Hydrogel-Based Sensors for Ethanol Detection in Alcoholic Beverages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Ethanol-Sensitive Polyacrylamide-Bisacrylamide Hydrogels
2.2. Preparation of Hydrogel-Based Piezoresistive Ethanol Sensors
2.3. Determination of the Measuring Range
2.4. Cross-Sensitivity to Salt Concentrations
2.5. Cross-Sensitivity to pH Value
2.6. Sensor Calibration and Measurements of a Vodka Sample
3. Results and Discussion
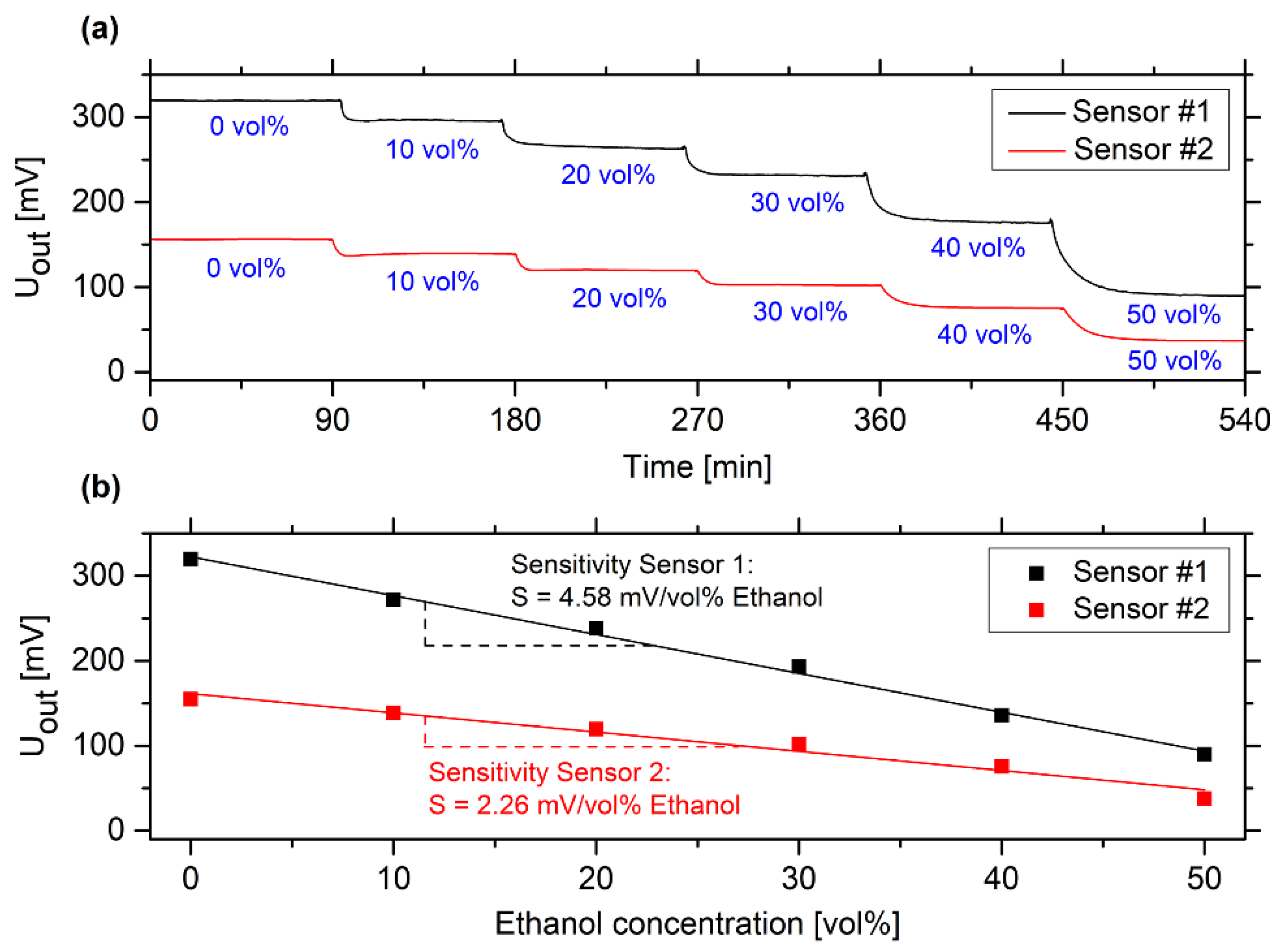
3.1. Determination of the Measuring Range
3.2. Cross-Sensitivity to Salt Concentrations
3.3. Cross-Sensitivity to pH Value
3.4. Calibration Curves and Measurement of a Vodka Sample
3.5. Limit of Detection (LoD) and Limit of Quantification (LoQ)
4. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Nomenclature of the Used Hydrogel-Based Ethanol Sensors
| Sensors | Sensors Were Used for |
|---|---|
| Sensor #1–#2 | Determination of the measuring range (Section 3.1) |
| Sensor #3–#5 | Cross-sensitivity to different salt concentrations (Section 3.2) |
| Sensor #6–#8 | Cross-sensitivity to different pH values (Section 3.3) |
| Sensor #9–#14 | Preparation of calibration curves, measurement of vodka samples (Section 3.4), and calculation of the LoD and LoQ (Section 3.5) |
Appendix B. Determination of the Limit of Detection (LoD) and Limit of Quantification (LoQ) according to DIN 32654
Appendix B.1. Limit of Detection (LoD)
Appendix B.2. Limit of Quantification (LoQ)
| Sensor | sL [mV] | b [mV/vol%] | LoD [vol%] | LoQ [vol%] |
|---|---|---|---|---|
| Sensor #9 | 1.46 | 2.28 | 2.11 | 3.78 |
| Sensor #10 | 0.16 | 1.72 | 0.31 | 0.55 |
| Sensor #11 | 0.13 | 2.60 | 0.17 | 0.31 |
| Sensor #12 | 0.17 | 3.13 | 0.18 | 0.32 |
| Sensor #13 | 0.04 | 2.61 | 0.06 | 0.10 |
| Sensor #14 | 0.53 | 2.66 | 0.65 | 1.17 |
References
- Wen, G.; Li, Z.; Choi, M.M.F. Detection of ethanol in food: A new biosensor based on bacteria. J. Food Eng. 2013, 118, 56–61. [Google Scholar] [CrossRef]
- Lachenmeier, D.W.; Godelmann, R.; Steiner, M.; Ansay, B.; Weigel, J.; Krieg, G. Rapid and mobile determination of alcoholic strength in wine, beer and spirits using a flow-through infrared sensor. Chem. Cent. J. 2010, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Osorio, D.; Ricardo Pérez-Correa, J.; Agosin, E.; Cabrera, M. Soft-sensor for on-line estimation of ethanol concentrations in wine stills. J. Food Eng. 2008, 87, 571–577. [Google Scholar] [CrossRef]
- Castellari, M.; Sartini, E.; Spinabelli, U.; Riponi, C.; Galassi, S. Determination of Carboxylic Acids, Carbohydrates, Glycerol, Ethanol, and 5-HMF in Beer by High-Performance Liquid Chromatography and UV-Refractive Index Double Detection. J. Chromatogr. Sci. 2001, 39, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Guenther, M.; Wallmersperger, T.; Gerlach, G. Piezoresistive Chemical Sensors Based on Functionalized Hydrogels. Chemosensors 2014, 2, 145–170. [Google Scholar] [CrossRef]
- Kumar, S.S.; Pant, B.D. Design principles and considerations for the ‘ideal’ silicon piezoresistive pressure sensor: A focused review. Microsyst. Technol. 2014, 20, 1213–1247. [Google Scholar] [CrossRef]
- Franke, D.; Binder, S.; Gerlach, G. Performance of Fast-Responsive, Porous Crosslinked Poly(N-Isopropylacrylamide) in a Piezoresistive Microsensor. IEEE Sens. Lett. 2017, 1, 1500904. [Google Scholar] [CrossRef]
- Guenther, M.; Gerlach, G.; Wallmersperger, T.; Avula, M.N.; Cho, S.H.; Xie, X.; Devener, B.V.; Solzbacher, F.; Tathireddy, P.; Magda, J.J.; et al. Smart Hydrogel-Based Biochemical Microsensor Array for Medical Diagnostics. Adv. Sci. Technol. 2013, 85, 47–52. [Google Scholar] [CrossRef]
- Schmidt, U.; Jorsch, C.; Guenther, M.; Gerlach, G. Biochemical piezoresistive sensors based on hydrogels for biotechnology and medical applications. J. Sens. Sens. Syst. 2016, 5, 409–417. [Google Scholar] [CrossRef]
- Erfkamp, J.; Guenther, M.; Gerlach, G. Hydrogel-based piezoresistive sensor for the detection of ethanol. J. Sens. Sens. Syst. 2018, 7, 219–226. [Google Scholar] [CrossRef]
- Richter, A.; Paschew, G.; Klatt, S.; Lienig, J.; Arndt, K.-F.; Adler, H.-J. Review on Hydrogel-based pH Sensors and Microsensors. Sensors 2008, 8, 561–581. [Google Scholar] [CrossRef] [PubMed]
- Çaykara, T.; Turan, E. Effect of the amount and type of the crosslinker on the swelling behavior of temperature-sensitive poly(N-tert-butylacrylamide-co-acrylamide) hydrogels. Colloid Polym. Sci. 2006, 284, 1038–1048. [Google Scholar] [CrossRef]
- Caykara, T.; Kiper, S.; Demirel, G. Thermosensitive poly(N-isopropylacrylamide-co-acrylamide) hydrogels: Synthesis, swelling and interaction with ionic surfactants. Eur. Polym. J. 2006, 42, 348–355. [Google Scholar] [CrossRef]
- Okay, O. General Properties of Hydrogels. In Hydrogel Sensors and Actuators: Engineering and Technology; Gerlach, G., Arndt, K.-F., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1–14. ISBN 978-3-540-75645-3. [Google Scholar]
- Anseth, K.S.; Bowman, C.N.; Brannon-Peppas, L. Mechanical properties of hydrogels and their experimental determination. Biomaterials 1996, 17, 1647–1657. [Google Scholar] [CrossRef]
- Guenther, M.; Kuckling, D.; Corten, C.; Gerlach, G.; Sorber, J.; Suchaneck, G.; Arndt, K.-F. Chemical sensors based on multiresponsive block copolymer hydrogels. Sens. Actuators B Chem. 2007, 126, 97–106. [Google Scholar] [CrossRef]
- Guenther, M.; Gerlach, G.; Corten, C.; Kuckling, D.; Muller, M.; Shi, Z.; Sorber, J.; Arndt, K.-F. Application of Polyelectrolytic Temperature-Responsive Hydrogels in Chemical Sensors. Macromol. Symp. 2007, 254, 314–321. [Google Scholar] [CrossRef]
- Guenther, M.; Gerlach, G.; Wallmersperger, T. Piezoresistive chemical sensors based on hydrogels. SPIE Proc. 7362 Smart Sens. Actuators MEMS IV 2009, 736218. [Google Scholar] [CrossRef]
- Sivanantham, M.; Tata, B.V.R. Swelling/deswelling of polyacrylamide gels in aqueous NaCl solution: Light scattering and macroscopic swelling study. Pramana 2012, 79, 457–469. [Google Scholar] [CrossRef]
- Guenther, M. Anwendung Polymerer Funktionsschichten in Piezoresistiven Chemischen und Feuchtesensoren; Dresdner Beiträge zur Sensorik; TUDpress, Verl. der Wiss: Dresden, Germany, 2009; ISBN 978-3-941298-38-5. [Google Scholar]
- Panteleit, B.; Hamer, K.; Kringel, R.; Kessels, W.; Schulz, H.D. Geochemical processes in the saltwater–freshwater transition zone: Comparing results of a sand tank experiment with field data. Environ. Earth Sci. 2011, 62, 77–91. [Google Scholar] [CrossRef]
- Tosca, N.J.; McLennan, S.M.; Lamb, M.P.; Grotzinger, J.P. Physicochemical properties of concentrated Martian surface waters. J. Geophys. Res. 2011, 116. [Google Scholar] [CrossRef]
- Lizarralde, I.; Fernández-Arévalo, T.; Brouckaert, C.; Vanrolleghem, P.; Ikumi, D.S.; Ekama, G.A.; Ayesa, E.; Grau, P. A new general methodology for incorporating physico-chemical transformations into multi-phase wastewater treatment process models. Water Res. 2015, 74, 239–256. [Google Scholar] [CrossRef] [PubMed]
- Drewag Netz GmbH. Medianwerte Reinwasser der Wasserwerke von Januar bis Dezember 2018. Available online: https://www.drewag.de/wps/wcm/connect/drewag/6056fb2d-fbd0-4e7d-9ea1-54dc3711d285/Durchschnittswerte-Reinwasser-Wasserwerke.pdf?MOD=AJPERES&CVID=m49zF7V&CVID=m49zF7V (accessed on 27 February 2019).
- Vanderhaegen, B.; Neven, H.; Verachtert, H.; Derdelinckx, G. The chemistry of beer aging—A critical review. Food Chem. 2006, 95, 357–381. [Google Scholar] [CrossRef]
- Choi, S.; Lee, J.-K.; Shukla, S.; Kim, M. Physiochemical properties and determination of biogenic amines in korean microbrewery beer products. J. Food Biochem. 2012, 36, 766–773. [Google Scholar] [CrossRef]
- Preedy, V.R. (Ed.) Comprehensive Handbook of Alcohol Related Pathology. Vol. 1; Elsevier: Amsterdam, The Netherlands, 2005; ISBN 978-0-12-564371-9. [Google Scholar]
- Schulz, V.; Gerlach, G.; Guenther, M.; Magda, J.J.; Solzbacher, F. Piezoresistive pH Microsensors Based on Stimuli-Sensitive Polyelectrolyte Hydrogels. Tm-Tech. Mess. Plattf. Für Methoden Syst. Anwend. Messtech. 2010, 77, 179–186. [Google Scholar] [CrossRef]
- Trinh, Q.T.; Gerlach, G.; Sorber, J.; Arndt, K.-F. Hydrogel-based piezoresistive pH sensors: Design, simulation and output characteristics. Sens. Actuators B Chem. 2006, 117, 17–26. [Google Scholar] [CrossRef]
- Sorber, J.; Steiner, G.; Schulz, V.; Guenther, M.; Gerlach, G.; Salzer, R.; Arndt, K.-F. Hydrogel-Based Piezoresistive pH Sensors: Investigations Using FT-IR Attenuated Total Reflection Spectroscopic Imaging. Anal. Chem. 2008, 80, 2957–2962. [Google Scholar] [CrossRef] [PubMed]
- Neyret, S.; Candau, F.; Selb, J. Synthesis in microemulsion and characterization of low charge density ampholytic terpolymers. Acta Polym. 1996, 47, 323–332. [Google Scholar] [CrossRef]
- Turan, E.; Çaykara, T. Swelling and network parameters of pH-sensitive poly(acrylamide-co-acrylic acid) hydrogels. J. Appl. Polym. Sci. 2007, 106, 2000–2007. [Google Scholar] [CrossRef]
- Erfkamp, J.; Guenther, M.; Gerlach, G. Piezoresistive Hydrogel-Based Sensors for the Detection of Ammonia. Sensors 2019, 19, 971. [Google Scholar] [CrossRef]
- Jin, X.; Hsieh, Y.-L. pH-responsive swelling behavior of poly(vinyl alcohol)/poly(acrylic acid) bi-component fibrous hydrogel membranes. Polymer 2005, 46, 5149–5160. [Google Scholar] [CrossRef]
- Nesrinne, S.; Djamel, A. Synthesis, characterization and rheological behavior of pH sensitive poly(acrylamide-co-acrylic acid) hydrogels. Arab. J. Chem. 2017, 10, 539–547. [Google Scholar] [CrossRef]
- International Organization for Standardization (ISO). International Electrotechnical Commission (IEC) Uncertainty of Measurement—Part 3: Guide to the Expression of Uncertainty in Measurement (GUM:1995); ISO-IEC Guide 98-3 2008; ISO and IEC: Geneva, Switzerland, 2008. [Google Scholar]
- Deutsches Insitiut für Normung e.V. (DIN). Chemische Analytik-Nachweis-, Erfassungs- und Bestimmungsgrenze unter Wiederholbedingungen-Begriffe, Verfahren, Auswertung (Chemical analysis-Decision Limit, Detection Limit and Determination Limit Under Repeatability Conditions-Terms, Methods, Evaluation); DIN 32645 2008-11; DIN: Berlin, Germany, 2008. [Google Scholar]
- Betz, J.M.; Nikelly, J.G. Determination of Ethanol in Alcoholic Beverages by Liquid Chromatography Using the UV Detector. J. Chromatogr. Sci. 1987, 25, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Buckee, G.K.; Mundy, A.P. Determination of Ethanol in Beer by Gas Chromatography (Direct Injection)—Collaborative Trial. J. Inst. Brew. 1993, 99, 381–384. [Google Scholar] [CrossRef]
- Wiśniewska, P.; Śliwińska, M.; Dymerski, T.; Wardencki, W.; Namieśnik, J. Application of Gas Chromatography to Analysis of Spirit-Based Alcoholic Beverages. Crit. Rev. Anal. Chem. 2015, 45, 201–225. [Google Scholar] [CrossRef] [PubMed]
- Petrova, S.; Kostov, Y.; Jeffris, K.; Rao, G. Optical Ratiometric Sensor for Alcohol Measurements. Anal. Lett. 2007, 40, 715–727. [Google Scholar] [CrossRef]
- Mohr, G.J.; Citterio, D.; Spichiger-Keller, U.E. Development of chromogenic reactands for optical sensing of alcohols. Sens. Actuators B Chem. 1998, 49, 226–234. [Google Scholar] [CrossRef]
- Mohr, G.J.; Lehmann, F.; Grummt, U.-W.; Spichiger-Keller, U.E. Fluorescent ligands for optical sensing of alcohols: Synthesis and characterisation of p-N,N-dialkylamino-trifluoroacetylstilbenes. Anal. Chim. Acta 1997, 344, 215–225. [Google Scholar] [CrossRef]
- Rotariu, L.; Bala, C.; Magearu, V. New potentiometric microbial biosensor for ethanol determination in alcoholic beverages. Anal. Chim. Acta 2004, 513, 119–123. [Google Scholar] [CrossRef]
- Kitagawa, Y.; Kitabatake, K.; Kubo, I.; Tamiya, E.; Karube, I. Alcohol sensor based on membrane-bound alcohol dehydrogenase. Anal. Chim. Acta 1989, 218, 61–68. [Google Scholar] [CrossRef]
- Schiefer, H.; Schiefer, F. Statistik für Ingenieure: Eine Einführung mit Beispielen aus der Praxis; Lehrbuch; Springer: Wiesbaden, Germany, 2018; ISBN 978-3-658-20639-0. [Google Scholar]







| Method | Advantages (+) and Disadvantages (−) | |
|---|---|---|
| Chromatographic methods [38,39] | (+) | Most sensitive and accurate method [40,41] |
| (−) | Very high acquisition and operating costs [40,41], especially for smaller companies | |
| (−) | Well-trained operator necessary due to difficult handling of the method [40] | |
| Optical sensors [42,43] | (+) | Wide fields of application due to large measuring ranges (2–50 vol% [42], 5–50 vol% [43]) |
| (−) | High LoD (1.5 vol% [42], 2 vol% [43]) | |
| (−) | Significant cross-sensitivity to pH [42] | |
| (−) | Dye leaching over time possible [41] | |
| Microbial [44] and enzymatic [45] biosensor | (+) | Measuring range: 0.05–5 mmol/L [44], 0.1–5 mmol/L [45], after dilution also usable for alcoholic beverages [44,45] |
| (−) | Microbial and enzymatic activity depends on different factors (e.g., temperature [44,45], pH [45]) | |
| (−) | Poor long-term stability due to loss of microbial and enzymatic activity over time [44,45] | |
| Hydrogel-based sensor (presented in this work) | (+) | Wide measuring range (up to 50 vol%) |
| (+) | Low LoD (0.060–0.56 vol%) | |
| (+) | No relevant salt or pH cross-sensitivity | |
| (+) | Low-cost sensor (~10€/Sensor) | |
| (+) | Small size, even more miniaturizable | |
| (+) | In-line process capability | |
| (−) | Measurement uncertainty must be improved | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erfkamp, J.; Guenther, M.; Gerlach, G. Hydrogel-Based Sensors for Ethanol Detection in Alcoholic Beverages. Sensors 2019, 19, 1199. https://doi.org/10.3390/s19051199
Erfkamp J, Guenther M, Gerlach G. Hydrogel-Based Sensors for Ethanol Detection in Alcoholic Beverages. Sensors. 2019; 19(5):1199. https://doi.org/10.3390/s19051199
Chicago/Turabian StyleErfkamp, Jan, Margarita Guenther, and Gerald Gerlach. 2019. "Hydrogel-Based Sensors for Ethanol Detection in Alcoholic Beverages" Sensors 19, no. 5: 1199. https://doi.org/10.3390/s19051199
APA StyleErfkamp, J., Guenther, M., & Gerlach, G. (2019). Hydrogel-Based Sensors for Ethanol Detection in Alcoholic Beverages. Sensors, 19(5), 1199. https://doi.org/10.3390/s19051199





