Improving In-Situ Estimation of Soil Profile Properties Using a Multi-Sensor Probe
Abstract
:1. Introduction
- Ten different spectral preprocessing techniques.
- Four calibration methods: PLSR, neural networks, regression trees, and random forests.
- All four sensors in combination compared to VNIR spectra alone.
- Single-field calibrations compared to those developed for multiple fields.
2. Materials and Methods
2.1. Study Fields
2.2. Sensor Data Collection
2.3. Soil Sampling and Laboratory Analysis
2.4. Alignment of Soil and Sensor Data
2.5. Analysis Methods
2.5.1. Spectral Preprocessing
- (1)
- Reflectance spectra (transformed from absorbance to reflectance);
- (2)
- Absorbance spectra (the default output format of the P4000 instrument);
- (3)
- Mean normalized spectra, smoothed with a 9-point moving average;
- (4)
- Spectra smoothed with a 9-point moving average and then mean normalized;
- (5)
- 30-point moving average;
- (6)
- 30-point Lowess smoothing;
- (7)
- 30-point Gaussian window smoothing;
- (8)
- 30-point Exponential smoothing;
- (9)
- Standard normal variate (SNV) transformation;
- (10)
- SNV plus 30-point Gaussian smoothing.
2.5.2. Calibration Methods
3. Results and Discussion
3.1. Comparison of Spectral Preprocessing Techniques
3.2. Comparison of Spectra and DECS
3.3. Model Calibration Methods
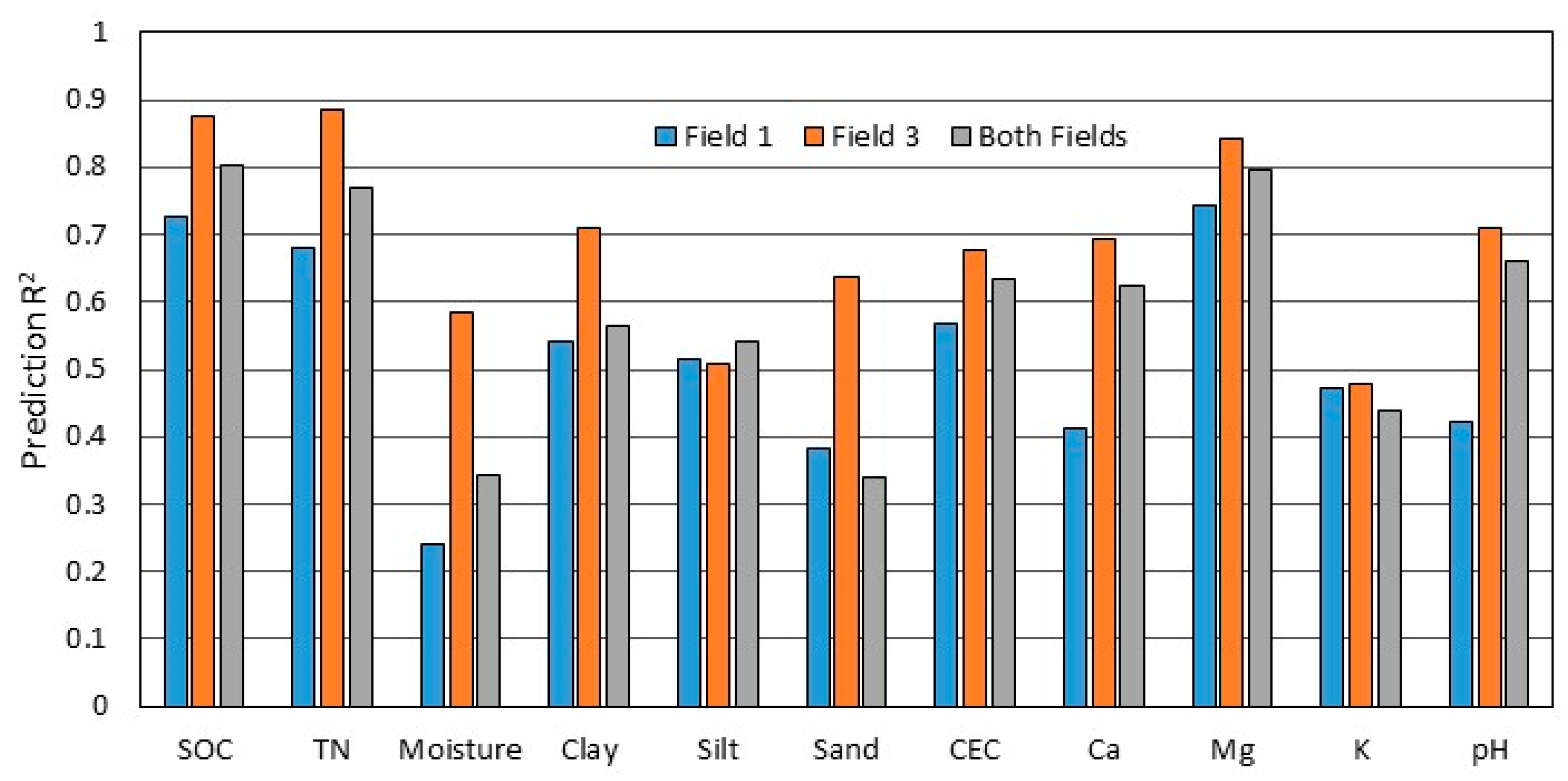
3.4. Comparison Among Fields
4. Conclusions
- Of the preprocessing techniques investigated, absorbance spectra smoothed with a 30-point Gaussian window produced the most consistently accurate estimates, but only slightly better than absorbance spectra with a SNV transformation. When averaged across all soil properties, there was little difference in accuracy (ΔR2 = 0.03) among the 10 preprocessing techniques.
- Spectra alone provided better estimates of some soil properties while the multiple sensor (DECS) dataset performed better for others. However, DECS estimates improved by more than 5% in RMSE only for Ca, a marginal improvement with the additional complexity of multiple sensors.
- Overall, PLSR was the best modeling method, providing most accurate results for six soil properties and second best for another four, out of the 11 properties investigated. Estimation accuracy was more strongly affected by choice of modeling method than by choice of sensor dataset or preprocessing method.
- Accuracy varied considerably between two fields with similar soils, suggesting that in this case field-specific characteristics or management activities may have influenced the relationship of sensor data to soil properties.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stenberg, B.; Viscarra Rossel, R.A.; Mouazen, A.M.; Wetterlind, J. Visible and near-infrared spectroscopy in soil science. Adv. Agron. 2010, 107, 163–215. [Google Scholar]
- Viscarra Rossel, R.A.; Walvoort, D.J.J.; McBratney, A.B.; Janik, L.J.; Skjemstad, J.O. Visible, near infrared, mid infrared or combined diffuse reflectance spectroscopy for simultaneous assessment of various soil properties. Geoderma 2006, 131, 59–75. [Google Scholar] [CrossRef]
- Mouazen, A.M.; Maleki, M.R.; de Baerdemaeker, J.; Ramon, H. On-line measurement of some soil properties using a VIS-NIR sensor. Soil Tillage Res. 2007, 93, 13–27. [Google Scholar] [CrossRef]
- Kusumo, B.H.; Hedley, C.B.; Hedley, M.J.; Hueni, A.; Tuohy, M.P.; Arnold, G.C. The use of diffuse reflectance spectroscopy for in situ carbon and nitrogen analysis of pastoral soils. Aust. J. Soil Res. 2008, 46, 623–635. [Google Scholar] [CrossRef]
- Kweon, G.; Lund, E.; Maxton, C.; Drummond, P.; Jensen, K. In Situ Measurement of Soil Properties Using a Probe-Based VIS-NIR Spectrophotometer; Paper No. 084399; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2008. [Google Scholar]
- Chang, C.W.; Laird, D.A.; Mausbach, M.J.; Hurburgh, C.R. Near-infrared reflectance spectroscopy-principal components regression analyses of soil properties. Soil Sci. Soc. Am. J. 2001, 65, 480–490. [Google Scholar] [CrossRef]
- Confalonieri, M.; Fornasier, F.; Ursino, A.; Boccardi, F.; Pintus, B.; Odoardi, M. The potential of near infrared reflectance spectroscopy as a tool for chemical characterization of agricultural soils. J. Near Infrared Spectrosc. 2001, 9, 123–131. [Google Scholar] [CrossRef]
- McCarty, G.W.; Reeves, J.B., III; Reeves, V.B.; Follett, R.F.; Kimble, J.M. Mid-infrared and near-infrared diffuse reflectance spectroscopy for soil carbon measurements. Soil Sci. Soc. Am. J. 2002, 66, 640–646. [Google Scholar]
- Lee, K.S.; Sudduth, K.A.; Drummond, S.T.; Lee, D.H.; Kitchen, N.R.; Chung, S.O. Calibration methods for soil property estimation using reflectance spectroscopy. Trans. ASABE 2010, 53, 675–684. [Google Scholar] [CrossRef]
- Sudduth, K.A.; Hummel, J.W. Soil organic matter, CEC, and moisture sensing with a portable NIR spectrophotometer. Trans. ASAE 1993, 36, 1571–1582. [Google Scholar] [CrossRef]
- Ben-Dor, E.; Banin, A. Near-infrared analysis as a rapid method to simultaneously evaluate several soil properties. Soil Sci. Soc. Am. J. 1995, 59, 364–372. [Google Scholar] [CrossRef]
- Shepherd, K.D.; Walsh, M.G. Development of reflectance spectral libraries for characterization of soil properties. Soil Sci. Soc. Am. J. 2002, 66, 988–998. [Google Scholar] [CrossRef]
- Cozzolino, D.; Moron, A. The potential of near-infrared reflectance spectroscopy to analyse soil chemical and physical characteristics. J. Agric. Sci. 2003, 140, 65–71. [Google Scholar] [CrossRef]
- Islam, K.; Singh, B.; McBratney, A. Simultaneous estimation of several soil properties by ultra-violet, visible, and near-infrared reflectance spectroscopy. Aust. J. Soil Res. 2003, 41, 1101–1114. [Google Scholar] [CrossRef]
- Lee, W.S.; Sanchez, J.F.; Mylavarapu, R.S.; Choe, J.S. Estimating chemical properties of Florida soils using spectral reflectance. Trans. ASAE 2003, 46, 1443–1453. [Google Scholar]
- Baker, J.M.; Ochsner, T.E.; Venterea, R.T.; Griffis, T.J. Tillage and soil carbon sequestration—What do we really know? Agric. Ecosyst. Environ. 2007, 118, 1–5. [Google Scholar] [CrossRef]
- Harrison, R.; Footen, P.; Strahm, B. Deep soil horizons: Contribution and importance to soil carbon pools and in assessing whole-ecosystem response to management and global change. For. Sci. 2011, 57, 67–76. [Google Scholar]
- Dalal, R.C.; Henry, R.J. Simultaneous determination of moisture, organic carbon, and total nitrogen by near-infrared reflectance spectrophotometry. Soil Sci. Soc. Am. J. 1986, 50, 120–123. [Google Scholar] [CrossRef]
- Hummel, J.W.; Sudduth, K.A.; Hollinger, S.E. Soil moisture and organic matter prediction of surface and subsurface soils using an NIR soil sensor. Comput. Electron. Agric. 2001, 32, 149–165. [Google Scholar] [CrossRef]
- Lee, K.S.; Sudduth, K.A.; Chung, S.O.; Kitchen, N.R.; Drummond, S.T. Wavelength identification and diffuse reflectance estimation for surface and profile soil properties. Trans. ASABE 2009, 52, 683–695. [Google Scholar] [CrossRef]
- Hedley, C.; Roudier, P.; Maddi, L. VNIR soil spectroscopy for field analysis. Commun. Soil Sci. Plant Anal. 2015, 46, 104–121. [Google Scholar] [CrossRef]
- Roudier, P.; Hedley, C.B.; Ross, C.W. Prediction of volumetric soil organic carbon from field-moist intact soil cores. Eur. J. Soil Sci. 2015, 66, 651–660. [Google Scholar] [CrossRef]
- Hummel, J.W.; Ahmad, I.S.; Newman, S.C.; Sudduth, K.A.; Drummond, S.T. Simultaneous soil moisture and cone index measurement. Trans. ASAE 2004, 47, 607–618. [Google Scholar] [CrossRef]
- Poggio, M.; Brown, D.J.; Bricklemyer, R.S. Comparison of Vis-NIR on in situ, intact core and dried, sieved soil to estimate clay content at field to regional scales. Eur. J. Soil Sci. 2017, 68, 434–448. [Google Scholar] [CrossRef]
- Ackerson, J.P.; Morgan, C.L.S.; Ge, Y. Penetrometer-mounted VisNIR spectroscopy: Application of EPO-PLS to in situ VisNIR spectra. Geoderma 2017, 286, 131–138. [Google Scholar] [CrossRef]
- Cho, Y.; Sudduth, K.A.; Drummond, S.T. Profile soil property estimation using a VIS-NIR-EC-force probe. Trans. ASABE 2017, 60, 683–692. [Google Scholar] [CrossRef]
- Wetterlind, J.; Piikki, K.; Stenberg, B.; Söderström, M. Exploring the predictability of soil texture and organic matter content with a commercial integrated soil profiling tool. Eur. J. Soil Sci. 2015, 66, 631–638. [Google Scholar] [CrossRef]
- Hodge, A.M.; Sudduth, K.A. Comparison of Two Spectrometers for Profile Soil Carbon Sensing; Paper No. 121338240; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2012. [Google Scholar]
- Veum, K.S.; Parker, P.A.; Sudduth, K.A.; Holan, S.H. Predicting profile soil properties with reflectance spectra via Bayesian covariate-assisted external parameter orthogonalization. Sensors 2018, 18, 3869. [Google Scholar] [CrossRef] [PubMed]
- Nduwamungu, C.; Ziadi, N.; Tremblay, G.F.; Parent, L.-E. Near-infrared reflectance spectroscopy prediction of soil properties: Effects of sample cups and preparation. Soil Sci. Soc. Am. J. 2009, 73, 1896–1903. [Google Scholar] [CrossRef]
- Igne, B.; Reeves, J.B., III; McCarty, G.; Hively, W.D.; Lund, E.; Hurburgh, C.R., Jr. Evaluation of spectral pretreatments, partial least squares, least squares support vector machines and locally weighted regression for quantitative spectroscopic analysis of soils. J. Near Infrared Spectrosc. 2010, 18, 167–176. [Google Scholar] [CrossRef]
- Xu, S.; Zhao, Y.; Wang, M.; Shi, X. Comparison of multivariate methods for estimating selected soil properties from intact cores of paddy fields by Vis-NIR spectroscopy. Geoderma 2018, 310, 29–43. [Google Scholar] [CrossRef]
- Viscarra Rossel, R.A.; Behrens, T. Using data mining to model and interpret soil diffuse reflectance spectra. Geoderma 2010, 158, 46–54. [Google Scholar] [CrossRef]
- Minasny, B.; McBratney, A.B.; Bellon-Maurel, V.; Roger, J.M.; Gobrecht, A.; Ferrand, L.; Joalland, S. Removing the effect of soil moisture from NIR diffuse reflectance spectra for the prediction of soil organic carbon. Geoderma 2011, 118–124. [Google Scholar] [CrossRef]
- Morgan, C.L.S.; Waiser, T.H.; Brown, D.J.; Hallmark, C.T. Simulated in situ characterization of soil organic and inorganic carbon with visible near-infrared diffuse reflectance spectroscopy. Geoderma 2009, 151, 249–256. [Google Scholar] [CrossRef]
- Reeves, J.B. Near- versus mid-infrared diffuse reflectance spectroscopy for soil analysis emphasizing carbon and laboratory versus on-site analysis: Where are we and what needs to be done? Geoderma 2010, 158, 3–14. [Google Scholar] [CrossRef]
- Viscarra Rossel, R.A.; Lobsey, C.R.; Sharman, C.; Flick, P.; McLachlan, G. Novel proximal sensing for monitoring soil organic C stocks and condition. Environ. Sci. Technol. 2017, 51, 5630–5641. [Google Scholar] [CrossRef] [PubMed]
- Sudduth, K.A.; Kitchen, N.R.; Bollero, G.A.; Bullock, D.G.; Wiebold, W.J. Comparison of electromagnetic induction and direct sensing of soil electrical conductivity. Agron. J. 2003, 95, 472–482. [Google Scholar] [CrossRef]
- McNeill, J.D. Rapid, accurate mapping of soil salinity by electromagnetic ground conductivity meters. In Advances in Measurement of Soil Physical Properties: Bringing Theory into Practice; Topp, G.C., Reynolds, W.D., Green, R.E., Eds.; SSSA: Madison, WI, USA, 1992; pp. 209–229. [Google Scholar]
- Rhoades, J.D.; Manteghi, N.A.; Shrouse, P.J.; Alves, W.J. Soil electrical conductivity and soil salinity: New formulations and calibrations. Soil Sci. Soc. Am. J. 1989, 53, 433–439. [Google Scholar] [CrossRef]
- ASABE Standards. Soil Cone Penetrometer; ASABE Standard S313.3; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2013. [Google Scholar]
- ASABE Standards. Procedures for Using and Reporting Data Obtained with the Soil Cone Penetrometer; ASABE Engineering Practice EP542; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2013. [Google Scholar]
- Elbanna, E.B.; Witney, B.D. Cone penetration resistance equation as a function of the clay ratio, soil moisture content and specific weight. J. Terramech. 1987, 24, 41–56. [Google Scholar] [CrossRef]
- Canarache, A. Factors and indices regarding excessive compactness of agricultural soils. Soil Tillage Res. 1991, 19, 145–164. [Google Scholar] [CrossRef]
- Chung, S.O.; Sudduth, K.A.; Plouffe, C.; Kitchen, N.R. Soil bin and field tests of an on-the-go soil strength profile sensor. Trans. ASABE 2008, 51, 5–18. [Google Scholar] [CrossRef]
- Sadler, E.J.; Lerch, R.N.; Kitchen, N.R.; Anderson, S.H.; Baffaut, C.; Sudduth, K.A.; Prato, A.A.; Kremer, R.J.; Vories, E.D.; Myers, D.B.; et al. Long-term agro-ecosystem research in the Central Mississippi River Basin: Introduction, establishment, and overview. J. Environ. Qual. 2015, 44, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon and organic matter. In Methods of Soil Analysis. Part 3—Chemical Methods; Sparks, D.L., Ed.; SSSA Book Ser. 5; SSSA: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- National Soil Survey Center. Soil Survey Laboratory Methods Manual; version 3.0; Soil Survey Investigations Report No. 42; USDA-NRCS National Soil Survey Center: Lincoln, NE, USA, 1996.
- Wold, S.; Sjöström, M.; Eriksson, L. PLS regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Cleveland, W.S. Robust locally weighted regression and smoothing scatterplots. J. Am. Stat. Assoc. 1979, 74, 829–836. [Google Scholar] [CrossRef]
- Cleveland, W.S.; Devlin, S.J. Locally weighted regression: An approach to regression analysis by local fitting. J. Am. Stat. Assoc. 1988, 83, 596–610. [Google Scholar] [CrossRef]
- Barnes, R.J.; Dhanoa, M.S.; Lister, S.J. Standard normal variate transformation and de-trending of near-infrared diffuse reflectance spectra. Appl. Spectrosc. 1989, 43, 772–777. [Google Scholar] [CrossRef]
- Beebe, K.R.; Kowalski, B.R. An introduction to multivariate calibration and analysis. Anal. Chem. 1987, 59, 1007A–1017A. [Google Scholar] [CrossRef]
- Breiman, L.; Friedman, J.; Olshen, R.; Stone, C. Classification and Regression Trees; Wadsworth: Belmont, CA, USA, 1984. [Google Scholar]
- Verbyla, D.L. Classification trees: A new discrimination tool. Can. J. For. Res. 1987, 17, 1150–1152. [Google Scholar] [CrossRef]
- Clark, L.A.; Pregibon, D. Tree-based models. In Statistical Models; Chambers, J.M., Hastie, T.J., Eds.; Wadsworth: Belmont, CA, USA, 1992; pp. 377–419. [Google Scholar]
- Therneau, T.M.; Atkinson, E.J. An Introduction to Recursive Partitioning Using the RPART Routines; Technical Report No. 61; Mayo Clinic: Rochester, MN, USA, 1997. [Google Scholar]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Brown, D.J.; Bricklemyer, R.S.; Miller, P.R. Validation requirements for diffuse reflectance soil characterization models with a case study of VNIR soil C prediction in Montana. Geoderma 2005, 129, 251–267. [Google Scholar] [CrossRef]
- Young, F.J.; Radatz, C.A.; Marshall, C.A. Soil Survey of Boone County, Missouri; USDA-NRCS: Washington, DC, USA, 2003.
- Yin, X.; Gupta, V.; Du, H.; Wang, X.; Miller, J.D. Surface charge and wetting characteristics of layered silicate minerals. Adv. Colloid Interface Sci. 2012, 179–182, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.; Shainberg, I. Calcium-magnesium exchange in montmorillonite and vermiculite. Clay Clay Miner. 1972, 20, 37–46. [Google Scholar] [CrossRef]


| Soil Property | Field 1 | Field 3 | Combination | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD † | Range | CV | Mean | SD | Range | CV | Mean | SD | Range | CV | |
| Samples from all soil horizons to 1.2 m profile depth (n = 148) | ||||||||||||
| SOC (%) | 0.69 | 0.40 | 1.29 | 57.3 | 0.74 | 0.48 | 1.59 | 64.6 | 0.71 | 0.43 | 1.61 | 60.5 |
| TN (%) | 0.07 | 0.04 | 0.12 | 54.6 | 0.07 | 0.04 | 0.13 | 64.4 | 0.07 | 0.04 | 0.13 | 58.4 |
| Moisture (%) | 22.2 | 2.7 | 12.8 | 12.2 | 21.0 | 2.8 | 12.5 | 13.4 | 21.8 | 2.8 | 13.0 | 12.9 |
| Clay fraction (%) | 35.8 | 14.2 | 47.1 | 39.5 | 33.1 | 11.0 | 43.7 | 33.3 | 34.7 | 13.0 | 47.1 | 37.4 |
| Silt fraction (%) | 60.6 | 12.5 | 46.5 | 20.6 | 60.9 | 9.1 | 40.0 | 14.9 | 60.7 | 11.2 | 46.5 | 18.4 |
| Sand fraction (%) | 3.6 | 3.2 | 15.0 | 88.4 | 6.0 | 4.7 | 17.3 | 77.6 | 4.6 | 4.0 | 17.8 | 87.9 |
| CEC (cmol·kg−1) | 28.2 | 9.2 | 31.7 | 32.5 | 28.0 | 8.2 | 36.6 | 29.4 | 28.1 | 8.8 | 36.6 | 31.2 |
| Ca (cmol·kg−1) | 10.6 | 3.4 | 18.2 | 31.8 | 14.0 | 3.9 | 19.5 | 28.0 | 12.0 | 4.0 | 21.3 | 33.2 |
| Mg (cmol·kg−1) | 3.74 | 1.99 | 6.90 | 53.3 | 4.65 | 2.38 | 7.20 | 51.3 | 4.11 | 2.20 | 7.20 | 53.5 |
| K (cmol·kg−1) | 0.41 | 0.17 | 0.80 | 41.8 | 0.40 | 0.14 | 0.60 | 35.4 | 0.41 | 0.16 | 0.80 | 39.3 |
| pH | 4.36 | 0.63 | 3.20 | 14.5 | 5.19 | 0.70 | 2.80 | 13.6 | 4.70 | 0.78 | 3.20 | 16.5 |
| Samples from surface horizon. Depth varied from 8 to 35.7 cm with a median of 21.8 cm (n = 33) | ||||||||||||
| SOC (%) | 1.23 | 0.13 | 0.43 | 10.3 | 1.44 | 0.18 | 0.59 | 12.6 | 1.31 | 0.18 | 0.75 | 13.8 |
| TN (%) | 0.12 | 0.01 | 0.05 | 11.1 | 0.13 | 0.01 | 0.04 | 10.0 | 0.12 | 0.01 | 0.05 | 11.8 |
| Moisture (%) | 20.6 | 1.27 | 4.2 | 6.2 | 18.7 | 1.9 | 5.36 | 10.1 | 19.83 | 1.78 | 6.56 | 9.0 |
| Clay fraction (%) | 20.1 | 4.5 | 15.8 | 22.2 | 22.7 | 3.8 | 14.1 | 16.6 | 21.2 | 4.3 | 17.4 | 20.5 |
| Silt fraction (%) | 73.8 | 5.9 | 21.1 | 8.0 | 69.6 | 4.2 | 13.6 | 6.1 | 72.1 | 5.6 | 22.5 | 7.8 |
| Sand fraction (%) | 6.1 | 3.0 | 10.8 | 49.7 | 7.7 | 1.6 | 5.10 | 20.8 | 6.7 | 2.6 | 10.8 | 39.1 |
| CEC (cmol·kg−1) | 18.7 | 3.4 | 12.3 | 18.2 | 22.1 | 2.8 | 11.1 | 12.7 | 20.1 | 3.6 | 15.5 | 17.7 |
| Ca (cmol·kg−1) | 9.6 | 4.0 | 17.9 | 41.1 | 15.0 | 2.17 | 8.0 | 14.5 | 11.8 | 4.3 | 17.9 | 36.0 |
| Mg (cmol·kg−1) | 1.55 | 0.68 | 2.80 | 43.7 | 2.14 | 0.71 | 2.30 | 33.0 | 1.79 | 0.74 | 2.80 | 41.2 |
| K (cmol·kg−1) | 0.25 | 0.08 | 0.20 | 30.6 | 0.44 | 0.13 | 0.40 | 30.2 | 0.33 | 0.14 | 0.50 | 41.9 |
| pH | 5.16 | 0.76 | 2.90 | 14.7 | 6.22 | 0.32 | 1.10 | 5.1 | 5.59 | 0.81 | 2.90 | 14.4 |
| Preprocessing Technique | Field 1 | Field 3 | Combination (F1 + F3) | Grand Mean R2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD † & Range | CV | Mean | SD & Range | CV | Mean | SD & Range | CV | ||
| Reflectance | 0.51 | 0.14 0.44 | 28.1 | 0.65 | 0.16 0.51 | 24.9 | 0.57 | 0.15 0.48 | 25.3 | 0.58 |
| Absorbance | 0.52 | 0.14 0.43 | 26.8 | 0.67 | 0.15 0.49 | 23.1 | 0.59 | 0.16 0.49 | 27.4 | 0.59 |
| Normalize + 9-point m.a. | 0.51 | 0.14 0.45 | 28.2 | 0.65 | 0.18 0.54 | 27.3 | 0.61 | 0.20 0.70 | 32.6 | 0.59 |
| 9-point m.a. then normalize | 0.52 | 0.13 0.39 | 25.8 | 0.62 | 0.18 0.55 | 28.4 | 0.59 | 0.14 0.45 | 24.1 | 0.58 |
| 30-point m.a. | 0.53 | 0.13 0.38 | 24.6 | 0.68 | 0.14 0.44 | 21.0 | 0.59 | 0.16 0.50 | 27.9 | 0.60 |
| 30-point Lowess smoothing | 0.52 | 0.15 0.50 | 28.8 | 0.65 | 0.16 0.48 | 25.3 | 0.60 | 0.16 0.47 | 25.8 | 0.59 |
| 30-point Gaussian smoothing | 0.51 | 0.15 0.53 | 29.8 | 0.71 | 0.12 0.40 | 16.9 | 0.60 | 0.16 0.47 | 26.0 | 0.61 |
| 30-point exponential smoothing | 0.52 | 0.13 0.42 | 25.7 | 0.64 | 0.17 0.47 | 26.2 | 0.60 | 0.15 0.45 | 24.2 | 0.59 |
| SNV (standard normal variate) | 0.52 | 0.16 0.42 | 29.9 | 0.68 | 0.14 0.43 | 27.4 | 0.61 | 0.14 0.44 | 23.7 | 0.60 |
| SNV + 30-pt Gaussian smoothing | 0.54 | 0.15 0.39 | 27.4 | 0.68 | 0.14 0.44 | 20.2 | 0.61 | 0.15 0.46 | 24.0 | 0.61 |
| Preprocessing Technique | SOC † | TN | Moisture | Clay | Silt | Sand | CEC | Ca | Mg | K | pH |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reflectance | 0.78 0.204 | 0.76 0.0201 | 0.43 2.103 | 0.60 8.264 | 0.54 7.638 | 0.31 3.360 | 0.60 5.560 | 0.49 2.839 | 0.79 1.005 | 0.48 0.117 | 0.57 0.506 |
| Absorbance | 0.79 0.198 | 0.77 0.0198 | 0.33 2.312 | 0.57 8.521 | 0.56 7.441 | 0.32 3.347 | 0.65 5.184 | 0.62 2.431 | 0.81 0.978 | 0.48 0.116 | 0.66 0.456 |
| Normalize then 9-point m.a. | 0.79 0.195 | 0.76 0.0199 | 0.38 2.205 | 0.57 8.493 | 0.45 8.265 | 0.29 3.411 | 0.61 5.508 | 0.62 2.453 | 0.80 0.982 | 0.47 0.118 | 0.63 0.476 |
| 9-point m.a. then normalize | 0.80 0.194 | 0.76 0.0198 | 0.39 2.202 | 0.55 8.755 | 0.53 7.654 | 0.35 3.267 | 0.58 5.688 | 0.61 2.485 | 0.76 1.081 | 0.49 0.116 | 0.65 0.462 |
| 30-point m.a. | 0.79 0.199 | 0.76 0.0198 | 0.31 2.333 | 0.61 8.130 | 0.51 7.825 | 0.34 3.282 | 0.64 5.261 | 0.63 2.438 | 0.81 0.977 | 0.43 0.122 | 0.62 0.479 |
| 30-point Lowess smoothing | 0.80 0.196 | 0.76 0.0197 | 0.36 2.439 | 0.59 8.425 | 0.56 7.429 | 0.34 3.274 | 0.63 5.300 | 0.64 2.414 | 0.81 0.958 | 0.44 0.120 | 0.65 0.462 |
| 30-point Gaussian smoothing | 0.80 0.194 | 0.77 0.0196 | 0.37 2.240 | 0.58 8.421 | 0.52 7.774 | 0.34 3.265 | 0.65 5.246 | 0.64 2.424 | 0.81 0.961 | 0.46 0.117 | 0.67 0.450 |
| 30-point exponential smoothing | 0.79 0.198 | 0.76 0.0200 | 0.40 2.180 | 0.61 8.147 | 0.54 7.579 | 0.35 3.270 | 0.62 5.379 | 0.63 2.461 | 0.80 0.985 | 0.45 0.119 | 0.65 0.463 |
| SNV (standard normal variate) | 0.81 0.188 | 0.78 0.0193 | 0.48 2.020 | 0.62 8.000 | 0.55 7.544 | 0.37 3.230 | 0.63 5.386 | 0.58 2.594 | 0.79 1.008 | 0.45 0.118 | 0.65 0.457 |
| SNV + 30-pt Gaussian smoothing | 0.81 0.186 | 0.77 0.0196 | 0.48 2.040 | 0.63 7.868 | 0.54 7.556 | 0.35 3.248 | 0.61 5.479 | 0.63 2.426 | 0.78 1.020 | 0.44 0.119 | 0.66 0.460 |
| SOC † | TN | Moisture | Clay | Silt | Sand | CEC | Ca | Mg | K | pH | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Spectra | 0.80 | 0.78 | 0.39 | 0.61 | 0.54 | 0.27 | 0.60 | 0.52 | 0.79 | 0.45 | 0.66 |
| 0.193 | 0.019 | 2.218 | 8.089 | 7.626 | 3.468 | 5.536 | 2.795 | 1.011 | 0.118 | 0.453 | |
| DECS | 0.80 | 0.77 | 0.34 | 0.57 | 0.54 | 0.34 | 0.63 | 0.62 | 0.80 | 0.44 | 0.66 |
| 0.193 | 0.020 | 2.281 | 8.551 | 7.557 | 3.297 | 5.337 | 2.439 | 0.989 | 0.121 | 0.453 |
| Field 1 | Field 3 | Combination | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soil Property | DECS | Spectra | DECS | Spectra | DECS | Spectra | ||||||
| R2 | RMSE | R2 | RMSE | R2 | RMSE | R2 | RMSE | R2 | RMSE | R2 | RMSE | |
| SOC † | 0.73 | 0.208 | 0.77 | 0.194 | 0.87 | 0.173 | 0.87 | 0.177 | 0.80 | 0.193 | 0.80 | 0.193 |
| TN | 0.68 | 0.022 | 0.73 | 0.020 | 0.89 | 0.015 | 0.85 | 0.017 | 0.77 | 0.020 | 0.78 | 0.019 |
| Moisture | 0.24 | 2.387 | 0.34 | 2.210 | 0.59 | 1.838 | 0.49 | 1.979 | 0.34 | 2.281 | 0.39 | 2.218 |
| Clay | 0.54 | 9.687 | 0.55 | 9.463 | 0.71 | 6.007 | 0.72 | 5.834 | 0.57 | 8.551 | 0.61 | 8.089 |
| Silt | 0.52 | 8.627 | 0.53 | 8.647 | 0.51 | 6.377 | 0.60 | 5.802 | 0.54 | 7.557 | 0.54 | 7.626 |
| Sand | 0.38 | 2.502 | 0.38 | 2.516 | 0.64 | 2.862 | 0.74 | 2.453 | 0.34 | 3.297 | 0.27 | 3.468 |
| CEC | 0.57 | 6.055 | 0.55 | 6.157 | 0.68 | 4.682 | 0.70 | 4.625 | 0.63 | 5.337 | 0.60 | 5.536 |
| Ca | 0.41 | 2.587 | 0.26 | 2.920 | 0.70 | 2.232 | 0.55 | 2.642 | 0.63 | 2.439 | 0.52 | 2.795 |
| Mg | 0.74 | 1.019 | 0.75 | 1.003 | 0.84 | 0.959 | 0.80 | 1.066 | 0.80 | 0.989 | 0.79 | 1.011 |
| K | 0.47 | 0.127 | 0.46 | 0.129 | 0.48 | 0.103 | 0.28 | 0.120 | 0.44 | 0.121 | 0.45 | 0.118 |
| pH | 0.42 | 0.487 | 0.45 | 0.470 | 0.71 | 0.381 | 0.71 | 0.386 | 0.66 | 0.453 | 0.66 | 0.453 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, X.; Sudduth, K.A.; Veum, K.S.; Li, M. Improving In-Situ Estimation of Soil Profile Properties Using a Multi-Sensor Probe. Sensors 2019, 19, 1011. https://doi.org/10.3390/s19051011
Pei X, Sudduth KA, Veum KS, Li M. Improving In-Situ Estimation of Soil Profile Properties Using a Multi-Sensor Probe. Sensors. 2019; 19(5):1011. https://doi.org/10.3390/s19051011
Chicago/Turabian StylePei, Xiaoshuai, Kenneth A. Sudduth, Kristen S. Veum, and Minzan Li. 2019. "Improving In-Situ Estimation of Soil Profile Properties Using a Multi-Sensor Probe" Sensors 19, no. 5: 1011. https://doi.org/10.3390/s19051011
APA StylePei, X., Sudduth, K. A., Veum, K. S., & Li, M. (2019). Improving In-Situ Estimation of Soil Profile Properties Using a Multi-Sensor Probe. Sensors, 19(5), 1011. https://doi.org/10.3390/s19051011





