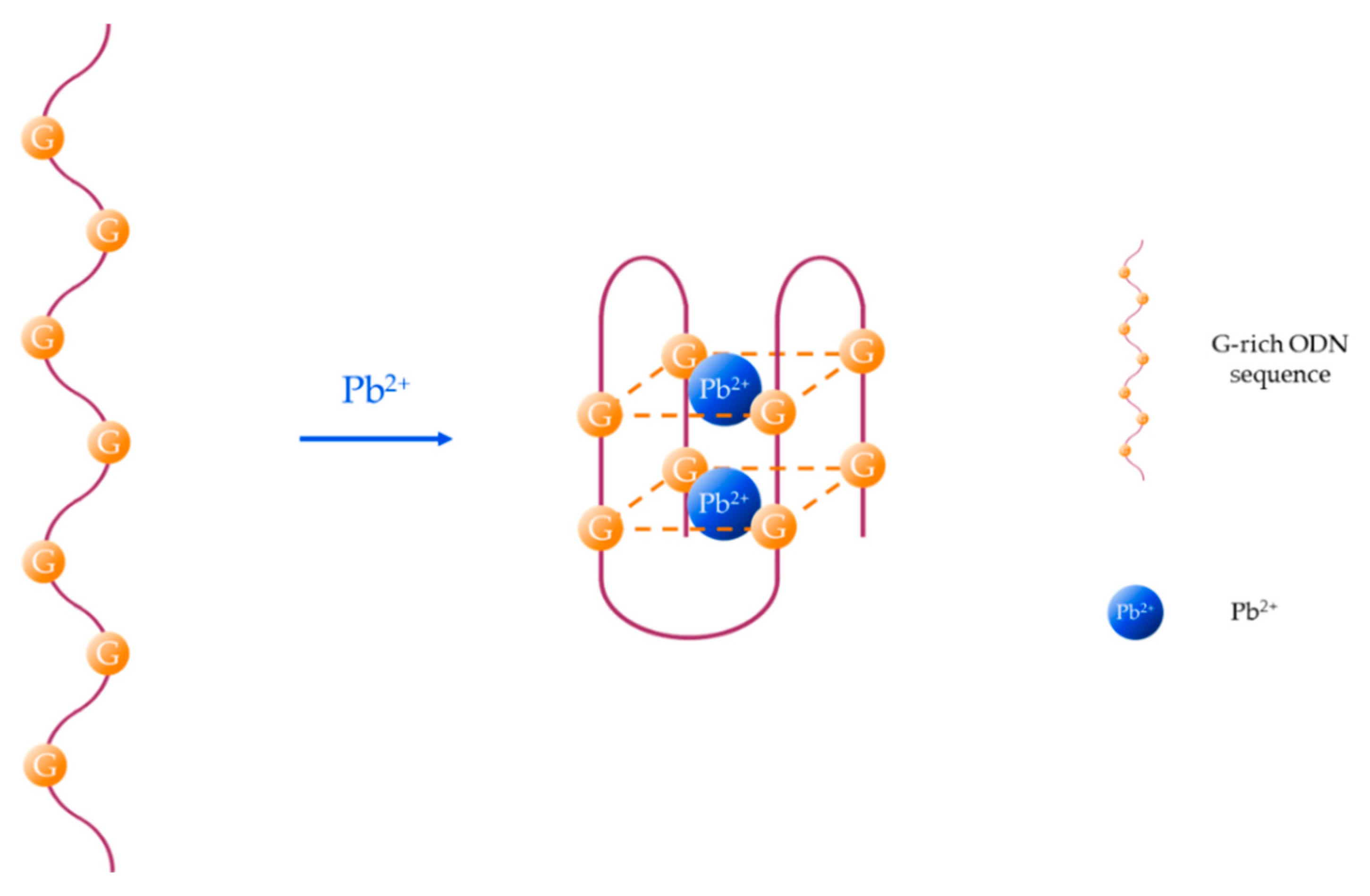
Another particular case is the formation of guanine (G)-quadruplexes induced by the presence of Pb2+ [56], as it is depicted in Figure 2. Although other metal ions, such as K+, Na+, or Ca2+ can slightly influence the conformation of the G-quadruplex structure [57], G-rich aptamers show good selectivity and specificity towards Pb2+ [58] owing to the high binding ability between Pb2+ and G bases [59].
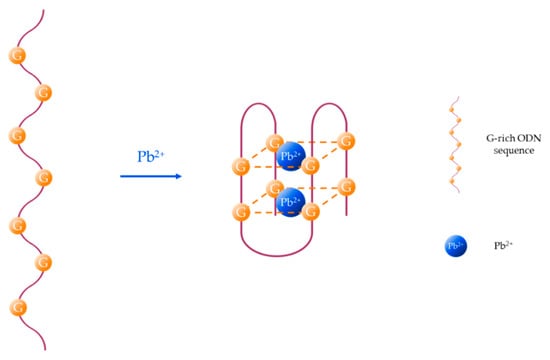
Figure 2.
Pb2+-induced formation of G-quadruplex structures.
Utilizing these sensing structures, different fluorescent detection strategies have been developed, all of them determined by the conformational change of the ON sequences in the presence of the target metal ion.
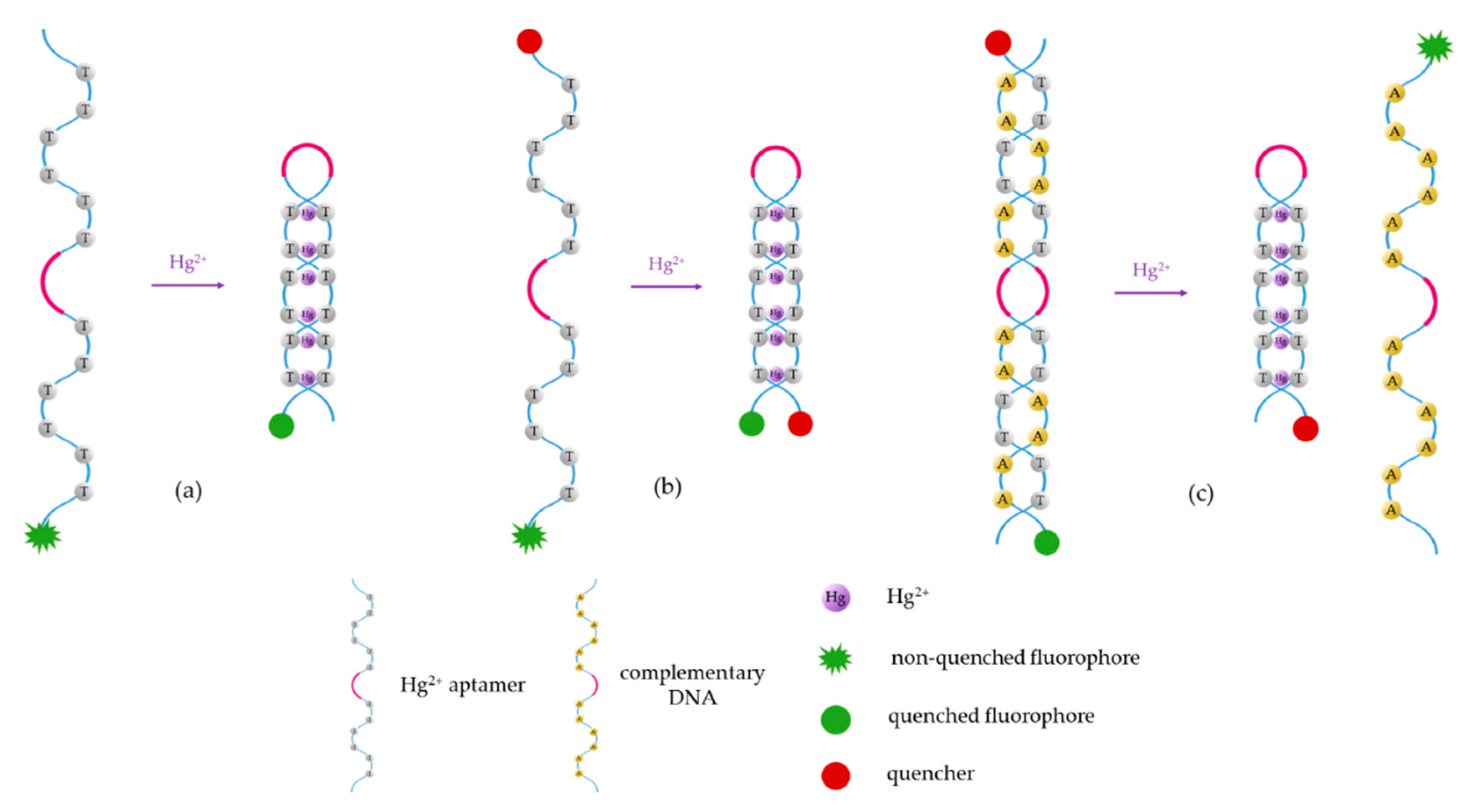
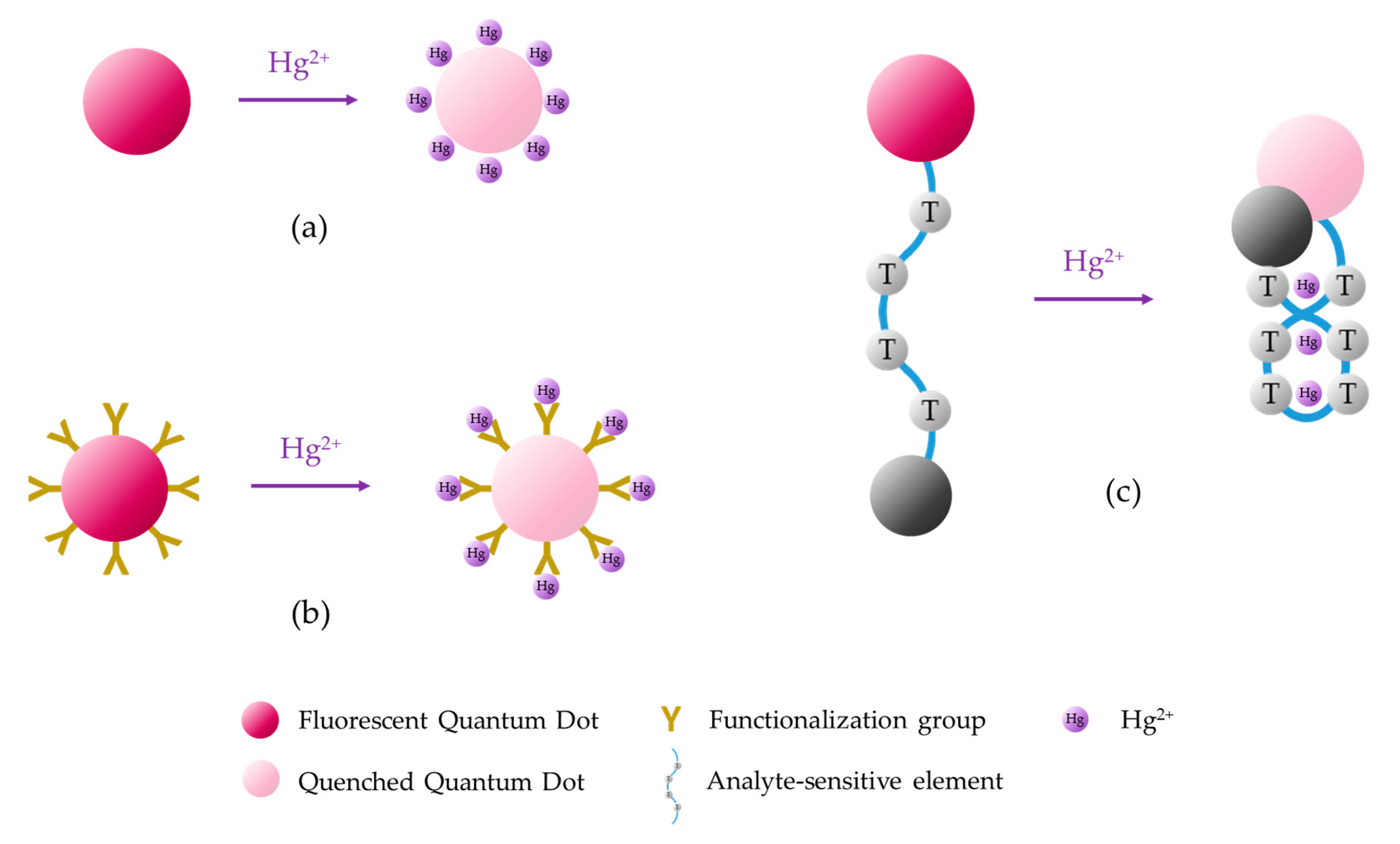
The main mechanisms for the monitoring of heavy metal ions with aptamers are shown in Figure 3. For the sake of simplicity, the sensing procedures exposed in this schematic are specific for the particular case of Hg2+, but they can be implemented for the detection of other metal ions as long as these compounds induce a conformational change of the aptamer. These detection procedures, as well as the labelling of the fluorophores (and, if necessary, the quenchers), depend on the utilization of one or two DNA strands, as explained below.
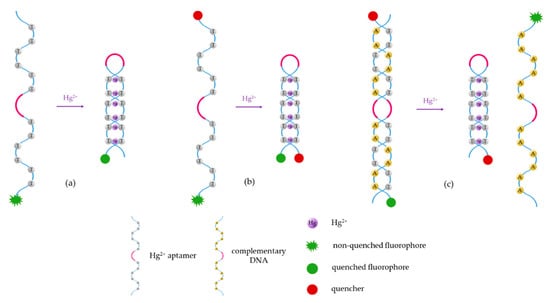
Figure 3.
Schematic of the different Hg2+ sensing procedures utilizing fluorescent aptasensors: (a) the fluorophore is labeled to the sensitive aptamer, (b) the fluorophore and the quencher are linked to the two terminis of the aptamer, and (c) the fluorophore and the quencher are labeled to the aptamer and the complementary DNA, or vice versa. These sensing procedures can be applied to detect other heavy metal ions just by substituting the T-rich sequences by the appropriate ON sequence.
In the case of utilizing a single T-rich ON sequence, the luminophore is usually labeled to one of its termini (5′ or 3′). Its fluorescent emission can be quenched either by the electron transfer to the T-Hg2+-T mismatches (Figure 3a) [60] or by a quencher linked to the other termini (3′ or 5′) of the sensitive strand (Figure 3b) [61]: as the T-Hg2+-T mismatches are formed, the ON sequence acquires a hairpin structure [62], decreasing the distance between the fluorophore and the quencher. This fact promotes the energy transfer between the first one (which acts as donor) and the second one (which acts as acceptor) [63].
The third procedure (Figure 3c) consists of the detection of Hg2+ by competitive binding: a T-rich aptamer (labeled to the fluorophore or the quencher) is usually linked to its complementary DNA (labeled to the quencher or the fluorophore). In the presence of Hg2+, the complementary DNA separates from the aptamer, to which the Hg2+ ions bind, forming the T-Hg2+-T mismatches that give rise the hairpin structure [64].
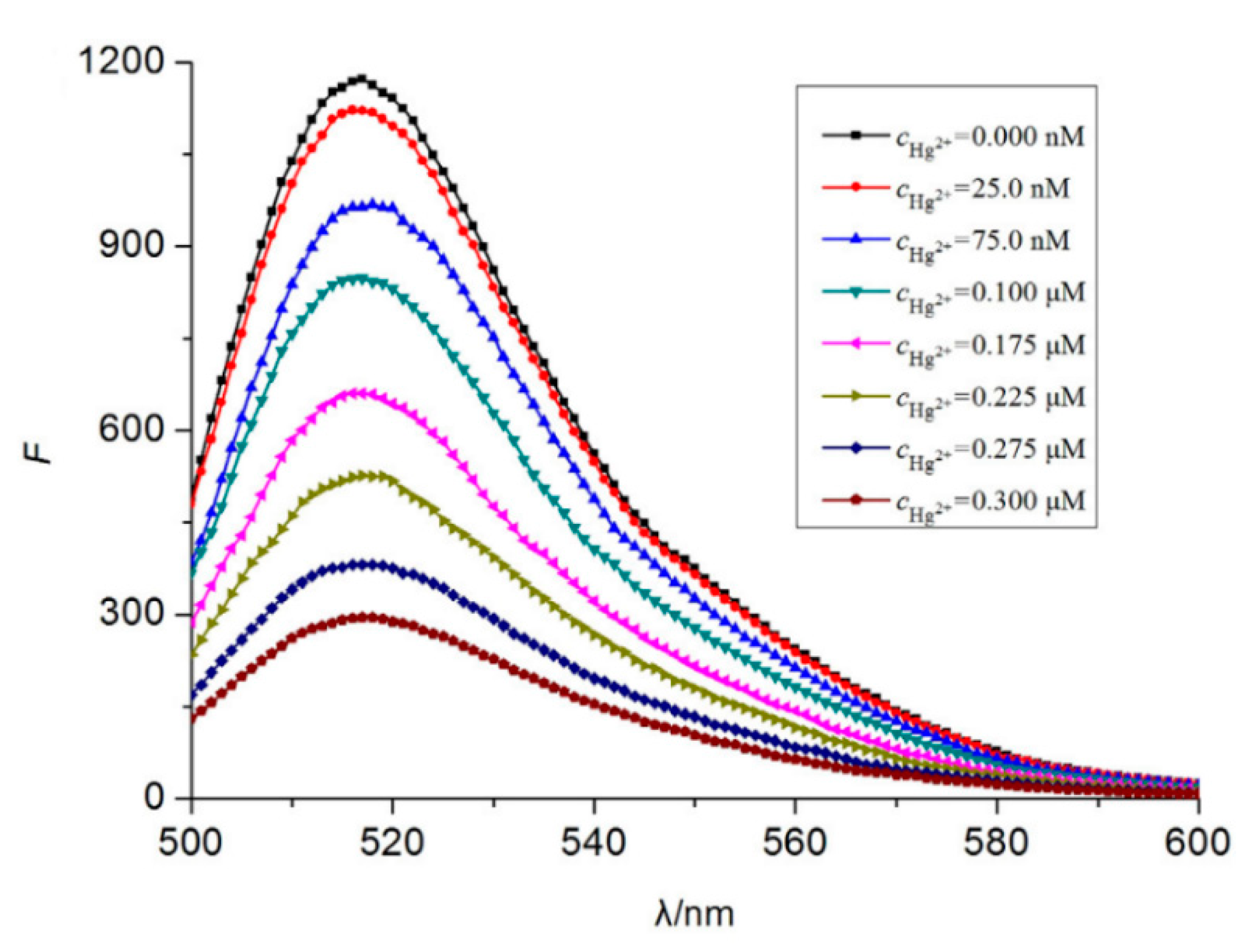
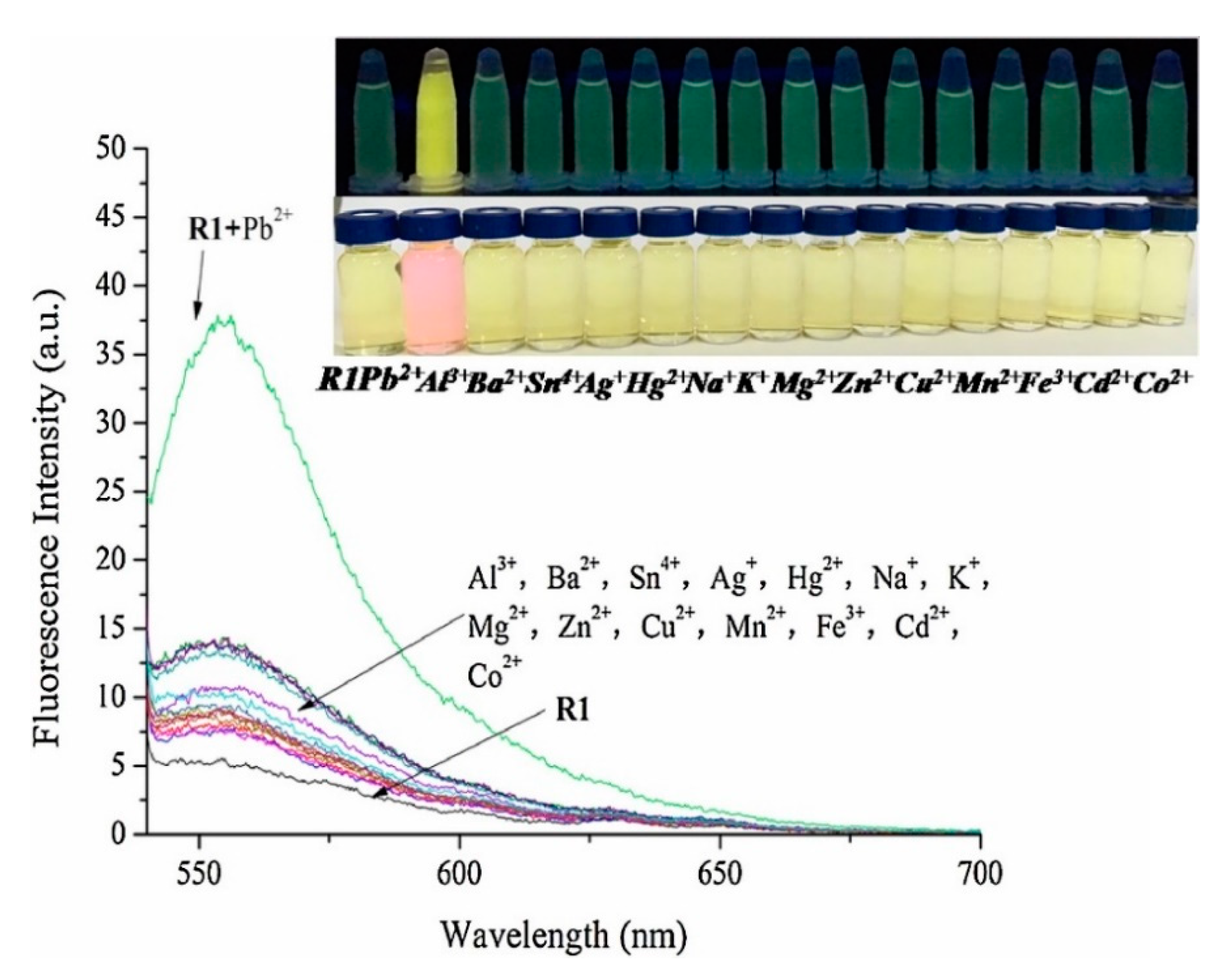
Typical fluorophores that are labeled to the sensitive DNA sequences are dyes, such as 6-carboxyfluorescein, (6-FAM), carboxytetramethylrhodamine (TAMRA), or Texas Red, among others, as well as fluorescent quantum dots and up-conversion nanoparticles. Chen et al. [65] developed a mercury-mediated aptamer-beacon by labeling a 6-carboxyfluorescein (FAM) to the 5′ termini of a T-rich oligonucleotide: 5′-FAM-CGC TTG TTT GTT CGC ACC CGT TCT TTC TT-3′. In the presence of Hg2+, this aptamer acquired a hairpin structure due to the formation of the T-Hg2+-T pairs. This led to the fluorescence resonance energy transfer (FRET) from the FAM to the T-Hg2+-T base pairs and, consequently, to the decrease of the fluorescence intensity, as displayed in Figure 4. The sensing system exhibited a limit of detection (LOD) of 4.28 nM Hg2+ and a linear detection range from 14.2 nM to 300 nM Hg2+. Furthermore, the selectivity of the sensor for Hg2+ over a series of metal ions (Pb2+, Ag+, Cu2+, Ca2+, Ba2+, Ni2+, K+, Cd2+, Co2+, Cr3+, Fe3+, Al3+, Mn2+, and Zn2+) was also analyzed: it was found that not one of them presented any kind of interference, even at 16–67 times higher concentrations than that of Hg2+.
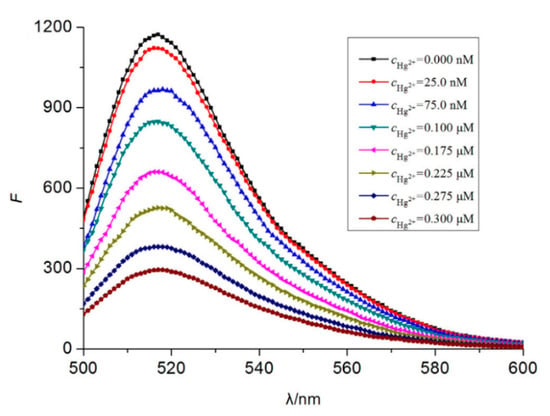
Figure 4.
Fluorescence spectra of 5′-FAM-CGC TTG TTT GTT CGC ACC CGT TCT TTC TT-3′ for different Hg2+ concentrations. Reprinted with permission from [65].
As explained previously, the fluorescent emission of the dyes can also be attenuated by a quencher linked to the opposite termini of the sensitive aptamer. One which has been widely employed with this aim is 4-([4-(dimethylamino)phenyl]azo)benzoic acid (DABCYL) [66], owing to its broad absorption spectrum [67]: the sensor reported by Li and co-authors [68] presented a linear calibration curve between 10 nM and 200 nM, with a LOD of 10 nM Hg2+. Furthermore, in order to avoid the biodegradation of the aptamer, it was encapsulated in a porous phospholipid nanoshell (PPN), allowing its utilization in human urine samples.
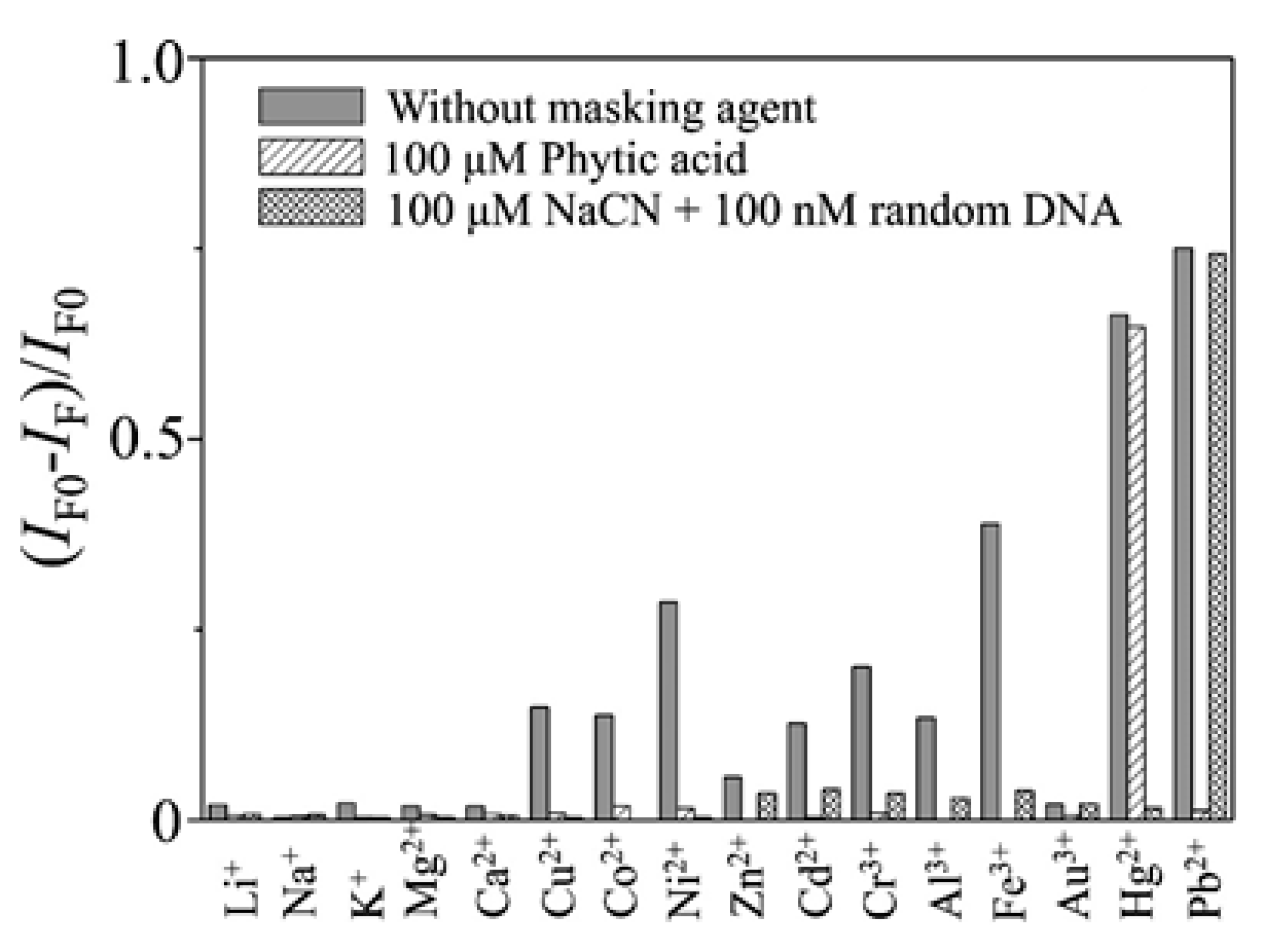
Additionally, by labeling FAM and DABCYL to the 5′ and 3′ terminus of a thrombin-binding aptamer (TBA), which is also a T-rich sequence, a Pb2+ and Hg2+ sensor was developed: it presented a LOD of 300 pM and 5 nM for Pb2+ and Hg2+, respectively, and linear detection ranges from 0.5 nM and 30 nM for Pb2+ and from 10 nM to 200 nM for Hg2+ [61]. As the TBA is a T- and G-rich aptamer [69], in order to develop a sensor for a specific metal ion, masking agents were used: the presence of Pb2+, and that of Cu2+, Co2+, Ni2+, Cd2+, Cr3+, Al3+, and Fe3+ ions as well, was masked by adding phytic acid to the samples. The interference of Hg2+ was avoided by utilizing CN− and a random DNA, as can be seen in Figure 5. Regarding to the reutilization of the sensor, it provided recoveries between the 95% and the 104%.
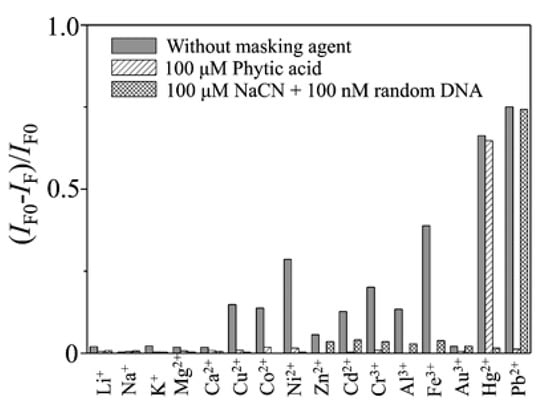
Figure 5.
Quenching efficiency (IF0 − IF)/IF0 of the fluorescence intensity (518 nm) of the TBA-based sensor in the presence of metal ions in the absence and presence of masking agents. Concentrations: Hg2+, 1.0 μM; Pb2+, 100 nM; Li+ and Na+, 100 μM; K+, Mg2+, and Ca2+, 10 μM; other ions, 1.0 μM. Reprinted with permission from [61]. Copyright 2009 American Chemical Society.
As gold nanoparticles (Au NPs) are good energy acceptors [70], they are another kind of fluorescence quenchers [71]: by covalently linking a Hg2+-sensitive aptamer labeled with FAM to an Au NP, it was possible to linearly monitor the Hg2+ concentration from 20 nM to 1000 nM [29], with a LOD of 16 nM. Furthermore, the utilization of Au NPs helped to stabilize the aptamer and decrease the LOD [72]. The quenching effect of Au NPs has also been utilized to fabricate “turn-on” fluorescent sensors: while the sensitive aptamer is linked to an Au NP (quencher), the complementary DNA sequence is labeled with a fluorophore [73], or vice versa [74]. In the absence of mercury (II) ions, the aptamer and the complementary strand are linked, so the fluorophore and the Au NP are in proximity and fluorescence transfer occurs, resulting in a negligible fluorescent emission. In the presence of Hg2+, due to the high specificity of the thymine groups to this metal ion, the sensitive aptamer acquires a hairpin structure, displacing the complementary strand away from the Au NP, which leads to an increase of the fluorescence [64].
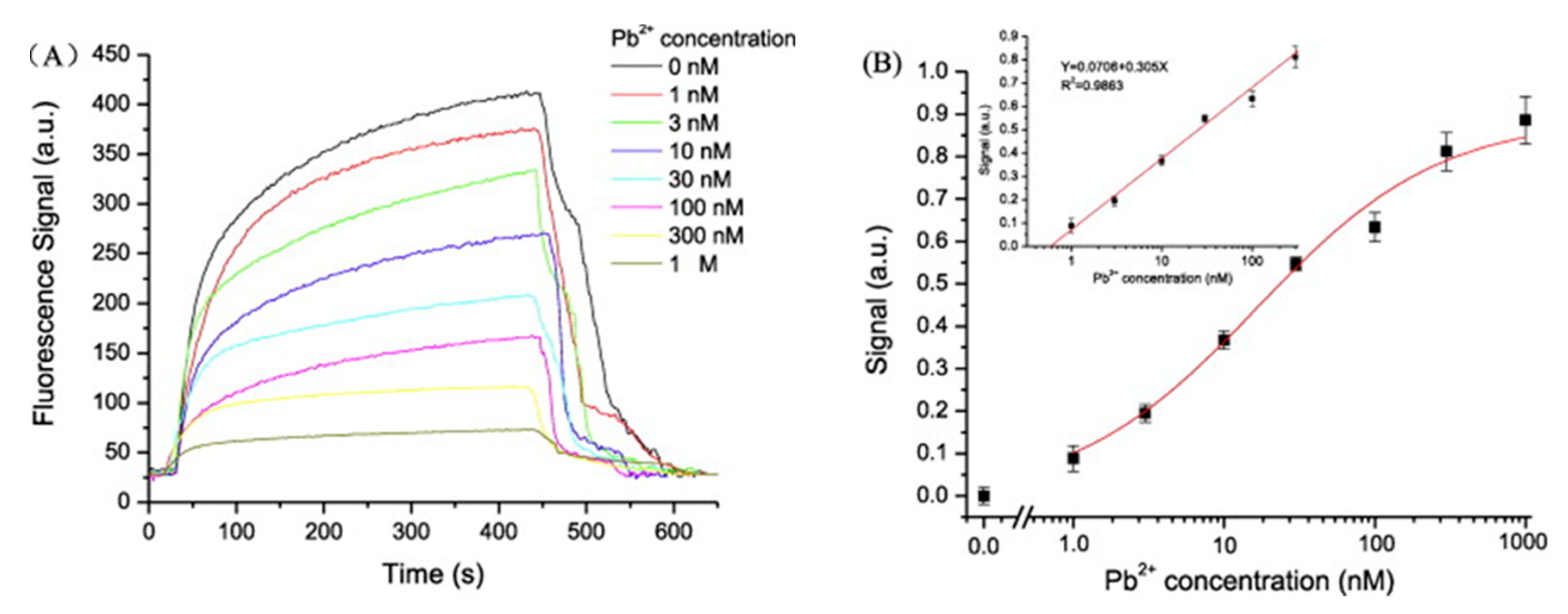
Based on the competitive binding mechanism, an optical fiber sensor for monitoring Pb2+ was fabricated [75]. The complementary strand was deposited onto the optical fiber: in the absence of Pb2+, when the Cy5.5-labeled aptamer bound to the complementary DNA, its fluorescent emission was coupled to the optical fiber. Oppositely, when Pb2+ was present, it induced the aptamer to form G-quadruplex structures, being detached from the complementary strand, which resulted in a decrease of the coupled fluorescent intensity, as can be observed in Figure 6.
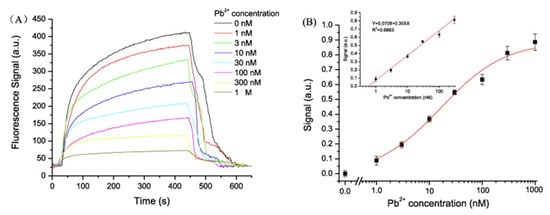
Figure 6.
(A) Response curve for different Pb2+ concentrations in the presence of the complementary DNA. (B) Logarithmic calibration curve of the sensor. Reprinted with permission from [75].
There also exist different dyes that are sensitive to the formation of double-stranded DNA (dsDNA), such as PicoGreen [76] and SYBR Green 1 [77]. Utilizing this feature, a fluorescent assay was developed for the detection of Hg2+ and Ag+ ions utilizing two complementary strands: 5′-TTCTTTCTTCCCCTTGTTTGTT-3′ and 5′-AACAAACAAGGGGAAGAAAGAA-3′ [78]. In the absence of both of these metal ions, the two strands formed a dsDNA, which led to the fluorescence emission by PicoGreen. As the Hg2+ or Ag+ concentration increased, the number of aptamer/cDNA sequences decreased, resulting in a diminution of the PicoGreen emission. The LOD was 50 nM for Hg2+ and 930 pM for Ag+, while the linear detection ranges were 50 nM to 4 µM and 930 pM to 930 nM for Hg2+ and Ag+, respectively. Furthermore, not one of the other analyzed metal ions, including Cu2+, Li+, Zn2+, Na+, Ca2+, Mg2+, K+, and Pb2+, interfered in the measurements and, finally, the sensor presented recoveries of the 80–105% for Ag+ and 104–114% for Hg2+.
As most of the aptamer-based fluorescent sensors for heavy metal ions are focused on Hg2+ and Pb2+ ions detection, the main ones are summarized in Table 1 (Hg2+) and Table 2 (Pb2+). Due to the high affinity of T-rich and G-rich ON sequences to these ions, and their high toxicity even at trace levels, less attention has been paid to other heavy metal ions. Thus, the development of sensitive and specific ON sequences for those ions is one of the main challenges for scientists.

Table 1.
Hg2+ fluorescent sensors based on aptamer detection.

Table 2.
Pb2+ fluorescent sensors based on aptamer detection.
3. Heavy Metal Ion Sensors Based on Fluorescent Quantum Dots
Quantum dots (QDs) are nanocrystals [93] that exhibit interesting optical properties, such as narrow and symmetric emission spectra and broad absorption band [94]: both parameters are tunable by modifying their material, shape, and size [95]. Thus, they can be used in a wide range of applications, for instance, photovoltaic devices [96], light-emitting diodes [97], or bioimaging [98].
Fluorescent QDs can be fabricated utilizing semiconductor materials [99,100], carbon [101] or carbon derivatives [102]. Sensing devices can be developed following three main strategies, which are shown in Figure 7: direct interaction between the analyte and the QDs [103], functionalization of the QDs [104,105], and integration of the QDs with other sensory materials [106].
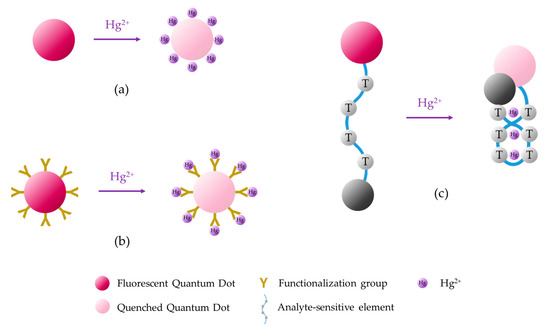
Figure 7.
Sensing mechanisms based on fluorescent quantum dots: (a) direct interaction between the analyte and the QDs, (b) interaction of the analyte with the functionalized QDs, and (c) integration of the QD with another sensory material.
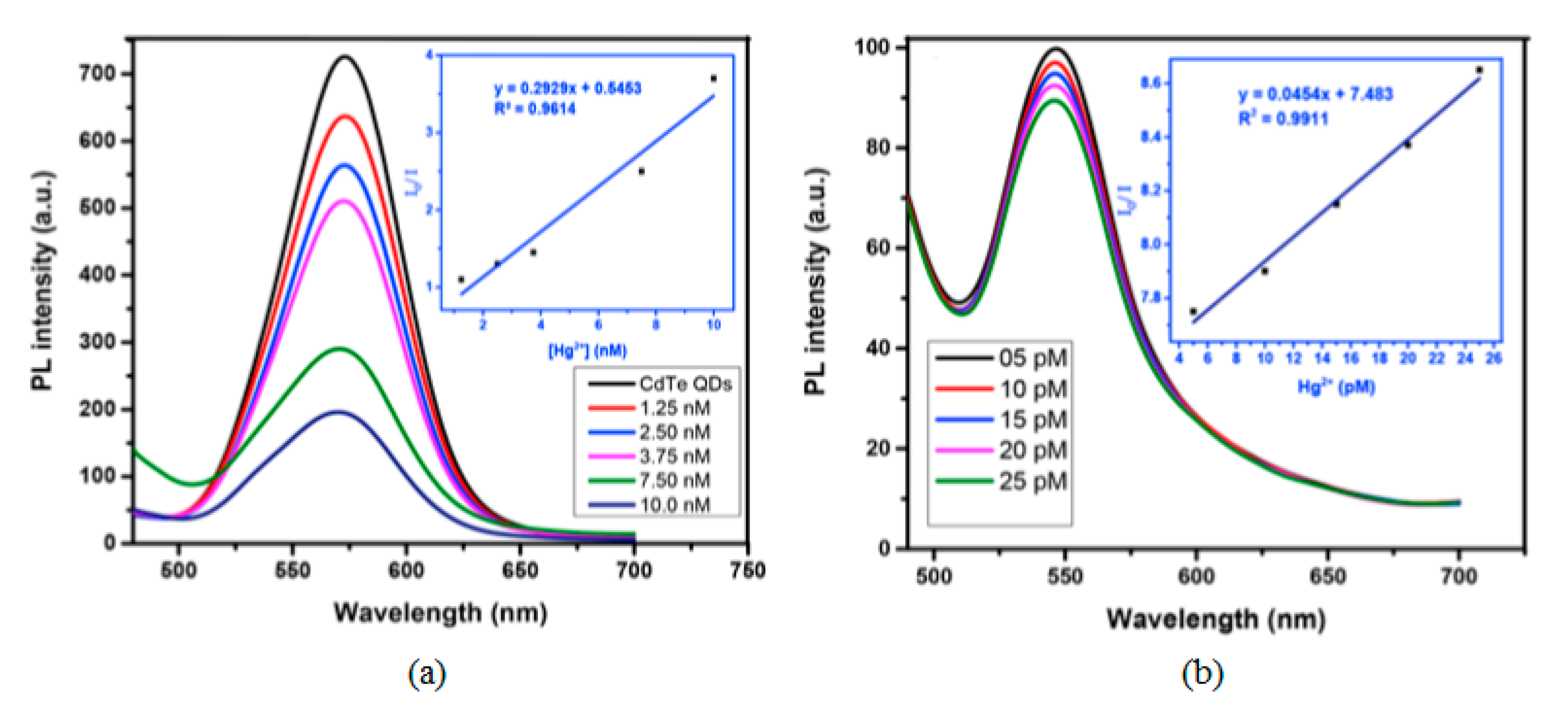
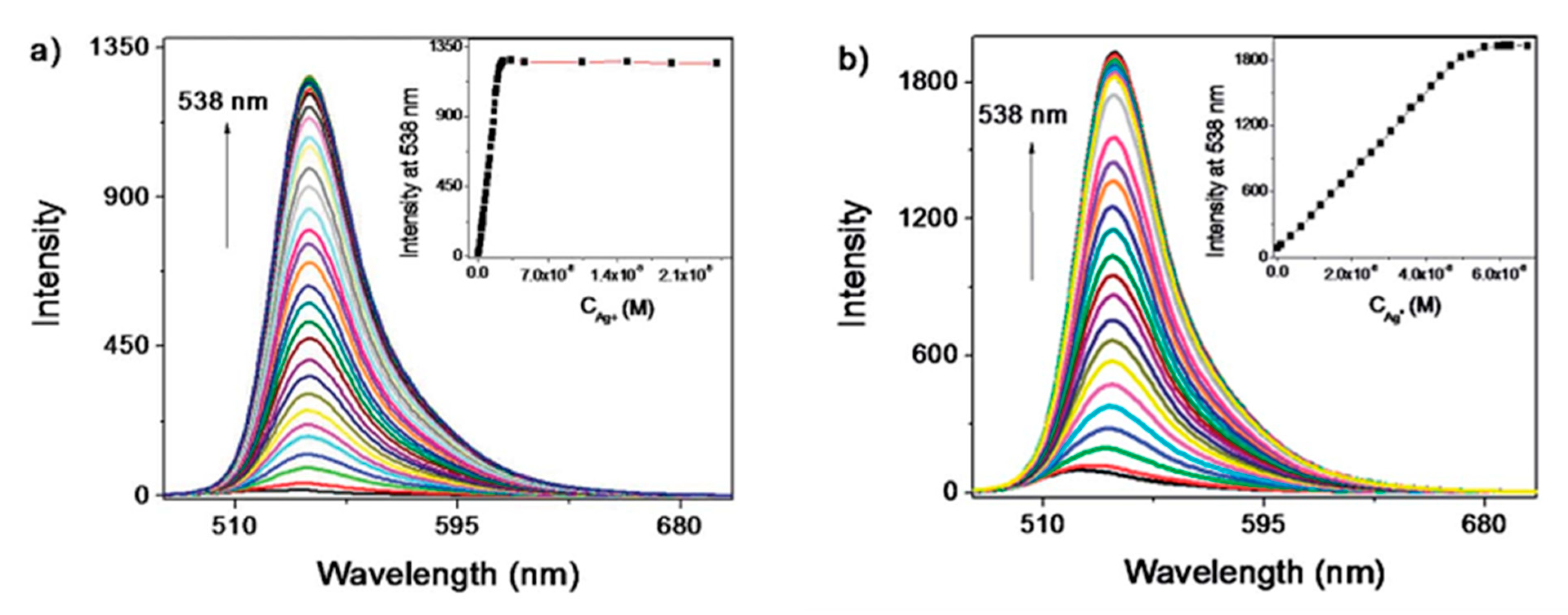
Among the semiconductor QDs, CdTe QDs have been widely employed for the monitoring of heavy metal ions [107,108]. Furthermore, their selectivity and sensitivity can be tuned by utilizing different capping agents [109], such as thioglycolic acid (TGA) or L-cysteine: in the first case, an electron transfer process occurs between the functional groups of TGA and Hg2+ ions, which quenches the luminescent intensity of the CdTe QDs. Thus, employing TGA capped CdTe QDs, it was possible to detect Hg2+ in the nanomolar range, from 1.25 × 10−9 M to 1 × 10−8 M, with a LOD of 3.5 × 10−10 M Hg2+, as it can be observed in Figure 8a. In the case of L-cysteine capped CdTe QDs, their interaction with Hg2+ ions depends on the concentration of the metal ion: for concentrations of Hg2+ in the picomolar range, these ions interact with the carboxylate moiety of the L-cysteine on the surface of CdTe QDs by electrostatic forces [110]. As a consequence, their luminescent intensity was linearly quenched by the Hg2+ ions from 5 × 10−12 M to 2.5 × 10−11 M, as it is displayed in Figure 8b. Furthermore, the LOD of this sensor was 2.7 × 10−12 M Hg2+. At higher concentrations of Hg2+, there is an electron transfer between the Hg2+ ions and the L-cysteine capped CdTe QDs [111] which induces not only a quenching of the luminescence, but also a red shift in the luminescence peak. Other QDs that show sensitivity to Hg2+ are hyperbranched-graft-copolymers-capped CdS QDs [112], L-cysteine-capped ZnS QDs [113] or polyethylene glycol-capped ZnO QDs [114].
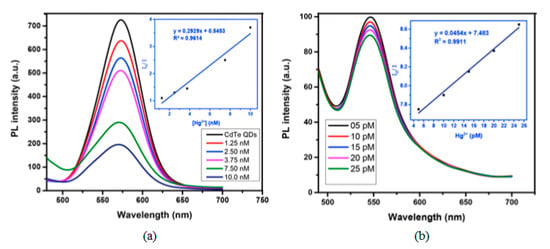
Figure 8.
Fluorescence spectra of (a) TGA and (b) L-cysteine capped CdTe QDs for Hg2+ concentrations in the nanomolar and picomolar ranges, respectively. Reprinted with permission from [109].
Sometimes, these QDs are functionalized with a sensitive element. That is the case of cysteamine-capped CdTe/ZnS core-shell QDs functionalized with T-rich aptamers [115] which, as explained previously, exhibit high affinity for Hg2+ ions. In the absence of Hg2+ ions, these aptamers act as an aggregator agent, resulting in a quenching of the fluorescence. As the Hg2+ concentration increases, T-rich aptamers acquire a hairpin structure and are detached from the QDs, which de-aggregate, giving rise to an increase of the fluorescent intensity. The LOD of this sensor was 8 × 10−11 M Hg2+, and it was capable of detecting Hg2+ linearly from 5 × 10−10 M to 1 × 10−6 M Hg2+. Taking advantage of the high affinity of thiourea for Hg2+, this compound was used to modify CdSe/CdS core-shell QDs for the development of a Hg2+ sensor [116] with a LOD of 2.79 × 10−9 M and a linear detection range from 5 × 10−9 M to 1.5 × 10−6 M. Although Cu2+ was found as an interferent ion, its presence could be masked with potassium cyanide. Apart from that, the recoveries of the fluorescent emission after removal of Hg2+ were between 83.8% and 95.4%.
Carbon QDs (CQDs) are a new kind of fluorescent nanomaterials [117] that exhibit several advantages over semiconductor QDs, such as good biocompatibility [118], low toxicity [119], good aqueous solubility [120], or facile synthesis [121].
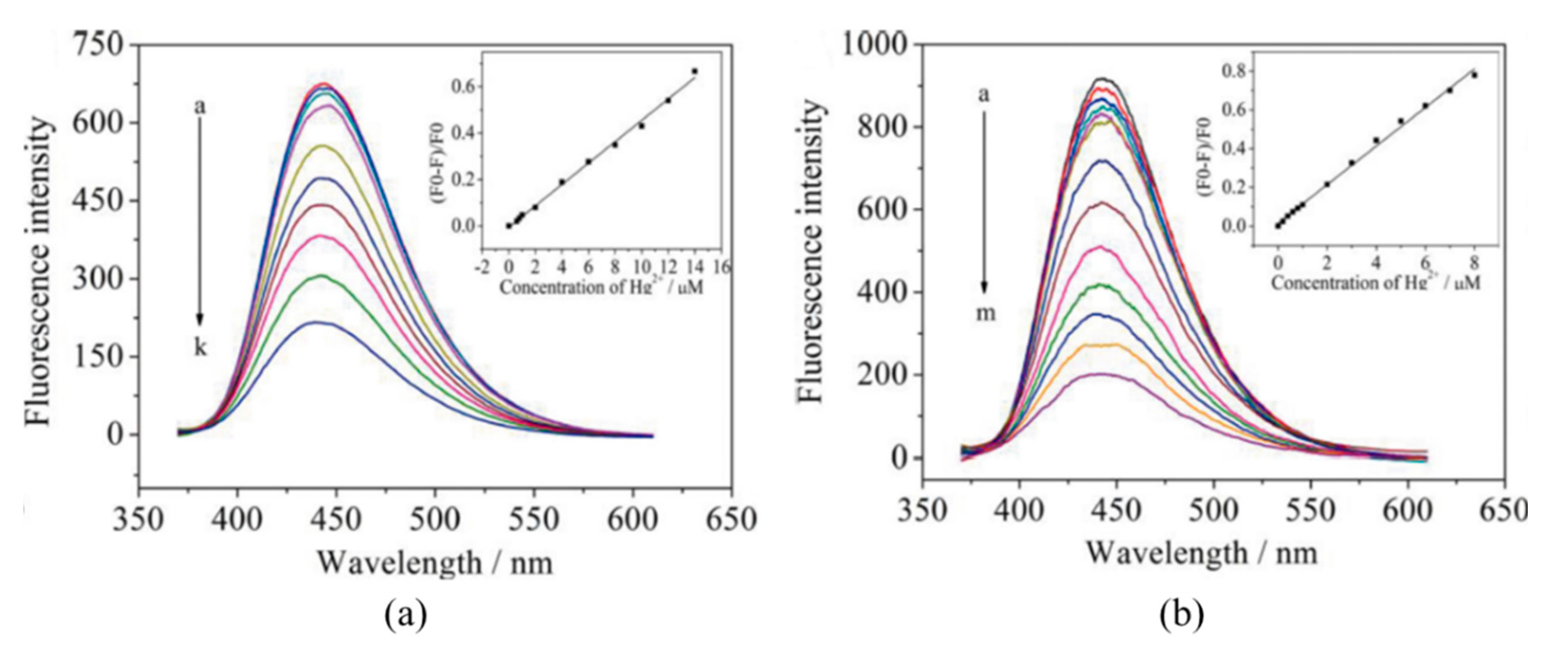
A common approach to tune their fluorescent properties is by doping them of other elements [122]: nitrogen (N) is the most commonly employed one [123,124,125], but boron (B), sulfur (S), and phosphorous (P) are also utilized [126,127,128]. Liu et al. [129] improved the performance of carbon dots by N-doping: although the first ones exhibited a larger linear detection range (from 6 × 10−7 to 1.4 × 10−5 M, while that of N-doped CQDs was from 0.2−8 µM), their LOD was much lower, 8.7 × 10−8 M, opposite to that of 2.5 × 10−7 M of the CQDs, as shown in Figure 9. Furthermore, the N-doping also enhanced the selectivity of the CQDs, avoiding the interference of Ag+, Fe3+, Cu2+ and Cd2+ cations. The potential use of N-CQDs in real applications was tested by determining Hg2+ concentrations in real water samples: in the case of mineral water, the recoveries of the N-CQDs were between 96.6% and 105.5%, while in tap water they ranged from 98.5% to 105%.
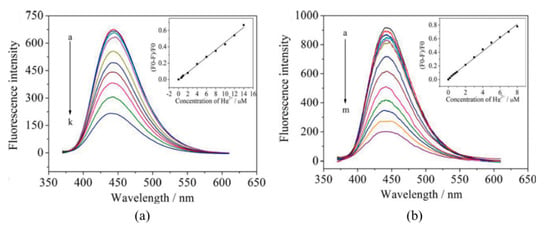
Figure 9.
Fluorescence spectra of 10 μg/mL carbon QDs (a) and N-carbon QDs (b) upon addition of different concentrations of Hg2+ (a–k: 0, 0.6, 0.8, 1, 2, 4, 6, 8, 10, 12, and 14 μM; a–m: 0, 0.2, 0.4, 0.6, 0.8, 1, 2, 3, 4, 5, 6, 7, and 8 μM). The linear calibration ranges of each one of the sensors are shown in the insets. Reprinted with permission from [129].
While N-dopants improve the quantum yield on CQDs [130], S-dopants are good ligands for metal ions [131]. Thus, S-doped carbon dots have been widely employed for the detection of Fe3+ ions [132,133,134]. One example is the sensor reported in [135], which exhibited high selectivity for Fe3+ at pH 0. In this acid media, the LOD of the sensor was 9.6 × 10−7 M, while the linear detection range was from 2.5 × 10−5 to 5 × 10−3 M.
In order to enhance the properties of CQDs, they can be doped of several compounds. N-, S-, co-doped carbon dots without any functionalization were fabricated for the linear detection of Hg2+ [136] between 0 and 20 µM, with a LOD of 1.7 × 10−7 M. Additionally, the fluorescent intensity can be linearly recovered by using cyanide anions.
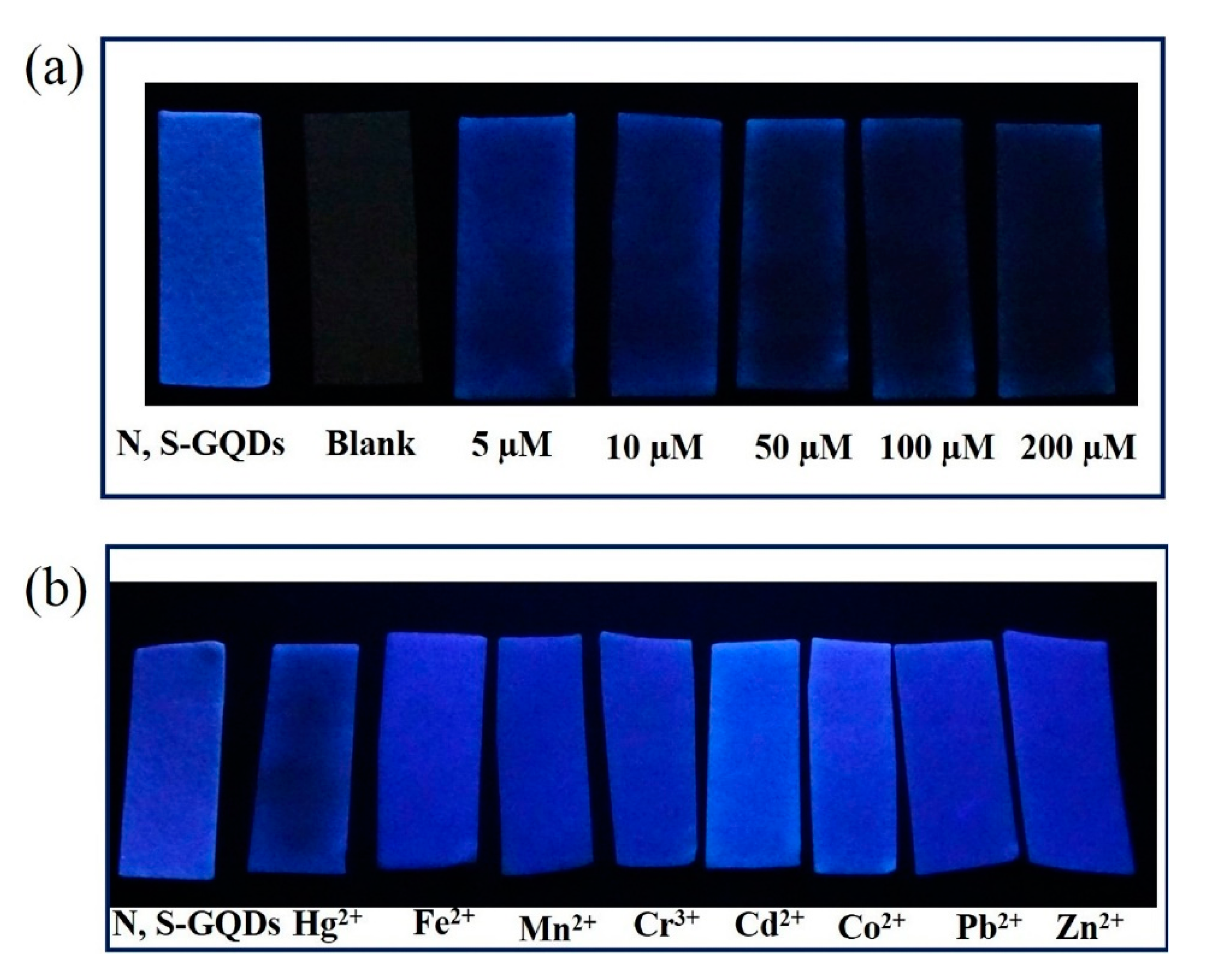
A particular kind of carbon QDs are based on graphene: their features are derived from graphene and carbon nanodots [137]. Hence, their sensing properties can also be modified with dopants such as nitrogen or sulfur [138,139]. In particular, N-, S-codoped graphene QDs-based paper strips have been used in real waste water for the detection of Hg2+ ions [140]: as it can be observed in Figure 10a, the luminescence intensity of the QDs-coated paper strips decreased as the Hg2+ concentration increased from 10 to 200 µM. Furthermore, concentrations of 100 µM of other metal ions (Fe2+, Mn2+, Cr3+, Cd2+, Co2+, and Zn2+) did not present any interference, as it is displayed in Figure 10b.
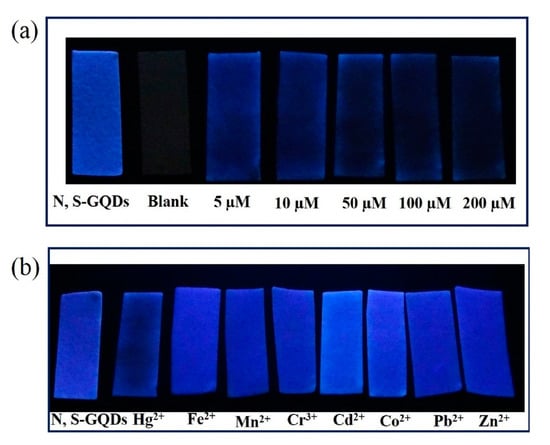
Figure 10.
(a) Luminescence of blank paper strips and paper strips coated with N-, S-codoped graphene QDs exposed to different Hg2+ concentrations and (b) in the presence of different metal ions in a 100 µM concentration. Reprinted with permission from [140].
Most of the sensors based on fluorescent QDs have been developed with the purpose of monitoring Hg2+ ions. Thus, Table 3 is focused on this kind of sensors, while devices for other metal ions, are summarized in Table 4.

Table 3.
Hg2+ fluorescent sensors based on QDs.

Table 4.
Fluorescent sensors for different metal ions based on QDs.
4. Heavy Metal Ion Sensors Based on Organic Dyes
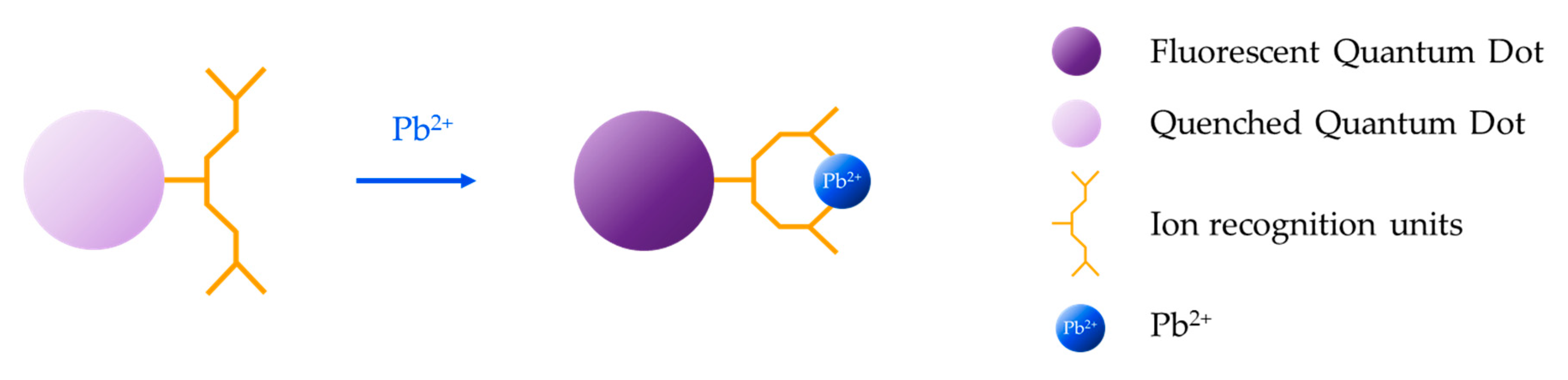
Organic dyes have been widely employed for the development of fluorescence-based sensors [166,167,168] because of their attractive features: high molar extinction coefficient [169], bright signal [170], ease of modification [171], and presence of many possible reactive sites in their skeletons [172]. For the detection of heavy metal ions, these fluorophores are modified with an ion recognition unit (ionophore), which serves as host for the target metal ion (guest) [173]. The interaction between the ionophore and the target analyte induces a modification of the photophysical features of the fluorophore that is translated into a change of its fluorescent emission [174], usually from “off” to “on”. Typically employed ionophores are crown ethers [175] and aliphatic or aromatic amines [176], which act as electron donors, that is, they quench the fluorescent emission through a photo-induced electron transfer (PET) mechanism with the fluorophore [177] in the absence of the target metal ion. However, in its presence, PET does not occur, giving rise to an enhancement of the fluorescence intensity, as shown in Figure 11.
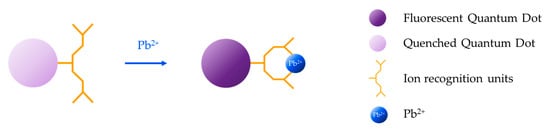
Figure 11.
Sensing mechanism based on organic dyes modified with an ion recognition unit: the interaction between the ionophore and the target analyte induces a change in the fluorescent emission of the fluorophore.
Among all of the organic dyes, rhodamine derivatives are the most utilized ones due to their structure-dependent properties [178,179,180]. Other dyes that are also widely employed for the fabrication of fluorescent sensors are fluorescein [181,182] and coumarin derivatives [183,184].
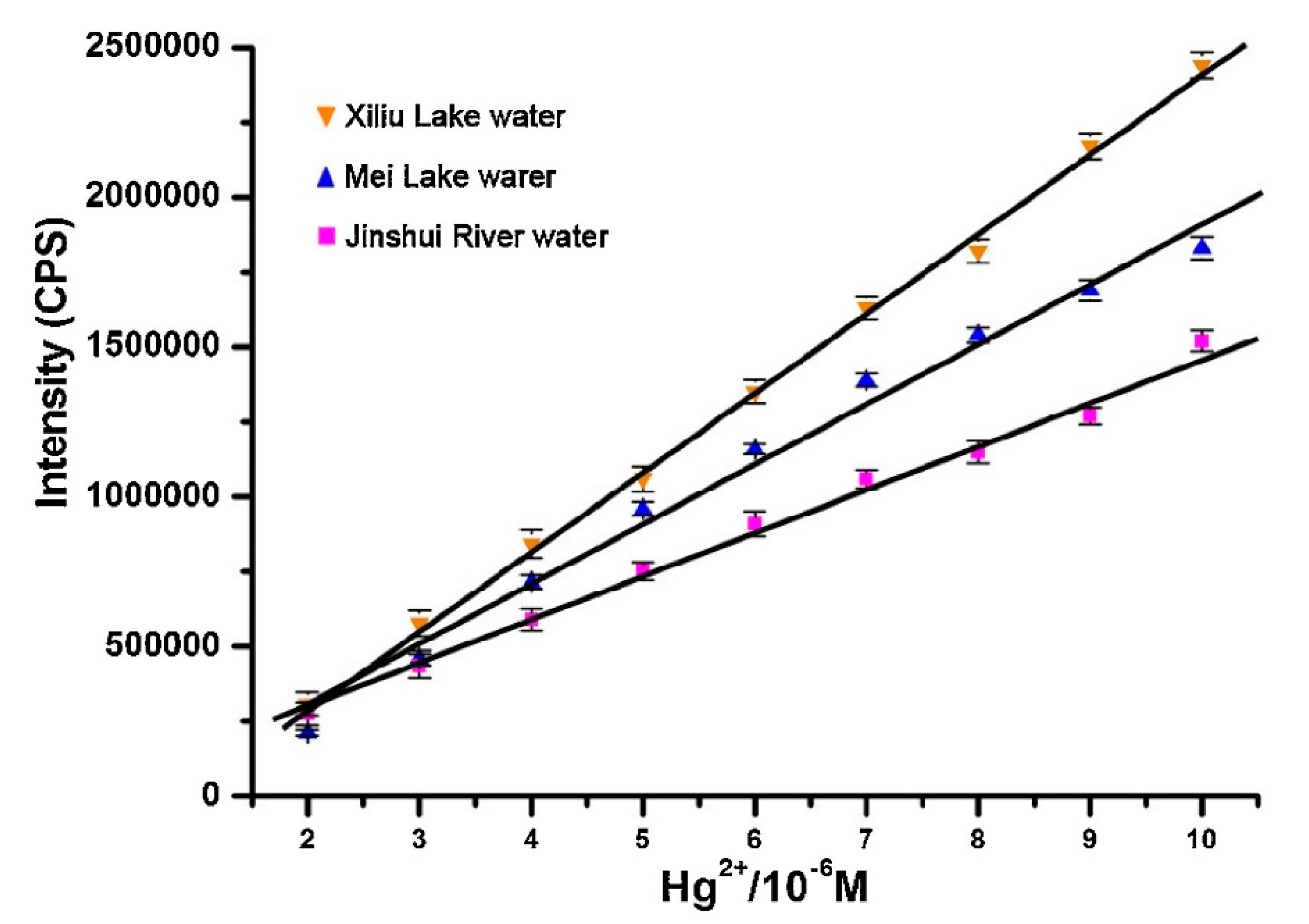
Li and co-workers [185] developed a turn-on fluorescent probe for Hg2+ based on a rhodamine B derivative (rhodamine B hydroxamate spirolactam) linked to a NS2 unit as a receptor that detected Hg2+ linearly from 0 to 1.6 × 10−5 M with a LOD of 2.36 × 10−6 M. The fluorescent response of the sensor towards Hg2+ was not interfered by any other metal ion and the probe was regenerated by using Na2S. Furthermore, the potential utilization of this sensor in real applications was tested by exposing the sensor to three natural water samples to which different Hg2+ concentrations were added, as shown in Figure 12.
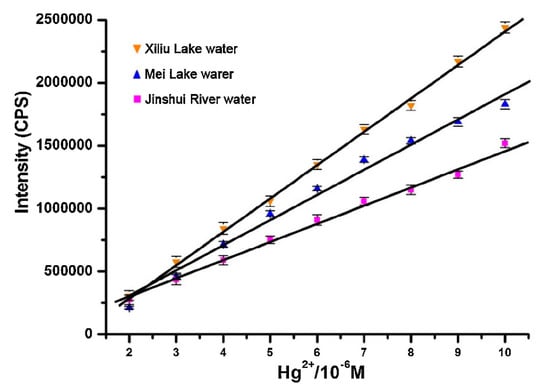
Figure 12.
Hg2+ detection carried out by the rhodamine B derivative in three different natural water samples. Reprinted with permission from [185].
Rhod-5N is another rhodamine derivative that consists of a 5N-BAPTA ionophore linked to a rhodamine fluorophore: it exhibits a fluorescence enhancement in the presence of Hg2+ and Cd2+ [186]. Ruan et al. [187] fabricated a Hg2+ sensor by immobilizing it in a silica sol-gel matrix onto the end of an optical fiber: its LOD was 1.25 × 10−7 M Hg2+, and it also presented a linear detection range up to 5 × 10−7 M Hg2+.
Apart from for the detection of Hg2+, rhodamine derivatives have been utilized for monitoring Cu2+, Cd2+, or Pb2+ ions [188,189]. For instance, a probe based on rhodamine 6G and p-Cresol derivatives [190] exhibited a fluorescence enhancement under the addition of Pb2+ ions when it was illuminated with UV light. Furthermore, the color change promoted by Pb2+ allowed the naked-eye detection of that ion, as shown in Figure 13.
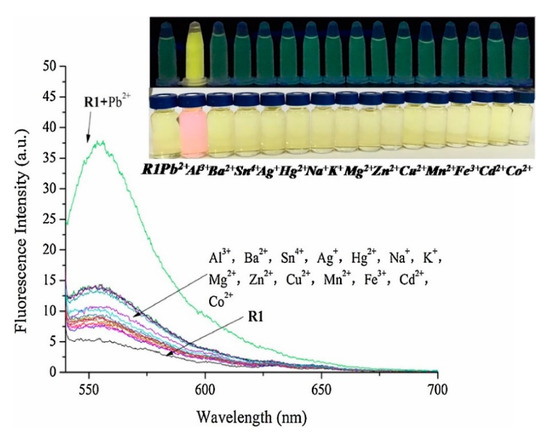
Figure 13.
Fluorescence spectra of the probe based on rhodamine 6G and p-Cresol derivatives in the presence of Pb2+ (1 × 10−5 M) and other metal ion (5 × 10−6 M). The inset shows the fluorescence under UV illumination and the color change of the probe upon the addition of Pb2+ and other metal ions. Reprinted with permission from [190].
Regarding to fluorescein, it has been shown that the modification of its sites with different functional groups gives rise to sensors of different sensitivities to Ag+ ions [191]: when the 4,5-positions of fluorescein were modified with N,Se-containing receptors, the LOD of the sensor was 3 × 10−8 M Ag+, whereas the introduction of a N,S-receptor decreased it up to 4 × 10−9 M. In both cases, the presence of Ag+ ions induced the opening of the spironolactone ring, which led to the increase of the fluorescence intensity, as shown in Figure 14.
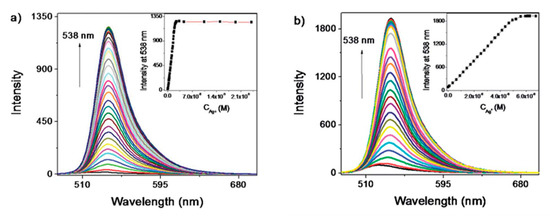
Figure 14.
Fluorescence spectra of N,S- and N,Se-modified fluorescein sensors for different Ag+ ions in ethanol. Reprinted with permission from [191]. Copyright 2014 Japan Society for Analytical Chemistry.
Another commonly used dye is coumarin, which has been functionalized with receptors that are sensitive to different heavy metal ions: in the case of utilizing a triazole substituted 8-hydroxyquinoline (8-HQ) receptor [192], which exhibits a high affinity towards Pb2+, a highly sensitive (LOD = 3.36 × 10−11 M) and selective sensor was developed. In the absence of Pb2+, due the PET mechanism from the receptor to coumarin, the fluorescent emission was weak. In the presence of that metal ion, the PET process did not occur, so the blue emission of coumarin was recovered and visually detectable.
As well as in the case of aptamer- or QDs-based heavy metal ions sensors, most of the devices based on organic dyes are devoted to the detection of Hg2+ ions, whereas those dedicated to the monitoring of other heavy metal ions are not so numerous.
Table 5 summarizes the sensors for Hg2+ detection based on organic dyes, whereas those developed for the detection of other metal ions are outlined in Table 6.

Table 5.
Hg2+ sensors for different metal ions based on organic dyes.

Table 6.
Fluorescent sensors for different metal ions based on organic dyes.
5. Comparison between Fluorescent Sensors for Heavy Metal Ions Based on Different Materials
Although this review is focused on those sensors fabricated with fluorescent aptamers, quantum dots, and organic dyes, other materials can be utilized for the detection of heavy metal ions, such as porphyrins and metal-organic frameworks (MOFs). Thus, in this section, a brief comparison between all the materials is carried out.
As it can be observed in Table 7, the sensors developed with fluorescent aptamers and quantum dots present the lowest limits of detection, oppositely to those fabricated with MOFs. Regarding the detection ranges, the sensors based on porphyrins and MOFs are capable of detecting heavy metal ions at higher concentration ranges (from nanomolar to hundreds of micromolar concentrations) than the rest of the sensors, which monitor concentrations from the picomolar range to the micromolar one. Although reversibility and specificity are not always analyzed, the obtained results are usually positive: the sensors recover their original fluorescence intensity once the contaminants are removed from the aqueous media, and the sensors are not or slightly affected by the presence of other heavy metal ions.

Table 7.
Fluorescent sensors for heavy metal ions based on different kind of materials.
6. Conclusions
As it has been shown in this review, fluorescence-based sensors exhibit interesting features for the monitoring of heavy metal ions in aqueous media: the devices presented here exhibit good sensitivity and selectivity, low detection limits as well as large detection ranges. Apart from that, some of them show recovery values close to the 100%, even after being tested in real water samples. Among the heavy metal ion species, special attention has been paid to the sensors devoted to Hg2+, as long as it is one of the most hazardous water pollutants and it presents an accumulative effect on human body through the food chain.
Depending on the application, different sensing materials can be utilized for the monitoring of heavy metal ions: on one side, the utilization of aptamers allows the development of sensors with low detection limits, good reversibility and outstanding specificity for Hg2+ or Pb2+, due to the high affinity that T and G bases present for these contaminants, respectively. Additionally, thanks to the functionalization of quantum dots, it is possible to fabricate sensors for monitoring a wide range of heavy metal ions: although their detection limits are not as low as those of the aptasensors, they present good selectivity and reversibility. Finally, the modification of organic dyes with ion recognition units also permits the detection of several metal ions in real water samples, presenting large detection ranges, good selectivity and reversibility. Although the three kinds of materials present appropriate features for sensing applications (low detection limits, acceptable selectivity and reversibility), those devices based on aptamers exhibit the lowest limits of detection and the highest selectivity, due to the high affinity of the T-rich and G-rich ODN sequences for Hg2+ and Pb2+, respectively. However, this specificity for these analytes does not allow their utilization for the detection of other metal ions, which can be done by QDs and organic dyes.
These facts, together with all the advantages that the optic technology presents nowadays, make custom-designed fluorescent sensors attractive tools for the monitoring of heavy metal ions in real applications.
Author Contributions
All authors contributed equally to this work.
Funding
This work was supported by the Spanish State Research Agency (AEI) and the Spanish Ministry of Economy and Competitiveness through the TEC2016-79367-C2-2-R project. Nerea de Acha would also like to acknowledge her pre-doctoral fellowship (reference BES-2014-069692) funded by the Spanish Ministry of Economy and Competitiveness through the TEC2013-43679-R project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization. Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Schriks, M.; Heringa, M.B.; van der Kooi, M.M.E.; de Voogt, P.; van Wezel, A.P. Toxicological relevance of emerging contaminants for drinking water quality. Water Res. 2010, 44, 461–476. [Google Scholar] [CrossRef] [PubMed]
- Gregory, M.R. Environmental implications of plastic debris in marine settings- entanglement, ingestion, smothering, hangers-on, hitch-hiking and alien invasions. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2013–2025. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, K.M.; King, A.M.; Harter, T. Identifying sources of groundwater nitrate contamination in a large alluvial groundwater basin with highly diversified intensive agricultural production. J. Contam. Hydrol. 2013, 151, 140–154. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, N. Contamination of soils with microbial pathogens originating from effluent water used for irrigation. In Proceedings of the EGU General Assembly 2009, Vienna, Austria, 19–24 April 2009. [Google Scholar]
- Järup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Goldhaber, S.B. Trace element risk assessment: Essentiality vs. toxicity. Regul. Toxicol. Pharmacol. 2003, 38, 232–242. [Google Scholar] [CrossRef]
- Xie, W.; Peng, C.; Wang, H.; Chen, W. Health risk assessment of trace metals in various environmental media, crops and human hair from a mining affected area. Int. J. Environ. Res. Public Health 2017, 14, 1595. [Google Scholar] [CrossRef]
- Stankovic, S.; Jovic, M.; Stankovic, A.R.; Katsikas, L. Heavy Metals in Seafood Mussels. Risks for Human Health; Springer: Dordrecht, The Netherlands, 2012; Volume 1, ISBN 9789400724396. [Google Scholar]
- Huff, J.; Lunn, R.M.; Waalkes, M.P.; Tomatis, L.; Infante, P.F. Cadmium-induced cancers in animals and in humans. Int. J. Occup. Environ. Health 2007, 13, 202–212. [Google Scholar] [CrossRef]
- Bhatti, P.; Stewart, P.A.; Hutchinson, A.; Rothman, N.; Linet, M.S.; Inskip, P.D.; Rajaraman, P. Lead exposure, polymorphisms in genes related to oxidative stress, and risk of adult brain tumors. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1841–1848. [Google Scholar] [CrossRef]
- Bansod, B.; Kumar, T.; Thakur, R.; Rana, S.; Singh, I. A review on various electrochemical techniques for heavy metal ions detection with different sensing platforms. Biosens. Bioelectron. 2017, 94, 443–455. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef]
- Jan, A.T.; Azam, M.; Siddiqui, K.; Ali, A.; Choi, I.; Haq, Q.M.R. Heavy metals and human health: Mechanistic insight into toxicity and counter defense system of antioxidants. Int. J. Mol. Sci. 2015, 16, 29592–29630. [Google Scholar] [CrossRef] [PubMed]
- Senthil Kumar, P.; Saravanan, A. Sustainable wastewater treatments in textile sector. Sustain. Fibres Text. 2017, 323–346. [Google Scholar]
- Suszcynsky, E.M.; Shann, J.R. Phytotoxicity and accumulation of mercury in tobacco subjected to different exposure routes. Environ. Toxicol. Chem. 1995, 14, 61–67. [Google Scholar] [CrossRef]
- Lough, G.C.; Schauer, J.J.; Park, J.-S.; Shafer, M.M.; Deminter, J.T.; Weinstein, J.P. Emissions of metals associated with motor vehicle roadways. Environ. Sci. Technol. 2005, 39, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Yuliusman, S.; Nurqomariah, A.; Fajaryanto, R. Recovery of cobalt and nickel from spent lithium ion batteries with citric acid using leaching process: Kinetics study. In Proceedings of the E3S Web of Conferences, Berdyansk, Ukraine, 4–8 September 2018; Volume 67. [Google Scholar]
- Arruti, A.; Fernández-Olmo, I.; Irabien, A. Evaluation of the contribution of local sources to trace metals levels in urban PM2.5 and PM10 in the Cantabria region (Northern Spain). J. Environ. Monit. 2010, 12, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- Farzin, L.; Shamsipur, M.; Sheibani, S. A review: Aptamer-based analytical strategies using the nanomaterials for environmental and human monitoring of toxic heavy metals. Talanta 2017, 174, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.-C.; Yin, B.-C. Highly sensitive detection of mercury(II) ions by fluorescence polarization enhanced by gold nanoparticles. Angew. Chem. Int. Ed. 2008, 47, 8386–8389. [Google Scholar] [CrossRef]
- Nolan, E.M.; Lippard, S.J. A “Turn-on” Fluorescent Sensor for the Selective Detection of Mercuric Ion in Aqueous Media. J. Am. Chem. Soc. 2003, 125, 14270–14271. [Google Scholar] [CrossRef]
- Simões Da Costa, A.M.; Delgadillo, I.; Rudnitskaya, A. Detection of copper, lead, cadmium and iron in wine using electronic tongue sensor system. Talanta 2014, 129, 63–71. [Google Scholar] [CrossRef]
- Alizadeh, T.; Ganjali, M.R.; Zare, M. Application of an Hg2+ selective imprinted polymer as a new modifying agent for the preparation of a novel highly selective and sensitive electrochemical sensor for the determination of ultratrace mercury ions. Anal. Chim. Acta 2011, 689, 52–59. [Google Scholar] [CrossRef]
- Kim, H.N.; Ren, W.X.; Kim, J.S.; Yoon, J. Fluorescent and colorimetric sensors for detection of lead, cadmium, and mercury ions. Chem. Soc. Rev. 2012, 41, 3210–3244. [Google Scholar] [CrossRef] [PubMed]
- Kuswandi, B.; Nuriman; Huskens, J.; Verboom, W. Optical sensing systems for microfluidic devices: A review. Anal. Chim. Acta 2007, 601, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Aiestaran, P.; Dominguez, V.; Arrue, J.; Zubia, J. A fluorescent linear optical fiber position sensor. Opt. Mater. 2009, 31, 1101–1104. [Google Scholar] [CrossRef]
- Vendrell, M.; Zhai, D.; Er, J.C.; Chang, Y.-T. Combinatorial strategies in fluorescent probe development. Chem. Rev. 2012, 112, 4391–4420. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; He, Y.; Xing, X.; Zhao, Y.; Tang, H.; Pang, D. Aptamer functionalized gold nanoparticles based fluorescent probe for the detection of mercury (II) ion in aqueous solution. Talanta 2013, 113, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Lakowicz, J.R., Ed.; Springer: Boston, MA, USA, 2006; ISBN 978-0-387-31278-1. [Google Scholar]
- Elosua, C.; de Acha, N.; Lopez-Torres, D.; Matias, I.R.; Arregui, F.J. Luminescent Optical Fiber Oxygen Sensor following Layer-by-layer Method. Procedia Eng. 2014, 87, 987–990. [Google Scholar] [CrossRef]
- Caselli, M. Porphyrin-based electrostatically self-assembled multilayers as fluorescent probes for mercury(ii) ions: A study of the adsorption kinetics of metal ions on ultrathin films for sensing applications. RSC Adv. 2015, 5, 1350–1358. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Z.; Li, Y.; Zhuang, Q.; Zhao, W.; Gu, J. Porphyrinic MOFs for reversible fluorescent and colorimetric sensing of mercury(II) ions in aqueous phase. RSC Adv. 2016, 6, 69807–69814. [Google Scholar] [CrossRef]
- He, J.-L.; Zhu, S.-L.; Wu, P.; Li, P.-P.; Li, T.; Cao, Z. Enzymatic cascade based fluorescent DNAzyme machines for the ultrasensitive detection of Cu(II) ions. Biosens. Bioelectron. 2014, 60, 112–117. [Google Scholar] [CrossRef]
- Zhu, Y.-F.; Wang, Y.-S.; Zhou, B.; Yu, J.-H.; Peng, L.-L.; Huang, Y.-Q.; Li, X.-J.; Chen, S.-H.; Tang, X.; Wang, X.-F. A multifunctional fluorescent aptamer probe for highly sensitive and selective detection of cadmium(II). Anal. Bioanal. Chem. 2017, 409, 4951–4958. [Google Scholar] [CrossRef]
- Xu, H.; Miao, R.; Fang, Z.; Zhong, X. Quantum dot-based turn-on fluorescent probe for detection of zinc and cadmium ions in aqueous media. Anal. Chim. Acta 2011, 687, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Lu, Y.; He, S.; Wei, F.; Zhao, L.; Zeng, X. A highly sensitive and selective turn-on fluorescent chemosensor for palladium based on a phosphine-rhodamine conjugate. Chem. Commun. 2013, 49, 822–824. [Google Scholar] [CrossRef] [PubMed]
- Lakhin, A.V.; Tarantul, V.Z.; Gening, L.V. Aptamers: Problems, solutions and prospects. Acta Nat. 2013, 5, 34–43. [Google Scholar]
- Lung Khung, Y.; Narducci, D. Synergizing nucleic acid aptamers with 1-dimensional nanostructures as label-free field-effect transistor biosensors. Biosens. Bioelectron. 2013, 50, 278–293. [Google Scholar] [CrossRef] [PubMed]
- Bozokalfa, G.; Akbulut, H.; Demir, B.; Guler, E.; Gumus, Z.P.; Odaci Demirkol, D.; Aldemir, E.; Yamada, S.; Endo, T.; Coskunol, H.; et al. Polypeptide Functional Surface for the Aptamer Immobilization: Electrochemical Cocaine Biosensing. Anal. Chem. 2016, 88, 4161–4167. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Niazi, J.H.; Gu, M.B. Specific detection of oxytetracycline using DNA aptamer-immobilized interdigitated array electrode chip. Anal. Chim. Acta 2009, 634, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Radom, F.; Jurek, P.M.; Mazurek, M.P.; Otlewski, J.; Jeleń, F. Aptamers: Molecules of great potential. Biotechnol. Adv. 2013, 31, 1260–1274. [Google Scholar] [CrossRef]
- Lassalle, H.-P.; Marchal, S.; Guillemin, F.; Reinhard, A.; Bezdetnaya, L. Aptamers as remarkable diagnostic and therapeutic agents in cancer treatment. Curr. Drug Metab. 2012, 13, 1130–1144. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, Z.; Xu, L.; Xia, T.; Li, N.; Liu, J.; Fang, X. Exonuclease i aided enzyme-linked aptamer assay for small-molecule detection. Anal. Bioanal. Chem. 2014, 406, 2949–2955. [Google Scholar] [CrossRef]
- Jiang, H.; Ling, K.; Tao, X.; Zhang, Q. Theophylline detection in serum using a self-assembling RNA aptamer-based gold nanoparticle sensor. Biosens. Bioelectron. 2015, 70, 299–303. [Google Scholar] [CrossRef]
- Liu, Y.; Lai, Y.; Yang, G.; Tang, C.; Deng, Y.; Li, S.; Wang, Z. Cd-aptamer electrochemical biosensor based on AuNPs/CS modified glass carbon electrode. J. Biomed. Nanotechnol. 2017, 13, 1253–1259. [Google Scholar] [CrossRef]
- Miyake, Y.; Togashi, H.; Tashiro, M.; Yamaguchi, H.; Oda, S.; Kudo, M.; Tanaka, Y.; Kondo, Y.; Sawa, R.; Fujimoto, T.; et al. MercuryII-mediated formation of thymine-HgII-thymine base pairs in DNA duplexes. J. Am. Chem. Soc. 2006, 128, 2172–2173. [Google Scholar] [CrossRef] [PubMed]
- Ono, A.; Cao, S.; Togashi, H.; Tashiro, M.; Fujimoto, T.; MacHinami, T.; Oda, S.; Miyake, Y.; Okamoto, I.; Tanaka, Y. Specific interactions between silver(i) ions and cytosine-cytosine pairs in DNA duplexes. Chem. Commun. 2008, 4825–4827. [Google Scholar] [CrossRef] [PubMed]
- Ono, A.; Togashi, H. Highly selective oligonucleotide-based sensor for mercury(II) in aqueous solutions. Angew. Chem. Int. Ed. 2004, 43, 4300–4302. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, B.; Qi, Y.; Jin, Y. Label-free aptamer-based colorimetric detection of mercury ions in aqueous media using unmodified gold nanoparticles as colorimetric probe. Anal. Bioanal. Chem. 2009, 393, 2051–2057. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chang, H.; Hirata, A.; Wu, H.; Xue, Q.-K.; Chen, M. Nanoporous gold based optical sensor for sub-ppt detection of mercury ions. ACS Nano 2013, 7, 4595–4600. [Google Scholar] [CrossRef]
- Liu, C.-W.; Tsai, T.-C.; Osawa, M.; Chang, H.-C.; Yang, R.-J. Aptamer-based sensor for quantitative detection of mercury (II) ions by attenuated total reflection surface enhanced infrared absorption spectroscopy. Anal. Chim. Acta 2018, 1033, 137–147. [Google Scholar] [CrossRef]
- Li, B.; Du, Y.; Dong, S. DNA based gold nanoparticles colorimetric sensors for sensitive and selective detection of Ag(I) ions. Anal. Chim. Acta 2009, 644, 78–82. [Google Scholar] [CrossRef]
- Wen, Y.; Xing, F.; He, S.; Song, S.; Wang, L.; Long, Y.; Li, D.; Fan, C. A graphene-based fluorescent nanoprobe for silver(i) ions detection by using graphene oxide and a silver-specific oligonucleotide. Chem. Commun. 2010, 46, 2596–2598. [Google Scholar] [CrossRef]
- Park, J.; Choi, W.; Jang, K.; Na, S. High-sensitivity detection of silver ions using oligonucleotide-immobilized oscillator. Biosens. Bioelectron. 2013, 41, 471–476. [Google Scholar] [CrossRef]
- Guo, L.; Nie, D.; Qiu, C.; Zheng, Q.; Wu, H.; Ye, P.; Hao, Y.; Fu, F.; Chen, G. A G-quadruplex based label-free fluorescent biosensor for lead ion. Biosens. Bioelectron. 2012, 35, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Jung, I.H.; Kang, M.; Shim, H.-K.; Woo, H.Y. Cationic conjugated polyelectrolytes-triggered conformational change of molecular beacon aptamer for highly sensitive and selective potassium ion detection. J. Am. Chem. Soc. 2012, 134, 3133–3138. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Chen, Y.; Li, X.; Fang, W. Pb2+ induced DNA conformational switch from hairpin to G-quadruplex: Electrochemical detection of Pb2+. Analyst 2011, 136, 2367–2372. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Feng, Y.; Zhao, C.; Tang, B. Crystal violet as a G-quadruplex-selective probe for sensitive amperometric sensing of lead. Chem. Commun. 2011, 47, 11909–11911. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Yin, N.; Chen, G. Photoinduced electron transfer mediated by π-stacked thymine-Hg2+-thymine base pairs. J. Phys. Chem. C 2011, 115, 4837–4842. [Google Scholar] [CrossRef]
- Liu, C.-W.; Huang, C.-C.; Chang, H.-T. Highly selective DNA-based sensor for lead(II) and mercury(II) ions. Anal. Chem. 2009, 81, 2383–2387. [Google Scholar] [CrossRef]
- Ono, A. Development of novel oligonucleotide-based sensors which are highly Hg(II) selective and are insensitive to other heavy metal ions. Nucleic Acids Symp. Ser. (Oxf.) 2004, 29–30. [Google Scholar] [CrossRef]
- Wang, Y.; Bao, L.; Liu, Z.; Pang, D.-W. Aptamer biosensor based on fluorescence resonance energy transfer from upconverting phosphors to carbon nanoparticles for thrombin detection in human plasma. Anal. Chem. 2011, 83, 8130–8137. [Google Scholar] [CrossRef]
- Chung, C.H.; Kim, J.H.; Jung, J.; Chung, B.H. Nuclease-resistant DNA aptamer on gold nanoparticles for the simultaneous detection of Pb2+ and Hg2+ in human serum. Biosens. Bioelectron. 2013, 41, 827–832. [Google Scholar] [CrossRef]
- Chen, S.-H.; Wang, Y.-S.; Chen, Y.-S.; Tang, X.; Cao, J.-X.; Li, M.-H.; Wang, X.-F.; Zhu, Y.-F.; Huang, Y.-Q. Dual-channel detection of metallothioneins and mercury based on a mercury-mediated aptamer beacon using thymidine-mercury-thymidine complex as a quencher. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 151, 315–321. [Google Scholar] [CrossRef]
- Zeng, X.; Zhang, F.S.; Zhu, B.; Zhu, L. Fluorescence Determination of Merucury(II) Using a Thymine Aptamer. Anal. Lett. 2015, 48, 2208–2216. [Google Scholar] [CrossRef]
- Johansson, M.K. Choosing reporter-quencher pairs for efficient quenching through formation of intramolecular dimers. Methods Mol. Biol. 2006, 335, 17–29. [Google Scholar] [PubMed]
- Li, Z.; Muhandiramlage, T.P.; Keogh, J.P.; Hall, H.K.; Aspinwall, C.A. Aptamer-functionalized porous phospholipid nanoshells for direct measurement of Hg(2+) in urine. Anal. Bioanal. Chem. 2015, 407, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Fialová, M.; Kypr, J.; Vorlíčková, M. The thrombin binding aptamer GGTTGGTGTGGTTGG forms a bimolecular guanine tetraplex. Biochem. Biophys. Res. Commun. 2006, 344, 50–54. [Google Scholar] [CrossRef]
- Huang, X.; Ren, J. Gold nanoparticles based chemiluminescent resonance energy transfer for immunoassay of alpha fetoprotein cancer marker. Anal. Chim. Acta 2011, 686, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Mayilo, S.; Kloster, M.A.; Wunderlich, M.; Lutich, A.; Klar, T.A.; Nichtl, A.; Kürzinger, K.; Stefani, F.D.; Feldmann, J. Long-range fluorescence quenching by gold nanoparticles in a sandwich immunoassay for cardiac troponin T. Nano Lett. 2009, 9, 4558–4563. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, J.; Jiao, K.; Yang, X. Colorimetric detection of mercury ion (Hg2+) based on DNA oligonucleotides and unmodified gold nanoparticles sensing system with a tunable detection range. Biosens. Bioelectron. 2009, 24, 3153–3158. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Niu, C.; Wang, X.; Lv, X.; Zeng, G. “Turn-on” fluorescent sensor for Hg2+ based on single-stranded DNA functionalized Mn:CdS/ZnS quantum dots and gold nanoparticles by time-gated mode. Anal. Chem. 2013, 85, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ouyang, Q.; Li, H.; Chen, M.; Zhang, Z.; Chen, Q. Turn-On Fluoresence Sensor for Hg2+ in Food Based on FRET between Aptamers-Functionalized Upconversion Nanoparticles and Gold Nanoparticles. J. Agric. Food Chem. 2018, 66, 6188–6195. [Google Scholar] [CrossRef]
- Long, F.; Zhu, A.; Wang, H. Optofluidics-based DNA structure-competitive aptasensor for rapid on-site detection of lead(II) in an aquatic environment. Anal. Chim. Acta 2014, 849, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Dragan, A.I.; Casas-Finet, J.R.; Bishop, E.S.; Strouse, R.J.; Schenerman, M.A.; Geddes, C.D. Characterization of PicoGreen interaction with dsDNA and the origin of its fluorescence enhancement upon binding. Biophys. J. 2010, 99, 3010–3019. [Google Scholar] [CrossRef] [PubMed]
- Singer, V.L.; Lawlor, T.E.; Yue, S. Comparison of SYBR® Green I nucleic acid gel stain mutagenicity and ethidium bromide mutagenicity in the Salmonella/mammalian microsome reverse mutation assay (Ames test). Mutat. Res. Genet. Toxicol. Environ. Mutagen. 1999, 439, 37–47. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.; Yan, J.; Zhu, C.; Zhang, C.; Chen, A. Dulplex analysis of mercury and silver ions using a label-free fluorescent aptasensor. Int. J. Environ. Anal. Chem. 2018, 98, 349–359. [Google Scholar] [CrossRef]
- Yan, Z.; Tian, C.; Qu, X.; Shen, W.; Ye, B. DNA-functionalized photonic crystal microspheres for multiplex detection of toxic metal ions. Colloids Surf. B Biointerfaces 2017, 154, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhou, X.; Ding, W.; Guo, S.; Wu, N. Fluorescent aptamer-functionalized graphene oxide biosensor for label-free detection of mercury(II). Biosens. Bioelectron. 2013, 41, 889–893. [Google Scholar] [CrossRef]
- Li, J.; Wang, H.; Guo, Z.; Wang, Y.; Ma, H.; Ren, X.; Du, B.; Wei, Q. A “turn-off” fluorescent biosensor for the detection of mercury (II) based on graphite carbon nitride. Talanta 2017, 162, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Duan, N.; Shi, Z.; Fang, C.; Wang, Z. Dual fluorescence resonance energy transfer assay between tunable upconversion nanoparticles and controlled gold nanoparticles for the simultaneous detection of Pb2+ and Hg2+. Talanta 2014, 128, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Niu, C.; Ruan, M.; Wang, X.; Zeng, G.; Deng, C. Highly sensitive strategy for Hg2+ detection in environmental water samples using long lifetime fluorescence quantum dots and gold nanoparticles. Environ. Sci. Technol. 2013, 47, 4392–4398. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Zhu, L.; Wu, J.; Hou, Y.; Wang, P.; Wang, Z.; Yang, M. A fluorescent biosensor based on carbon dots-labeled oligodeoxyribonucleotide and graphene oxide for mercury (II) detection. Biosens. Bioelectron. 2015, 63, 506–512. [Google Scholar] [CrossRef]
- Zhang, L.; Mi, N.; Zhang, Y.; Wei, M.; Li, H.; Yao, S. Label-free DNA sensor for Pb2+ based on a duplex-quadruplex exchange. Anal. Methods 2013, 5, 6100–6105. [Google Scholar] [CrossRef]
- Huang, Y.; Yan, J.; Fang, Z.; Zhang, C.; Bai, W.; Yan, M.; Zhu, C.; Gao, C.; Chen, A. Highly sensitive and selective optical detection of lead(ii) using a label-free fluorescent aptasensor. RSC Adv. 2016, 6, 90300–90304. [Google Scholar] [CrossRef]
- Liu, C.; Huang, C.-Z. Detection of lead ions in water based on the surface energy transfer between gold nanoparticles and fluorescent dyes. Fenxi Huaxue/Chin. J. Anal. Chem. 2014, 42, 1195–1199. [Google Scholar] [CrossRef]
- Taghdisi, S.M.; Emrani, S.S.; Tabrizian, K.; Ramezani, M.; Abnous, K.; Emrani, A.S. Ultrasensitive detection of lead (II) based on fluorescent aptamer-functionalized carbon nanotubes. Environ. Toxicol. Pharmacol. 2014, 37, 1236–1242. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.S.; Shan, X.Y.; Chai, L.J.; Chen, J.R.; Feng, H. A fluorescent nanosensor based on graphene quantum dots-aptamer probe and graphene oxide platform for detection of lead (II) ion. Biosens. Bioelectron. 2015, 68, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhou, X.; Guo, S.; Wu, N. Detection of lead (II) with a “turn-on” fluorescent biosensor based on energy transfer from CdSe/ZnS quantum dots to graphene oxide. Biosens. Bioelectron. 2013, 43, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Zhu, C.; Huang, Y.; Yan, J.; Chen, A. Ultrasensitive detection of lead(II) using a turn-on probe based on the use of an aptamer and a water-soluble fluorescent perylene probe. Microchim. Acta 2017, 184, 2439–2444. [Google Scholar] [CrossRef]
- Niu, X.; Zhong, Y.; Chen, R.; Wang, F.; Liu, Y.; Luo, D. A “turn-on” fluorescence sensor for Pb2+ detection based on graphene quantum dots and gold nanoparticles. Sens. Actuators B Chem. 2018, 255, 1577–1581. [Google Scholar] [CrossRef]
- Frasco, M.F.; Chaniotakis, N. Semiconductor quantum dots in chemical sensors and biosensors. Sensors 2009, 9, 7266–7286. [Google Scholar] [CrossRef]
- Bera, D.; Qian, L.; Tseng, T.-K.; Holloway, P.H. Quantum dots and their multimodal applications: A review. Materials (Basel) 2010, 3, 2260–2345. [Google Scholar] [CrossRef]
- Hardman, R. A toxicologic review of quantum dots: Toxicity depends on physicochemical and environmental factors. Environ. Health Perspect. 2006, 114, 165–172. [Google Scholar] [CrossRef]
- Renugopalakrishnan, V.; Barbiellini, B.; King, C.; Molinari, M.; Mochalov, K.; Sukhanova, A.; Nabiev, I.; Fojan, P.; Tuller, H.L.; Chin, M.; et al. Engineering a robust photovoltaic device with quantum dots and bacteriorhodopsin. J. Phys. Chem. C 2014, 118, 16710–16717. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.; Jun, S.; Jang, H.; Lim, J.; Kim, B.; Kim, Y. White-light-emitting diodes with quantum dot color converters for display backlights. Adv. Mater. 2010, 22, 3076–3080. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Su, H.; Wang, K.; Wong, W.-K.; Zhu, X. Facile synthesis of N-rich carbon quantum dots from porphyrins as efficient probes for bioimaging and biosensing in living cells. Int. J. Nanomed. 2017, 12, 7375–7391. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Chen, F.; Chen, C.; Chen, X.; Gong, H.; Cai, C. A novel CdTe quantum dots probe amplified resonance light scattering signals to detect microRNA-122. Talanta 2017, 165, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Carrión, C.; Simonet, B.M.; Valcárcel, M. Colistin-functionalised CdSe/ZnS quantum dots as fluorescent probe for the rapid detection of Escherichia coli. Biosens. Bioelectron. 2011, 26, 4368–4374. [Google Scholar] [CrossRef] [PubMed]
- Sidorov, A.I.; Lebedev, V.F.; Kobranova, A.A.; Nashchekin, A.V. Formation of carbon quantum dots and nanodiamonds in laser ablation of a carbon film. Quantum Electron. 2018, 48, 45–48. [Google Scholar] [CrossRef]
- Kelarakis, A. Graphene quantum dots: In the crossroad of graphene, quantum dots and carbogenic nanoparticles. Curr. Opin. Colloid Interface Sci. 2015, 20, 354–361. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, L.; Zhang, S.; Yang, Y.; Chen, X.; Zhang, M. Fluorescent carbon nanoparticles for the fluorescent detection of metal ions. Biosens. Bioelectron. 2015, 63, 61–71. [Google Scholar] [CrossRef]
- Cai, J.; Sun, B.; Gou, X.; Gou, Y.; Li, W.; Hu, F. A novel way for analysis of calycosin via polyaniline functionalized graphene quantum dots fabricated electrochemical sensor. J. Electroanal. Chem. 2018, 816, 123–131. [Google Scholar] [CrossRef]
- Constantine, C.A.; Gattás-Asfura, K.M.; Mello, S.V.; Crespo, G.; Rastogi, V.; Cheng, T.-C.; DeFrank, J.J.; Leblanc, R.M. Layer-by-layer biosensor assembly incorporating functionalized quantum dots. Langmuir 2003, 19, 9863–9867. [Google Scholar] [CrossRef]
- Lin, X.; Gao, G.; Zheng, L.; Chi, Y.; Chen, G. Encapsulation of strongly fluorescent carbon quantum dots in metal-organic frameworks for enhancing chemical sensing. Anal. Chem. 2014, 86, 1223–1228. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Qu, L.; Yang, R.; Zhou, Y.; Li, J. A highly selective and simple fluorescent sensor for mercury (II) ion detection based on cysteamine-capped CdTe quantum dots synthesized by the reflux method. Luminescence 2015, 30, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Guo, J.; Yang, C. Ratiometric fluorescence sensor for Fe3+ ions detection based on quantum dot-doped hydrogel optical fiber. Sens. Actuators B Chem. 2018, 264, 52–58. [Google Scholar] [CrossRef]
- Labeb, M.; Sakr, A.-H.; Soliman, M.; Abdel-Fettah, T.M.; Ebrahim, S. Effect of capping agent on selectivity and sensitivity of CdTe quantum dots optical sensor for detection of mercury ions. Opt. Mater. (Amst.) 2018, 79, 331–335. [Google Scholar] [CrossRef]
- Gong, T.; Liu, J.; Liu, X.; Liu, J.; Xiang, J.; Wu, Y. A sensitive and selective sensing platform based on CdTe QDs in the presence of L-cysteine for detection of silver, mercury and copper ions in water and various drinks. Food Chem. 2016, 213, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Hao, J.; Yi, T.; Xu, Y.; Niu, X.; Ren, C.; Chen, H.; Chen, X. Probing the mechanism of the interaction between l-cysteine-capped-CdTe quantum dots and Hg2+ using capillary electrophoresis with ensemble techniques. Electrophoresis 2015, 36, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Cai, Y.-Q.; Liu, H.-J.; Chen, Y. CdS quantum dots capped with hyperbranched graft copolymers: Role of hyperbranched shell in fluorescence and selective mercury-sensing. Sens. Actuators B Chem. 2017, 251, 171–179. [Google Scholar] [CrossRef]
- Chen, G.-F.; Tsai, H.-P.; Lai, P.-S.; Liao, M.-Y. Functionalized Mn2+-doped zinc sulfide quantum dots as a metal ion sensor for industrial wastes. Sens. Mater. 2013, 25, 437–442. [Google Scholar] [CrossRef]
- Geng, S.; Lin, S.M.; Li, N.B.; Luo, H.Q. Polyethylene glycol capped ZnO quantum dots as a fluorescent probe for determining copper(II) ion. Sens. Actuators B Chem. 2017, 253, 137–143. [Google Scholar] [CrossRef]
- Rezaei, B.; Shahshahanipour, M.; Ensafi, A.A.; Farrokhpour, H. Development of highly selective and sensitive fluorimetric label-free mercury aptasensor based on cysteamine@CdTe/ZnS quantum dots, experimental and theoretical investigation. Sens. Actuators B Chem. 2017, 247, 400–407. [Google Scholar] [CrossRef]
- Xi, L.-L.; Ma, H.-B.; Tao, G.-H. Thiourea functionalized CdSe/CdS quantum dots as a fluorescent sensor for mercury ion detection. Chin. Chem. Lett. 2016, 27, 1531–1536. [Google Scholar] [CrossRef]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef] [PubMed]
- Esteves da Silva, J.C.G.; Gonçalves, H.M.R. Analytical and bioanalytical applications of carbon dots. TrAC Trends Anal. Chem. 2011, 30, 1327–1336. [Google Scholar] [CrossRef]
- Baker, S.N.; Baker, G.A. Luminescent carbon nanodots: Emergent nanolights. Angew. Chem. Int. Ed. 2010, 49, 6726–6744. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Lei, Y. Fluorescent carbon dots and their sensing applications. TrAC Trends Anal. Chem. 2017, 89, 163–180. [Google Scholar] [CrossRef]
- Shi, Y.; Li, C.; Liu, S.; Liu, Z.; Zhu, J.; Yang, J.; Hu, X. Facile synthesis of fluorescent carbon dots for determination of curcumin based on fluorescence resonance energy transfer. RSC Adv. 2015, 5, 64790–64796. [Google Scholar] [CrossRef]
- Lin, L.; Luo, Y.; Tsai, P.; Wang, J.; Chen, X. Metal ions doped carbon quantum dots: Synthesis, physicochemical properties, and their applications. TrAC Trends Anal. Chem. 2018, 103, 87–101. [Google Scholar] [CrossRef]
- Wang, H.; Sun, P.; Cong, S.; Wu, J.; Gao, L.; Wang, Y.; Dai, X.; Yi, Q.; Zou, G. Nitrogen-doped carbon dots for “green” quantum dot solar cells. Nanoscale Res. Lett. 2016, 11, 27. [Google Scholar] [CrossRef]
- Barati, A.; Shamsipur, M.; Arkan, E.; Hosseinzadeh, L.; Abdollahi, H. Synthesis of biocompatible and highly photoluminescent nitrogen doped carbon dots from lime: Analytical applications and optimization using response surface methodology. Mater. Sci. Eng. C 2015, 47, 325–332. [Google Scholar] [CrossRef]
- Wang, C.; Hu, T.; Wen, Z.; Zhou, J.; Wang, X.; Wu, Q.; Wang, C. Concentration-dependent color tunability of nitrogen-doped carbon dots and their application for iron(III) detection and multicolor bioimaging. J. Colloid Interface Sci. 2018, 521, 33–41. [Google Scholar] [CrossRef]
- Xiao, N.; Liu, S.G.; Mo, S.; Li, N.; Ju, Y.J.; Ling, Y.; Li, N.B.; Luo, H.Q. Highly selective detection of p-nitrophenol using fluorescence assay based on boron, nitrogen co-doped carbon dots. Talanta 2018, 184, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, J.; Li, J.; Xu, L.; Qiao, Y. One-pot synthesis of nitrogen and sulfur co-doped carbon dots and its application for sensor and multicolor cellular imaging. J. Colloid Interface Sci. 2017, 485, 167–174. [Google Scholar] [CrossRef]
- Zhi, B.; Gallagher, M.J.; Frank, B.P.; Lyons, T.Y.; Qiu, T.A.; Da, J.; Mensch, A.C.; Hamers, R.J.; Rosenzweig, Z.; Fairbrother, D.H.; et al. Investigation of phosphorous doping effects on polymeric carbon dots: Fluorescence, photostability, and environmental impact. Carbon N. Y. 2018, 129, 438–449. [Google Scholar] [CrossRef]
- Liu, Y.; Liao, M.; He, X.; Liu, X.; Kou, X.; Xiao, D. One-step synthesis of highly luminescent nitrogen-doped carbon dots for selective and sensitive detection of mercury(ii) ions and cellular imaging. Anal. Sci. 2015, 31, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, Y.; Liang, M.; Xu, L.; Qi, S.; Chen, H.; Chen, X. Solid-phase synthesis of highly fluorescent nitrogen-doped carbon dots for sensitive and selective probing ferric ions in living cells. Anal. Chem. 2014, 86, 9846–9852. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, Y.; Cao, J.; Zhu, J.; Fan, L.; Li, X. Sulfur-doped graphene quantum dots as a novel fluorescent probe for highly selective and sensitive detection of Fe3+. Anal. Chem. 2014, 86, 10201–10207. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Pu, P.; Zhao, J.; Dong, C.; Gao, C.; Chen, Y.; Chen, J.; Liu, Y.; Zhou, H. Preparation of highly photoluminescent sulfur-doped carbon dots for Fe(iii) detection. J. Mater. Chem. A 2015, 3, 542–546. [Google Scholar] [CrossRef]
- Wu, F.; Yang, M.; Zhang, H.; Zhu, S.; Zhu, X.; Wang, K. Facile synthesis of sulfur-doped carbon quantum dots from vitamin B1 for highly selective detection of Fe3+ ion. Opt. Mater. (Amst.) 2018, 77, 258–263. [Google Scholar] [CrossRef]
- Naik, V.M.; Gunjal, D.B.; Gore, A.H.; Pawar, S.P.; Mahanwar, S.T.; Anbhule, P.V.; Kolekar, G.B. Quick and low cost synthesis of sulphur doped carbon dots by simple acidic carbonization of sucrose for the detection of Fe3+ ions in highly acidic environment. Diam. Relat. Mater. 2018, 88, 262–268. [Google Scholar] [CrossRef]
- Yang, G.; Wan, X.; Su, Y.; Zeng, X.; Tang, J. Acidophilic S-doped carbon quantum dots derived from cellulose fibers and their fluorescence sensing performance for metal ions in an extremely strong acid environment. J. Mater. Chem. A 2016, 4, 12841–12849. [Google Scholar] [CrossRef]
- Wang, Y.; Kim, S.-H.; Feng, L. Highly luminescent N, S- Co-doped carbon dots and their direct use as mercury(II) sensor. Anal. Chim. Acta 2015, 890, 134–142. [Google Scholar] [CrossRef]
- Sun, H.; Wu, L.; Wei, W.; Qu, X. Recent advances in graphene quantum dots for sensing. Mater. Today 2013, 16, 433–442. [Google Scholar] [CrossRef]
- Shi, B.; Zhang, L.; Lan, C.; Zhao, J.; Su, Y.; Zhao, S. One-pot green synthesis of oxygen-rich nitrogen-doped graphene quantum dots and their potential application in pH-sensitive photoluminescence and detection of mercury(II) ions. Talanta 2015, 142, 131–139. [Google Scholar] [CrossRef]
- Bian, S.; Shen, C.; Hua, H.; Zhou, L.; Zhu, H.; Xi, F.; Liu, J.; Dong, X. One-pot synthesis of sulfur-doped graphene quantum dots as a novel fluorescent probe for highly selective and sensitive detection of lead(II). RSC Adv. 2016, 6, 69977–69983. [Google Scholar] [CrossRef]
- Anh, N.T.N.; Chowdhury, A.D.; Doong, R. Highly sensitive and selective detection of mercury ions using N, S-codoped graphene quantum dots and its paper strip based sensing application in wastewater. Sens. Actuators B Chem. 2017, 252, 1169–1178. [Google Scholar] [CrossRef]
- Zhu, J.; Chang, H.; Li, J.-J.; Li, X.; Zhao, J.-W. Using silicon-coated gold nanoparticles to enhance the fluorescence of CdTe quantum dot and improve the sensing ability of mercury (II). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 188, 170–178. [Google Scholar] [CrossRef]
- Wan, X.; Li, S.; Zhuang, L.; Tang, J. l-Tryptophan-capped carbon quantum dots for the sensitive and selective fluorescence detection of mercury ion in aqueous solution. J. Nanoparticle Res. 2016, 18, 202. [Google Scholar] [CrossRef]
- Ke, J.; Li, X.; Zhao, Q.; Hou, Y.; Chen, J. Ultrasensitive quantum dot fluorescence quenching assay for selective detection of mercury ions in drinking water. Sci. Rep. 2014, 4, 5624. [Google Scholar] [CrossRef]
- Yang, R.; Ding, X.; Zhou, Y.; Li, J.; Qu, L.; Zhang, K. A novel fluorescent sensor for mercury (ii) ion using self-assembly of poly(diallyl dimethyl ammonium)chloride functionalized CdTe quantum dots. Anal. Methods 2015, 7, 436–442. [Google Scholar] [CrossRef]
- Hua, M.; Wang, C.; Qian, J.; Wang, K.; Yang, Z.; Liu, Q.; Mao, H.; Wang, K. Preparation of graphene quantum dots based core-satellite hybrid spheres and their use as the ratiometric fluorescence probe for visual determination of mercury(II) ions. Anal. Chim. Acta 2015, 888, 173–181. [Google Scholar] [CrossRef]
- Fu, H.; Ji, Z.; Chen, X.; Cheng, A.; Liu, S.; Gong, P.; Li, G.; Chen, G.; Sun, Z.; Zhao, X.; et al. A versatile ratiometric nanosensing approach for sensitive and accurate detection of Hg2+ and biological thiols based on new fluorescent carbon quantum dots. Anal. Bioanal. Chem. 2017, 409, 2373–2382. [Google Scholar] [CrossRef]
- Sun, X.; Yang, S.; Guo, M.; Ma, S.; Zheng, M.; He, J. Reversible fluorescence probe based on N-doped carbon dots for the determination of mercury ion and glutathione in waters and living cells. Anal. Sci. 2017, 33, 761–767. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, W. Nitrogen-doped carbon quantum dots: Facile synthesis and application as a “turn-off” fluorescent probe for detection of Hg2+ ions. Biosens. Bioelectron. 2013, 55, 83–90. [Google Scholar] [CrossRef]
- Li, L.-L.; Ni, G.; Wang, J.-N.; Li, J.; Li, W. Synthesis of nitrogen-doped carbon quantum dots and its application as fluorescent sensor for Hg2+. Guang Pu Xue Yu Guang Pu Fen Xi/Spectrosc. Spectr. Anal. 2016, 36, 2846–2851. [Google Scholar]
- Patir, K.; Gogoi, S.K. Facile Synthesis of Photoluminescent Graphitic Carbon Nitride Quantum Dots for Hg2+ Detection and Room Temperature Phosphorescence. ACS Sustain. Chem. Eng. 2018, 6, 1732–1743. [Google Scholar] [CrossRef]
- Wang, B.; Zhuo, S.; Chen, L.; Zhang, Y. Fluorescent graphene quantum dot nanoprobes for the sensitive and selective detection of mercury ions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 131, 384–387. [Google Scholar] [CrossRef]
- Zhao, Q.; Rong, X.; Chen, L.; Ma, H.; Tao, G. Layer-by-layer self-assembly xylenol orange functionalized CdSe/CdS quantum dots as a turn-on fluorescence lead ion sensor. Talanta 2013, 114, 110–116. [Google Scholar] [CrossRef]
- Zhu, H.; Yu, T.; Xu, H.; Zhang, K.; Jiang, H.; Zhang, Z.; Wang, Z.; Wang, S. Fluorescent nanohybrid of gold nanoclusters and quantum dots for visual determination of lead ions. ACS Appl. Mater. Interfaces 2014, 6, 21461–21467. [Google Scholar] [CrossRef]
- Qu, H.; Cao, L.; Su, G.; Liu, W.; Gao, R.; Xia, C.; Qin, J. Silica-coated ZnS quantum dots as fluorescent probes for the sensitive detection of Pb2+ ions. J. Nanoparticle Res. 2014, 16. [Google Scholar] [CrossRef]
- Xu, J.; Jie, X.; Xie, F.; Yang, H.; Wei, W.; Xia, Z. Flavonoid moiety-incorporated carbon dots for ultrasensitive and highly selective fluorescence detection and removal of Pb2+. Nano Res. 2018, 11, 3648–3657. [Google Scholar] [CrossRef]
- Xu, S.; Xu, S.; Zhu, Y.; Xu, W.; Zhou, P.; Zhou, C.; Dong, B.; Song, H. A novel upconversion, fluorescence resonance energy transfer biosensor (FRET) for sensitive detection of lead ions in human serum. Nanoscale 2014, 6, 12573–12579. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, X.; Zhan, G.; Li, C. Fluorescent sensor for selective determination of copper ion based on N-acetyl-l-cysteine capped CdHgSe quantum dots. Biosens. Bioelectron. 2014, 54, 311–316. [Google Scholar] [CrossRef]
- Elmizadeh, H.; Soleimani, M.; Faridbod, F.; Bardajee, G.R. Ligand-Capped CdTe Quantum Dots as a Fluorescent Nanosensor for Detection of Copper Ions in Environmental Water Sample. J. Fluoresc. 2017, 27, 2323–2333. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, X.; Zhu, T.; Deng, M.; Ikechukwu, I.P.; Huang, W.; Yin, G.; Bai, Y.; Qu, D.; Huang, X.; et al. All-inorganic CsPbBr3 perovskite quantum dots as a photoluminescent probe for ultrasensitive Cu2+ detection. J. Mater. Chem. C 2018, 6, 4793–4799. [Google Scholar] [CrossRef]
- Qin, J.; Dong, B.; Gao, R.; Su, G.; Han, J.; Li, X.; Liu, W.; Wang, W.; Cao, L. Water-soluble silica-coated ZnS: Mn nanoparticles as fluorescent sensors for the detection of ultratrace copper(II) ions in seawater. Anal. Methods 2017, 9, 322–328. [Google Scholar] [CrossRef]
- Xie, Z.; Sun, X.; Jiao, J.; Xin, X. Ionic liquid-functionalized carbon quantum dots as fluorescent probes for sensitive and selective detection of iron ion and ascorbic acid. Colloids Surfaces A Physicochem. Eng. Asp. 2017, 529, 38–44. [Google Scholar] [CrossRef]
- Liu, Y.; Duan, W.; Song, W.; Liu, J.; Ren, C.; Wu, J.; Liu, D.; Chen, H. Red Emission B, N, S-co-Doped Carbon Dots for Colorimetric and Fluorescent Dual Mode Detection of Fe3+ Ions in Complex Biological Fluids and Living Cells. ACS Appl. Mater. Interfaces 2017, 9, 12663–12672. [Google Scholar] [CrossRef]
- Satnami, M.L.; Vaishanav, S.K.; Nagwanshi, R.; Ghosh, K.K. Spectrofluorometric Determination of Mercury and Lead by Colloidal CdS Nanomaterial. J. Dispers. Sci. Technol. 2016, 37, 196–204. [Google Scholar] [CrossRef]
- Elmizadeh, H.; Soleimani, M.; Faridbod, F.; Bardajee, G.R. A sensitive nano-sensor based on synthetic ligand-coated CdTe quantum dots for rapid detection of Cr(III) ions in water and wastewater samples. Colloid Polym. Sci. 2018, 296, 1581–1590. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, C.; Wang, X.; Wang, L.; Chen, A.; Hu, J.; Luo, Z. Fabrication of an “ion-imprinting” dual-emission quantum dot nanohybrid for selective fluorescence turn-on and ratiometric detection of cadmium ions. Analyst 2016, 141, 5886–5892. [Google Scholar] [CrossRef]
- Liu, M.; Hu, M.; Jiang, Q.; Lu, Z.; Huang, Y.; Tan, Y.; Jiang, Q. A novel coumarin derivative as a sensitive probe for tracing intracellular pH changes. RSC Adv. 2015, 5, 15778–15783. [Google Scholar] [CrossRef]
- Tan, J.-L.; Zhang, M.-X.; Zhang, F.; Yang, T.-T.; Liu, Y.; Li, Z.-B.; Zuo, H. A novel “off-on” colorimetric and fluorescent rhodamine-based pH chemosensor for extreme acidity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 140, 489–494. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, W.; Mu, L.; Zeng, X.; Xue, S.; Tao, Z.; Yamatob, T. A novel rhodamine-based thiacalix[4]arene fluorescent sensor for Fe3+ and Cr3+. J. Incl. Phenom. Macrocycl. Chem. 2010, 68, 139–146. [Google Scholar] [CrossRef]
- Chen, H.; Huang, H.; Huang, X.; Clifford, J.N.; Forneli, A.; Palomares, E.; Zheng, X.; Zheng, L.; Wang, X.; Shen, P.; et al. High molar extinction coefficient branchlike organic dyes containing Di(p-tolyl)phenylamine donor for dye-sensitized solar cells applications. J. Phys. Chem. C 2010, 114, 3280–3286. [Google Scholar] [CrossRef]
- Sun, W.-C.; Gee, K.R.; Klaubert, D.H.; Haugland, R.P. Synthesis of fluorinated fluoresceins. J. Org. Chem. 1997, 62, 6469–6475. [Google Scholar] [CrossRef]
- Zheng, H.; Zhan, X.-Q.; Bian, Q.-N.; Zhang, X.-J. Advances in modifying fluorescein and rhodamine fluorophores as fluorescent chemosensors. Chem. Commun. 2013, 49, 429–447. [Google Scholar] [CrossRef]
- Chang, P.V.; Bertozzi, C.R. Imaging beyond the proteome. Chem. Commun. 2012, 48, 8864–8879. [Google Scholar] [CrossRef]
- Wanichacheva, N. Design and Synthesis of Ionophores and Fluoroionophores for the Detection of Lithium and Ammoniums Ions; Worcester Polytechnic Institute: Worcester, MA, USA, 2006. [Google Scholar]
- Kaur, M.; Choi, D.H. Diketopyrrolopyrrole: Brilliant red pigment dye-based fluorescent probes and their applications. Chem. Soc. Rev. 2015, 44, 58–77. [Google Scholar] [CrossRef]
- Valeur, B.; Leray, I. Design principles of fluorescent molecular sensors for cation recognition. Coord. Chem. Rev. 2000, 205, 3–40. [Google Scholar] [CrossRef]
- Doludda, M.; Kastenholz, F.; Lewitzki, E.; Grell, E. Time-resolved response of fluorescent alkali ion indicators and detection of short-lived intermediates upon binding to molecular cavities. J. Fluoresc. 1996, 6, 159–163. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Wren, S.P.; Sun, T.; Grattan, K.T.V. Development of a fiber-optic chemical sensor for the detection of cadmium. In Proceedings of the IEEE Sensors, Glasgow, Scotland, 29 October–1 November 2017. [Google Scholar]
- Lee, H.Y.; Swamy, K.M.K.; Jung, J.Y.; Kim, G.; Yoon, J. Rhodamine hydrazone derivatives based selective fluorescent and colorimetric chemodosimeters for Hg2+ and selective colorimetric chemosensor for Cu2+. Sens. Actuators B Chem. 2013, 182, 530–537. [Google Scholar] [CrossRef]
- Niu, Y.; Qian, Y. Synthesis and aggregation-induced emission enhancement of naphthalimide-rhodamine dye. J. Photochem. Photobiol. A Chem. 2016, 329, 88–95. [Google Scholar] [CrossRef]
- Biswal, B.; Mallick, D.; Thirunavoukkarasu, M.; Mohanty, R.; Bag, B. A pyridine and pyrrole coupled rhodamine derivative for Co(II) ion detection and its imaging application in plant tissues. Sens. Actuators B Chem. 2016, 232, 410–419. [Google Scholar] [CrossRef]
- Chen, X.; Ma, H. A selective fluorescence-on reaction of spiro form fluorescein hydrazide with Cu(II). Anal. Chim. Acta 2006, 575, 217–222. [Google Scholar] [CrossRef]
- Du, X.-L.; Zhang, H.-S.; Guo, X.-F.; Deng, Y.-H.; Wang, H. 6-Oxy-(acetyl piperazine) fluorescein as a new fluorescent labeling reagent for free fatty acids in serum using high-performance liquid chromatography. J. Chromatogr. A 2007, 1169, 77–85. [Google Scholar] [CrossRef]
- Wu, W.-N.; Wu, H.; Wang, Y.; Mao, X.-J.; Zhao, X.-L.; Xu, Z.-Q.; Fan, Y.-C.; Xu, Z.-H. A highly sensitive and selective off–on fluorescent chemosensor for hydrazine based on coumarin β-diketone. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 188, 80–84. [Google Scholar] [CrossRef]
- Karaoglu, K.; Yilmaz, F.; Menteşe, E. A New Fluorescent “Turn-Off” Coumarin-Based Chemosensor: Synthesis, Structure and Cu-Selective Fluorescent Sensing in Water Samples. J. Fluoresc. 2017, 27, 1293–1298. [Google Scholar] [CrossRef]
- Li, M.; Sun, Y.; Dong, L.; Feng, Q.-C.; Xu, H.; Zang, S.-Q.; Mak, T.C.W. Colorimetric recognition of Cu2+ and fluorescent detection of Hg2+ in aqueous media by a dual chemosensor derived from rhodamine B dye with a NS2 receptor. Sens. Actuators B Chem. 2016, 226, 332–341. [Google Scholar] [CrossRef]
- Haugland, R.P. The Handbook: A Guide to Fluorescent Probes and Labeling Technologies, 10th ed.; Invitrogen Corp.: Carlsbad, CA, USA, 2005. [Google Scholar]
- Ruan, S.; Ebendorff-Heidepriem, H.; Ruan, Y. Optical fibre turn-on sensor for the detection of mercury based on immobilized fluorophore. Measurement 2018, 121, 122–126. [Google Scholar] [CrossRef]
- Li, M.; Jiang, X.-J.; Wu, H.-H.; Lu, H.-L.; Li, H.-Y.; Xu, H.; Zang, S.-Q.; Mak, T.C.W. A dual functional probe for “turn-on” fluorescence response of Pb2+ and colorimetric detection of Cu2+ based on a rhodamine derivative in aqueous media. Dalton Trans. 2015, 44, 17326–17334. [Google Scholar] [CrossRef]
- Su, W.; Yuan, S.; Wang, E. A Rhodamine-Based Fluorescent Chemosensor for the Detection of Pb2+, Hg2+ and Cd2+. J. Fluoresc. 2017, 27, 1871–1875. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Zhang, K.; Li, C.; Li, Y.; Niu, S. A novel fluorescent chemosensor based on a rhodamine 6G derivative for the detection of Pb2+ ion. Sens. Actuators B Chem. 2017, 246, 696–702. [Google Scholar] [CrossRef]
- Liu, C.; Huang, S.; Yao, H.; He, S.; Lu, Y.; Zhao, L.; Zeng, X. Preparation of fluorescein-based chemosensors and their sensing behaviors toward silver ions. RSC Adv. 2014, 4, 16109–16114. [Google Scholar] [CrossRef]
- Wu, G.; Li, M.; Zhu, J.; Lai, K.W.C.; Tong, Q.; Lu, F. A highly sensitive and selective turn-on fluorescent probe for Pb(II) ions based on a coumarin-quinoline platform. RSC Adv. 2016, 6, 100696–100699. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhang, L.; Zhou, P. A rhodamine B-based fluorescent sensor toward highly selective mercury (II) ions detection. Talanta 2016, 150, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fang, Z. A novel “turn on” glucose-based rhodamine B fluorescent chemosensor for mercury ions recognition in aqueous solution. Spectrosc. Lett. 2015, 48, 578–585. [Google Scholar] [CrossRef]
- Gao, T.; Lee, K.M.; Kim, S.H.; Heo, J.; Yang, S.I. A Mercuric Ion Selective Fluorescent Sensor Based on Rhodamine B with an Ethylene Unit. Bull. Korean Chem. Soc. 2017, 38, 292–295. [Google Scholar] [CrossRef]
- Long, Y.; Yang, M.-P.; Yang, B.-Q. Development and applications of two colorimetric and fluorescent indicators for Hg2+ detection. J. Inorg. Biochem. 2017, 172, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-M.; Zhao, R.-R.; Wei, Y.-L.; Yang, D.; Zhou, Z.-J.; Zhang, J.-F.; Zhou, Y. A rhodamine derivative for Hg2+-selective colorimetric and fluorescent sensing and its application to in vivo imaging. Chin. Chem. Lett. 2016, 27, 813–816. [Google Scholar] [CrossRef]
- Yan, F.; Cao, D.; Wang, M.; Yang, N.; Yu, Q.; Dai, L.; Chen, L. A new rhodamine-based “off-on” fluorescent chemosensor for Hg (II) ion and its application in imaging Hg (II) in living cells. J. Fluoresc. 2012, 22, 1249–1256. [Google Scholar] [CrossRef]
- Yan, F.; Wang, M.; Cao, D.; Yang, N.; Fu, Y.; Chen, L.; Chen, L. New fluorescent and colorimetric chemosensors based on the rhodamine detection of Hg2+ and Al3+ and application of imaging in living cells. Dye Pigment. 2013, 98, 42–50. [Google Scholar] [CrossRef]
- Bera, K.; Das, A.K.; Nag, M.; Basak, S. Development of a rhodamine-rhodanine-based fluorescent mercury sensor and its use to monitor real-time uptake and distribution of inorganic mercury in live zebrafish larvae. Anal. Chem. 2014, 86, 2740–2746. [Google Scholar] [CrossRef] [PubMed]
- Wanichacheva, N.; Hanmeng, O.; Kraithong, S.; Sukrat, K. Dual optical Hg2+-selective sensing through FRET system of fluorescein and rhodamine B fluorophores. J. Photochem. Photobiol. A Chem. 2014, 278, 75–81. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Wren, S.P.; Sun, T.; Grattan, K.T.V. Fluorescent optical fibre chemosensor for the detection of mercury. In Proceedings of the SPIE—The International Society for Optical Engineering, Baltimore, MD, USA, 18–19 April 2016; Volume 10013. [Google Scholar]
- Huang, K.; Jiao, X.; Liu, C.; Wang, Q.; Qiu, X.; Zheng, D.; He, S.; Zhao, L.; Zeng, X. Highly selective and sensitive fluorescent probe for mercury ions based on a novel rhodol-coumarin hybrid dye. Dye Pigment. 2017, 142, 437–446. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, Z.; Xiao, Y.; Zhang, T.-T.; Miao, J.-Y.; Zhao, B.-X. A new fluorescent turn-on chemodosimeter for mercury ions in solution and its application in cells and organisms. Anal. Chim. Acta 2014, 807, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Ncube, P.; Krause, R.W.M.; Ndinteh, D.T.; Mamba, B.B. Fluorescent sensing and determination of mercury (II) ions in water. Water SA 2014, 40, 175–182. [Google Scholar] [CrossRef]
- Han, Y.; Yang, C.; Wu, K.; Chen, Y.; Zhou, B.; Xia, M. A facile naphthalene-based fluorescent chemodosimeter for mercury ions in aqueous solution. RSC Adv. 2015, 5, 16723–16726. [Google Scholar] [CrossRef]
- Aliberti, A.; Vaiano, P.; Caporale, A.; Consales, M.; Ruvo, M.; Cusano, A. Fluorescent chemosensors for Hg2+ detection in aqueous environment. Sens. Actuators B Chem. 2017, 247, 727–735. [Google Scholar] [CrossRef]
- Sunnapu, O.; Kotla, N.G.; Maddiboyina, B.; Singaravadivel, S.; Sivaraman, G. A rhodamine based “turn-on” fluorescent probe for Pb(II) and live cell imaging. RSC Adv. 2015, 6, 656–660. [Google Scholar] [CrossRef]
- Shaily; Kumar, A.; Parveen, I.; Ahmed, N. Highly selective and sensitive coumarin–triazole-based fluorometric ‘turn-off’ sensor for detection of Pb2+ ions. Luminescence 2018, 33, 713–721. [Google Scholar] [CrossRef]
- Liu, J.; Wu, K.; Li, S.; Song, T.; Han, Y.; Li, X. A highly sensitive and selective fluorescent chemosensor for Pb2+ ions in an aqueous solution. Dalton Trans. 2013, 42, 3854–3859. [Google Scholar] [CrossRef] [PubMed]
- Karak, D.; Banerjee, A.; Lohar, S.; Sahana, A.; Mukhopadhyay, S.K.; Adhikari, S.S.; Das, D. Xanthone based Pb2+ selective turn on fluorescent probe for living cell staining. Anal. Methods 2013, 5, 169–172. [Google Scholar] [CrossRef]
- Sinha, S.; Rani Koner, R.; Kumar, S.; Mathew, J.; Roy, A.; Kanti Mukhopadhyay, S.; Nandi, C.K.; Ghosh, S. Structurally tuned benzo[h]chromene derivative as Pb2+ selective “turn-on” fluorescence sensor for living cell imaging. J. Lumin. 2013, 143, 355–360. [Google Scholar] [CrossRef]
- Saleem, M.; Lee, K.-H. Selective fluorescence detection of Cu2+ in aqueous solution and living cells. J. Lumin. 2014, 145, 843–848. [Google Scholar] [CrossRef]
- Karuk Elmas, Ş.N.; Ozen, F.; Koran, K.; Yilmaz, I.; Gorgulu, A.O.; Erdemir, S. Coumarin Based Highly Selective “off-on-off” Type Novel Fluorescent Sensor for Cu2+ and S2− in Aqueous Solution. J. Fluoresc. 2017, 27, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Cao, Q.; Wu, X.; Shu, H.; Zhou, B.; Geng, Y.; Zhu, J. Design and synthesis of a new selective fluorescent chemical sensor for Cu2+ based on a Pyrrole moiety and a Fluorescein conjugate. Tetrahedron Lett. 2016, 57, 942–948. [Google Scholar] [CrossRef]
- Yang, X.; Zeng, W.; Wang, L.; Lu, X.; Yan, Y.; Qu, J.; Liu, R. A new fluorescent probe based on styrylcyanine dye containing pyridine: Dissimilar fluorescent response to Cu2+ and Pb2+. RSC Adv. 2014, 4, 22613–22616. [Google Scholar] [CrossRef]
- An, J.-M.; Yan, M.-H.; Yang, Z.-Y.; Li, T.-R.; Zhou, Q.-X. A turn-on fluorescent sensor for Zn(II) based on fluorescein-coumarin conjugate. Dye Pigment 2013, 99, 1–5. [Google Scholar] [CrossRef]
- Ashwin, B.C.M.A.; Sivaraman, G.; Stalin, T.; Yuvakkumar, R.; Muthu Mareeswaran, P. Selective and sensitive fluorescent sensor for Pd2+ using coumarin 460 for real-time and biological applications. J. Photochem. Photobiol. B Biol. 2018, 183, 302–308. [Google Scholar] [CrossRef]
- Wu, D.; Huang, Y.; Hu, S.; Yi, X.; Wang, J. Sensitive Hg2+ Sensing via Quenching the Fluorescence of the Complex between Polythymine and 5,10,15,20-tetrakis(N-methyl-4-pyridyl) Porphyrin (TMPyP). Sensors (Basel) 2018, 18, 3998. [Google Scholar] [CrossRef]
- Huang, W.-B.; Gu, W.; Huang, H.-X.; Wang, J.-B.; Shen, W.-X.; Lv, Y.-Y.; Shen, J. A porphyrin-based fluorescent probe for optical detection of toxic Cd2+ ion in aqueous solution and living cells. Dye Pigment 2017, 143, 427–435. [Google Scholar] [CrossRef]
- Zhang, X.; Xia, T.; Jiang, K.; Cui, Y.; Yang, Y.; Qian, G. Highly sensitive and selective detection of mercury (II) based on a zirconium metal-organic framework in aqueous media. J. Solid State Chem. 2017, 253, 277–281. [Google Scholar] [CrossRef]
- Yang, C.-X.; Ren, H.-B.; Yan, X.-P. Fluorescent metal-organic framework MIL-53(Al) for highly selective and sensitive detection of Fe3+ in aqueous solution. Anal. Chem. 2013, 85, 7441–7446. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xia, T.; Zhang, X.; Zhang, Q.; Cui, Y.; Yang, Y.; Qian, G. A turn-on fluorescent probe for Cd2+ detection in aqueous environments based on an imine functionalized nanoscale metal-organic framework. RSC Adv. 2017, 7, 54892–54897. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).