Enhanced Auditory Steady-State Response Using an Optimized Chirp Stimulus-Evoked Paradigm
Abstract
:1. Introduction
1.1. Background
1.2. The Auditory Steady-State Responses (ASSRs)
1.3. The Chirp Stimulus
1.4. Literature Review
1.5. The Present Study
2. Materials and Methods
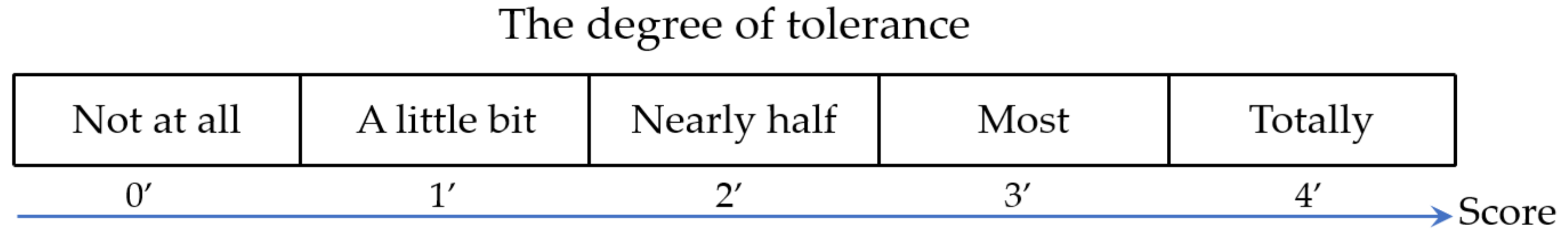
2.1. Subject
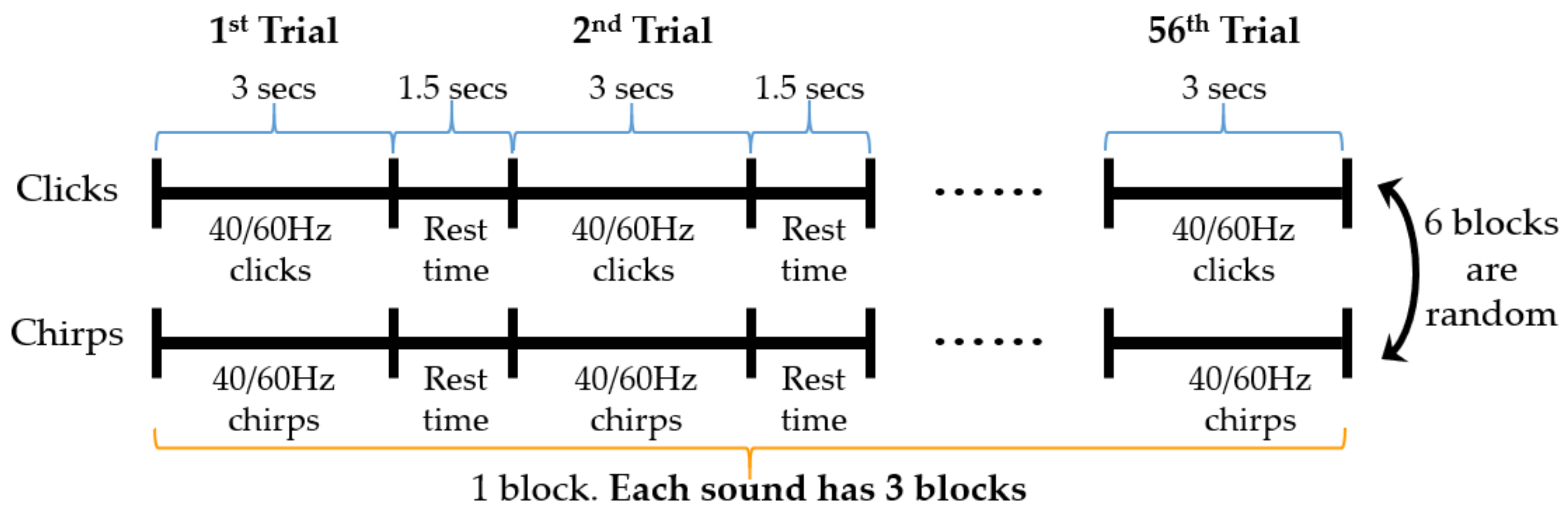
2.2. Stimuli
2.3. Design of the Evoked Paradigm
2.4. Data Acquisition and Processing
2.4.1. Data Acquisition
2.4.2. Data Processing
3. Results
3.1. Statistical Analyses
3.2. The ASSR Waves in Time Domain
3.3. Event-Related Spectral Perturbation (ERSP) Analysis
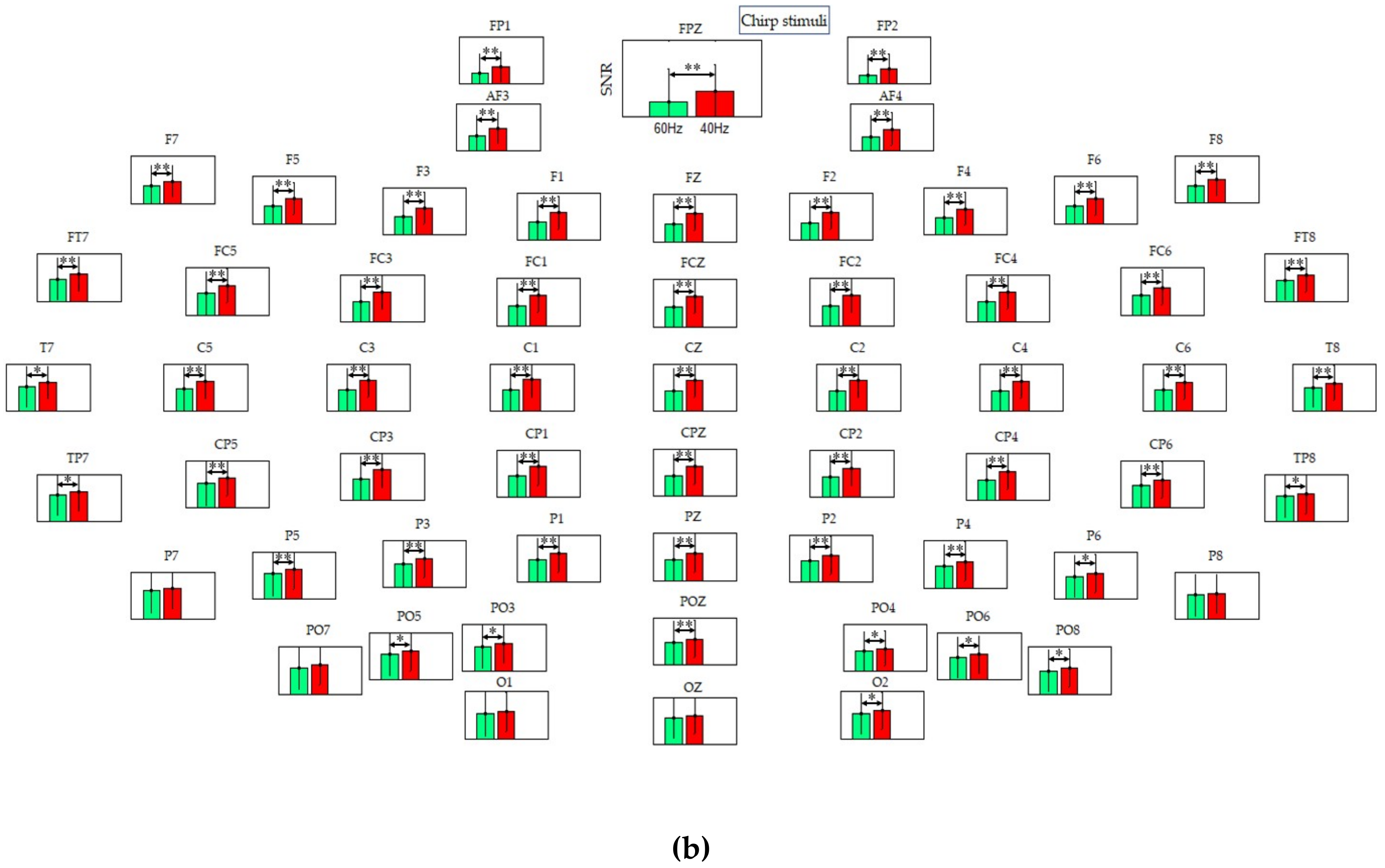
3.4. Signal-to-Noise Ratio (SNR) Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Muthukumaraswamy, S.D. High-frequency brain activity and muscle artifacts in MEG/EEG: A review and recommendations. Front. Hum. Neurosci. 2013, 7, 138. [Google Scholar] [PubMed]
- Fitzgerald, P.J.; Watson, B.O. Gamma oscillations as a biomarker for major depression: An emerging topic. Transl. Psychiatry 2018, 8, 177. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, C.S.; Demiralp, T. Human EEG gamma oscillations in neuropsychiatric disorders. Clin. Neurophysiol. 2005, 116, 2719–2733. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, R.; Genton, P. Epileptic syndromes and visually induced seizures. Epilepsia 2010, 45, 14–18. [Google Scholar] [CrossRef]
- Spencera, K.M.; Salisburya, D.F. Gamma-band auditory steady-state responses are impaired in first episode psychosis. Biol. Psychiatry 2008, 64, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Isomura, S.; Onitsuka, T.; Tsuchimoto, R.; Nakamura, I.; Hirano, S.; Oda, Y.; Oribe, N.; Hirano, Y.; Ueno, T.; Kanba, S. Differentiation between major depressive disorder and bipolar disorder by auditory steady-state responses. J. Affect. Disord. 2016, 190, 800. [Google Scholar] [CrossRef] [PubMed]
- Mcneer, R.; Bohorquez, J.O. Influence of auditory stimulation rates on evoked potentials during general anesthesia: Relation between the transient auditory middle-latency response and the 40-Hz auditory steady state response. Anesthesiology 2009, 110, 1026. [Google Scholar] [CrossRef] [PubMed]
- Rosburg, T.; Sörös, P. The response decrease of auditory evoked potentials by repeated stimulation—Is there evidence for an interplay between habituation and sensitization? Clin. Neurophysiol. 2016, 127, 397–408. [Google Scholar] [CrossRef]
- Tanaka, K.; Kuriki, S.; Nemoto, I.; Uchikawa, Y. Auditory Steady-State Responses in Magnetoencephalogram and Electroencephalogram: Phenomena, Mechanisms, and Applications. Adv. Biomed. Eng. 2013, 2, 55–62. [Google Scholar] [CrossRef]
- Onitsuka, T.; Oribe, N.; Nakamura, I.; Kanba, S. Review of neurophysiological findings in patients with schizophrenia. Psychiatry Clin. Neurosci. 2013, 67, 461–470. [Google Scholar] [CrossRef]
- Picton, T.W.; M Sasha, J.; Andrew, D.; David, P. Human auditory steady-state responses. Int. J. Audiol. 2003, 42, 177–219. [Google Scholar] [CrossRef]
- Tsuchimoto, R.; Kanba, S.; Hirano, S.; Oribe, N.; Ueno, T.; Hirano, Y.; Nakamura, I.; Oda, Y.; Miura, T.; Onitsuka, T. Reduced high and low frequency gamma synchronization in patients with chronic schizophrenia. Schizophr. Res. 2011, 133, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Yuko, O.; Toshiaki, O.; Rikako, T.; Shogo, H.; Naoya, O.; Takefumi, U.; Yoji, H.; Itta, N.; Tomofumi, M.; Shigenobu, K. Gamma band neural synchronization deficits for auditory steady state responses in bipolar disorder patients. PLoS One 2012, 7, e39955. [Google Scholar]
- Dau, T.; Wegner, O.; Mellert, V.; Kollmeier, B. Auditory brainstem responses with optimized chirp signals compensating basilar-membrane dispersion. J. Acoust. Soc. Am. 2000, 107, 1530–1540. [Google Scholar] [CrossRef] [PubMed]
- Rass, O.; Krishnan, G.; Brenner, C.A.; Hetrick, W.P.; Merrill, C.C.; Shekhar, A.; O’Donnell, B.F. Auditory steady state response in bipolar disorder: Relation to clinical state, cognitive performance, medication status, and substance disorders. Bipolar Disord. 2011, 12, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Alzaidi, A. Reduction noise in noncontact physical system. In Proceedings of the 2015 Long Island Systems, Applications and Technology, Farmingdale, NY, USA, 1 May 2015. [Google Scholar]
- Smith, S.B.; Lichtenhan, J.T.; Cone, B.K. Contralateral Inhibition of Click- and Chirp-Evoked Human Compound Action Potentials. Front. Neurosci. 2017, 11, 189. [Google Scholar] [CrossRef]
- Mourtzouchos, K.; Riga, M.; Cebulla, M.; Danielides, V.; Naxakis, S. Comparison of click Auditory Brainstem Response and chirp Auditory Steady-State Response Thresholds in Children. Int. J. Pediatr Otorhinolaryngol. 2018, 112, 91–96. [Google Scholar] [CrossRef]
- Eriko, A.; Minoru, T.; Noriko, N.; Seiji, N. Accuracy of synchrony judgment between two pulses: Effects of variations in cochlear delay amount. J. Acoust. Soc. Am. 2013, 133, 3428. [Google Scholar]
- Artieda, J.; Valencia, M.; Alegre, M.; Olaziregi, O.; Urrestarazu, E.; Iriarte, J. Potentials evoked by chirp-modulated tones: A new technique to evaluate oscillatory activity in the auditory pathway. Clin. Neurophysiol. 2004, 115, 699–709. [Google Scholar] [CrossRef]
- Elberling, C.; Don, M. Auditory brainstem responses to a chirp stimulus designed from derived-band latencies in normal-hearing subjects. J. Acoust. Soc. Am. 2008, 124, 3022. [Google Scholar] [CrossRef]
- Claus, E.; Manuel, D.; Mario, C.; Ekkehard, S. Auditory steady-state responses to chirp stimuli based on cochlear traveling wave delay. J. Acoust. Soc. Am. 2007, 122, 2772. [Google Scholar]
- Xu, Z.M.; Cheng, W.X.; Yao, Z.H. Prediction of frequency-specific hearing threshold using chirp auditory brainstem response in infants with hearing losses. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 812–816. [Google Scholar] [CrossRef] [PubMed]
- Muehler, R.; Verhey, J.L.; Rahne, T. Auditory brainstem responses to broad-band chirps: Amplitude growth; functions in sedated and anaesthetised infants. Int. J. Pediatr. Otorhinolaryngol. 2013, 77, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Petoe, M.A.; Bradley, A.P.; Wilson, W.J. On chirp stimuli and neural synchrony in the suprathreshold auditory brainstem response. J. Acoust. Soc. Am. 2010, 128, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Kuriki, S.; Kobayashi, Y.; Kobayashi, T.; Tanaka, K.; Uchikawa, Y. Steady-state MEG responses elicited by a sequence of amplitude-modulated; short tones of different carrier frequencies. Hear. Res. 2013, 296, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Thuné, H.; Recasens, M.; Uhlhaas, P.J. The 40-Hz Auditory Steady-State Response in Patients With Schizophrenia: A Meta-analysis. Jama Psychiatry 2016, 73, 1145–1153. [Google Scholar] [CrossRef]
- Griskova-Bulanova, I.; Dapsys, K.; Melynyte, S.; Voicikas, A.; Maciulis, V.; Andruskevicius, S.; Korostenskaja, M. 40Hz auditory steady-state response in schizophrenia: Sensitivity to stimulation type (clicks versus flutter amplitude-modulated tones). Neurosci. Lett. 2017, 662, 152–157. [Google Scholar] [CrossRef]
- Junius, D.; Dau, T. Influence of cochlear traveling wave and neural adaptation on auditory brainstem responses. Hear. Res. 2005, 205, 53–67. [Google Scholar] [CrossRef]
- Melynyte, S.; Pipinis, E.; Genyte, V.; Voicikas, A.; Rihs, T.; Griskovabulanova, I. 40 Hz Auditory Steady-State Response: The Impact of Handedness and Gender. Brain Topogr. 2017, 31, 1–11. [Google Scholar] [CrossRef]
- Fu, L.; Xiang, D.; Subodh, D.; Xiao, J.; Yao, L.; Wang, Y.; Wang, H.; Wang, G.; Liu, Z. Auditory P300 study in patients with convalescent bipolar depression and bipolar depression. Neuroreport 2018, 29, 968. [Google Scholar] [CrossRef]
- Oliver, F.; Torsten, D. Searching for the optimal stimulus eliciting auditory brainstem responses in humans. J. Acoust. Soc. Am. 2004, 116, 2213–2222. [Google Scholar]
- Keefe, D.H.; Feeney, M.P.; Hunter, L.L.; Fitzpatrick, D.F. Comparisons of transient evoked otoacoustic emissions using chirp and click stimuli. J. Acoust. Soc. Am. 2016, 140, 1949. [Google Scholar] [CrossRef] [PubMed]
- Goodman, S.S.; Withnell, R.H.; Boer, E.D.; Lilly, D.J.; Nuttall, A.L. Cochlear delays measured with amplitude-modulated tone-burst-evoked OAEs. Hear. Res. 2004, 188, 57–69. [Google Scholar] [CrossRef]
- Elberling, C.; Don, M. A direct approach for the design of chirp stimuli used for the recording of auditory brainstem responses. J. Acoust. Soc. Am. 2010, 128, 2955–2964. [Google Scholar] [CrossRef] [PubMed]
- Grandchamp, R.; Delorme, A. Single-Trial Normalization for Event-Related Spectral Decomposition Reduces Sensitivity to Noisy Trials. Front. Psychol. 2011, 2, 236. [Google Scholar] [CrossRef]
- Lee, M.; Sehatpour, P.; Hoptman, M.J.; Lakatos, P.; Dias, E.C.; Kantrowitz, J.T.; Martinez, A.M.; Javitt, D.C. Neural mechanisms of mismatch negativity dysfunction in schizophrenia. Int. J. Psychophysiol. 2016, 108, 1585–1593. [Google Scholar] [CrossRef]
- Onton, J.; Makeig, S. Information-based modeling of event-related brain dynamics. Prog. Brain Res. 2006, 159, 99–120. [Google Scholar]
- Allen, D.P.; Mackinnon, C.D. Time–frequency analysis of movement-related spectral power in EEG during repetitive movements: A comparison of methods. J. Neurosci. Methods 2010, 186, 107–115. [Google Scholar] [CrossRef]
- Wu, W.; Nagarajan, S.; Chen, Z. Bayesian Machine Learning: EEG/MEG Signal Processing Measurements. IEEE Signal Process Mag. 2016, 33, 14–36. [Google Scholar] [CrossRef]
- Bennett, C.L.; Mihajloski, T.; Özdamar, Ö. Signal-to-noise ratio improvement of swept-tone-generated transient otoacoustic emissions. Med. Biol. Eng. Comput. 2016, 55, 1–10. [Google Scholar] [CrossRef]
- Bewick, V.; Cheek, L.; Ball, J. Statistics review 9: One-way analysis of variance. Crit. Care 2004, 8, 130. [Google Scholar] [CrossRef] [PubMed]
- Margarita-Minou, B.M.; Lilia-María, M.C.; Iván, G.M.; Bárbara, E.Á.D.; Lourdes, L.P.; María-Eugenia, G.; Reynaldo, G.; Bender, J.E.; Ivette, C.A.; Yamila, P.T. Temporal lobe epilepsy surgery modulates the activity of auditory pathway. Epilepsy Res. 2014, 108, 748–754. [Google Scholar]
- Schecklmann, M.; Engel, S.; Markewitz, R.; Langguth, B. V34. Paired associative stimulation of the temporal cortex. Clin. Neurophysiol. 2015, 126, e83–e84. [Google Scholar] [CrossRef]
- Kumar, K.; Sinha, S.K.; Bhat, J.S. Tone-evoked brainstem responses and auditory steady state responses to 40hz and 80hz amplitude modulated stimuli with different frequencies—A comparative study. Indian J. Otolaryngol. Head Neck Surg. 2008, 60, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Jorge, B.; Ozcan, O. Generation of the 40-Hz auditory steady-state response (ASSR) explained using convolution. Clin. Neurophysiol. 2008, 119, 2598–2607. [Google Scholar]
- Hinsey, J.C. Symposium: Neural mechanism of hearing: I.—Anatomy and physiology.(c)—Central auditory pathways to the temporal lobes. Laryngoscope 1937, 47, 378–388. [Google Scholar]
- Binder, J.R.; Frost, J.A.; Hammeke, T.A.; Bellgowan, P.S.; Springer, J.A.; Kaufman, J.N.; Possing, E.T. Human temporal lobe activation by speech and nonspeech sounds. Cereb. Cortex 2000, 10, 512–528. [Google Scholar] [CrossRef]
- Keitel, A.; Ince, R.A.A.; Gross, J.; Kayser, C. Auditory cortical delta-entrainment interacts with oscillatory power in multiple fronto-parietal networks. Neuroimage 2016, 147, 32. [Google Scholar] [CrossRef]
- Hall, D.A. Auditory Pathways: Are ‘What’ and ‘Where’ Appropriate? Curr. Biol. 2003, 13, R406–R408. [Google Scholar] [CrossRef]
- Gandal, M.J.; Christopher, E.; Kerstin, K.; Siegel, S.J. Gamma synchrony: towards a translational biomarker for the treatment-resistant symptoms of schizophrenia. Neuropharmacology 2012, 62, 1504–1518. [Google Scholar] [CrossRef]
- Zhou, T.H.; Mueller, N.E.; Spencer, K.M.; Mallya, S.G.; Lewandowski, K.E.; Norris, L.A.; Levy, D.L.; Cohen, B.M.; Öngür, D.; Hall, M.H. Auditory steady state response deficits are associated with symptom severity and poor functioning in patients with psychotic disorder. Schizophr. Res. 2018, 201, 278–286. [Google Scholar] [CrossRef] [PubMed]





| MHS (N=10) | FMHS (N = 10) | F | Significance. | |
|---|---|---|---|---|
| Age (years) | 23.40 ± 1.174 | 24.30 ± 1.829 | 1.715 | 0.207 |
| Education (years) | 17.50 ± 0.527 | 18.50 ± 1.581 | 3.600 | 0.074 |
| Number of completed blocks | 60 | 60 | - | - |
| Tolerance score (total score) | 40 | 40 | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Liu, S.; Guo, D.; Sheng, Y.; Ke, Y.; An, X.; He, F.; Ming, D. Enhanced Auditory Steady-State Response Using an Optimized Chirp Stimulus-Evoked Paradigm. Sensors 2019, 19, 748. https://doi.org/10.3390/s19030748
Liu X, Liu S, Guo D, Sheng Y, Ke Y, An X, He F, Ming D. Enhanced Auditory Steady-State Response Using an Optimized Chirp Stimulus-Evoked Paradigm. Sensors. 2019; 19(3):748. https://doi.org/10.3390/s19030748
Chicago/Turabian StyleLiu, Xiaoya, Shuang Liu, Dongyue Guo, Yue Sheng, Yufeng Ke, Xingwei An, Feng He, and Dong Ming. 2019. "Enhanced Auditory Steady-State Response Using an Optimized Chirp Stimulus-Evoked Paradigm" Sensors 19, no. 3: 748. https://doi.org/10.3390/s19030748
APA StyleLiu, X., Liu, S., Guo, D., Sheng, Y., Ke, Y., An, X., He, F., & Ming, D. (2019). Enhanced Auditory Steady-State Response Using an Optimized Chirp Stimulus-Evoked Paradigm. Sensors, 19(3), 748. https://doi.org/10.3390/s19030748




