Robust Smartphone Assisted Biosensing Based on Asymmetric Nanofluidic Grating Interferometry
Abstract
:1. Introduction
2. Concept
3. Design and Implementation
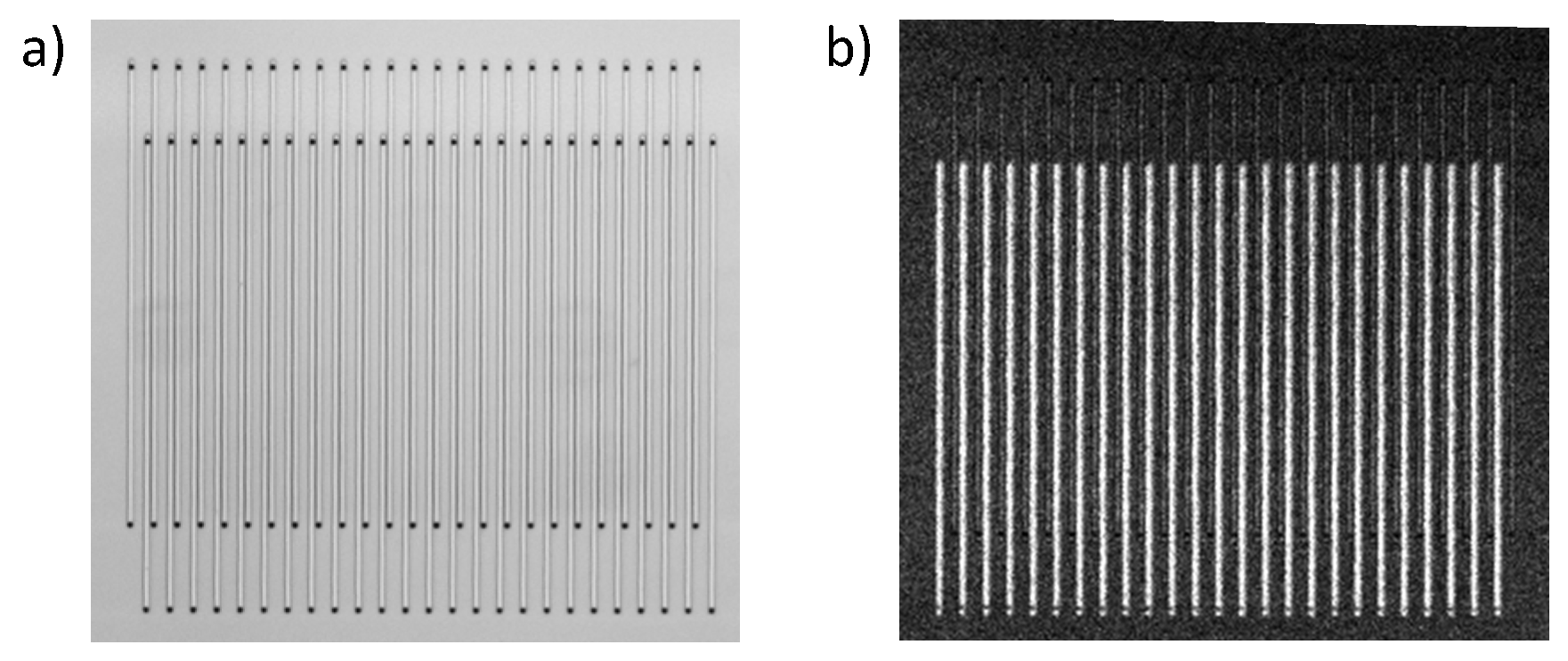
3.1. Fabrication
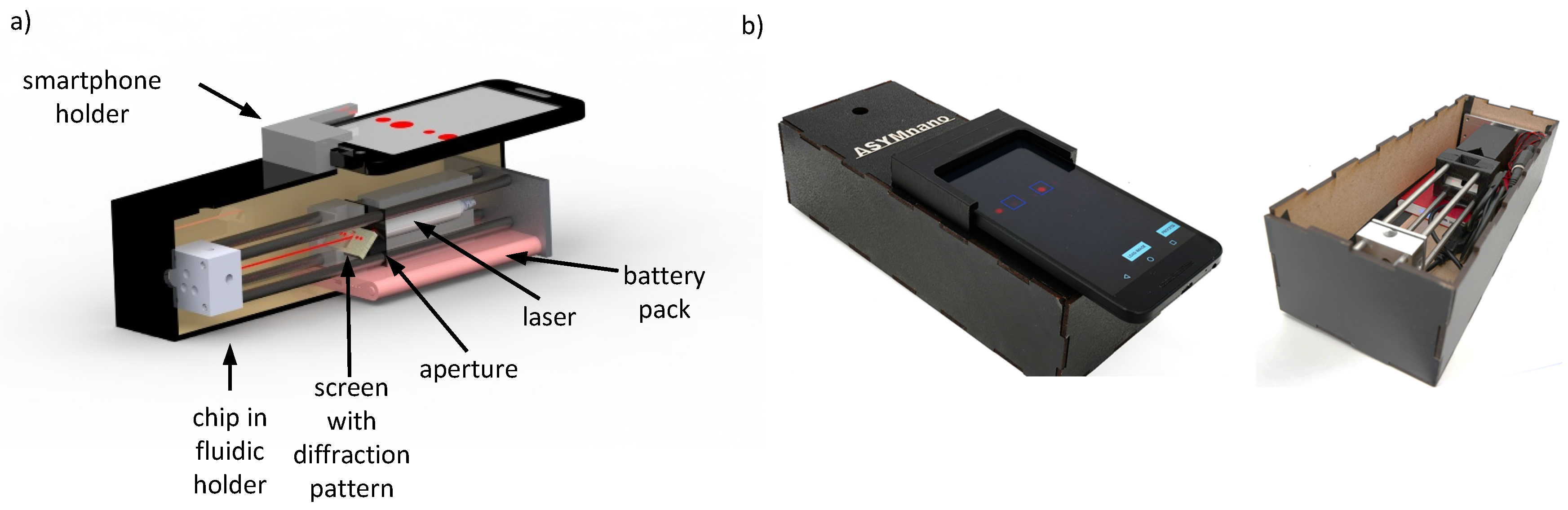
3.2. Mobile Optical Setup
3.3. Smartphone Application
4. Materials and Methods
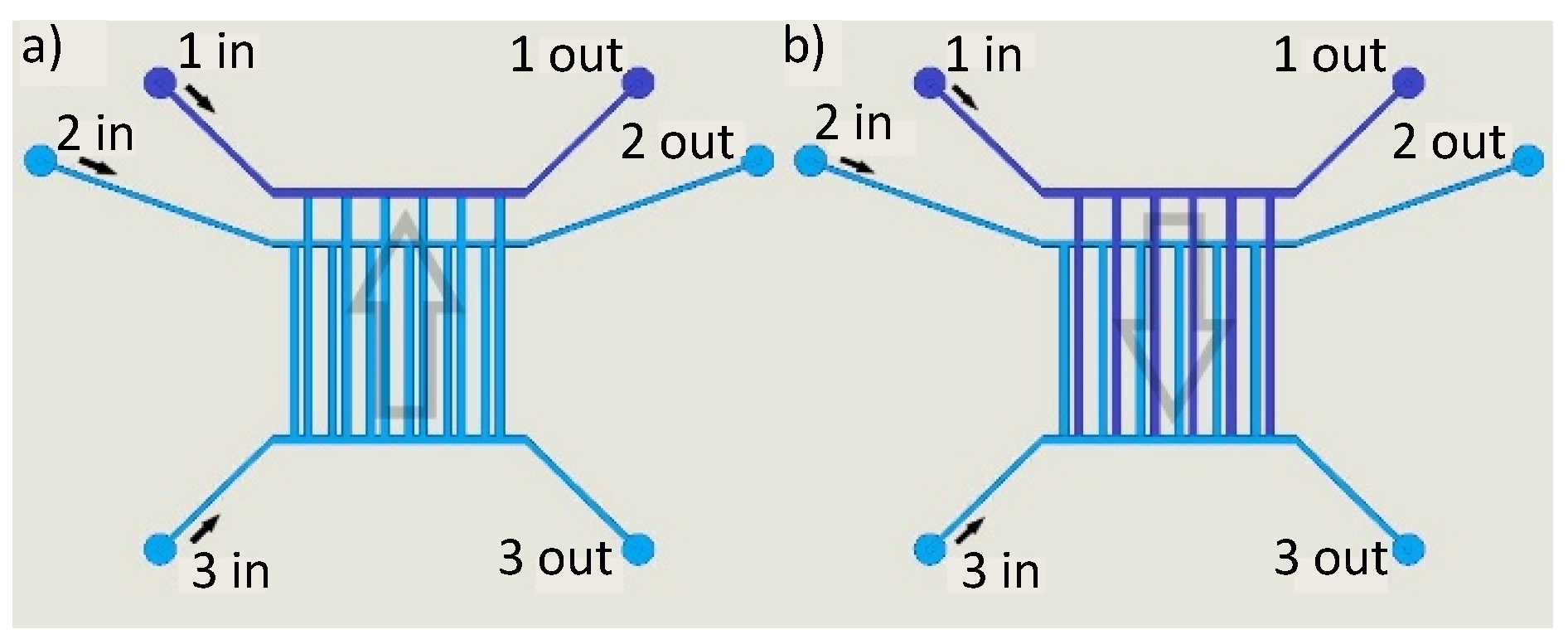
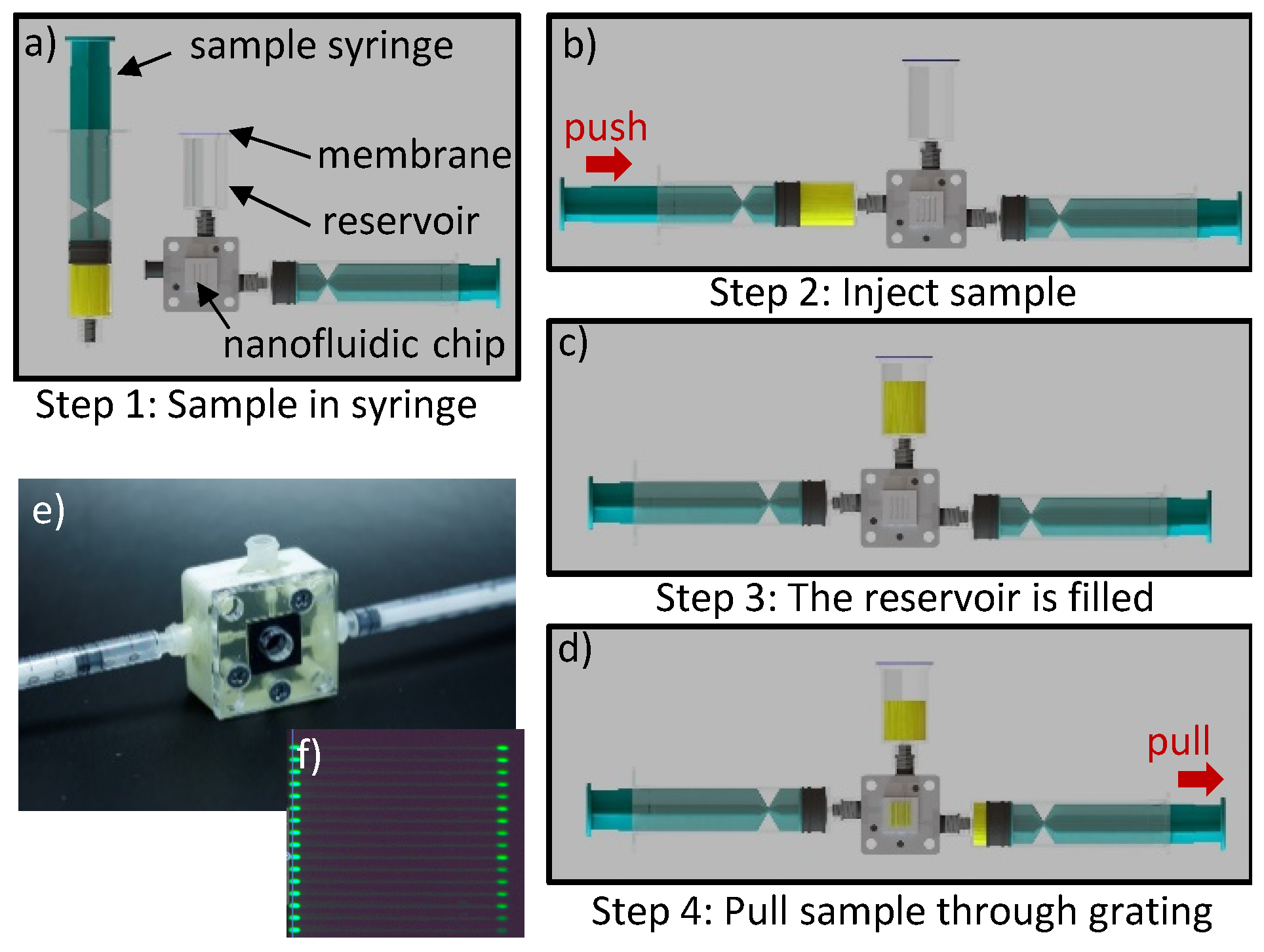
Fluidics
Test Solutions
5. Results and Discussion
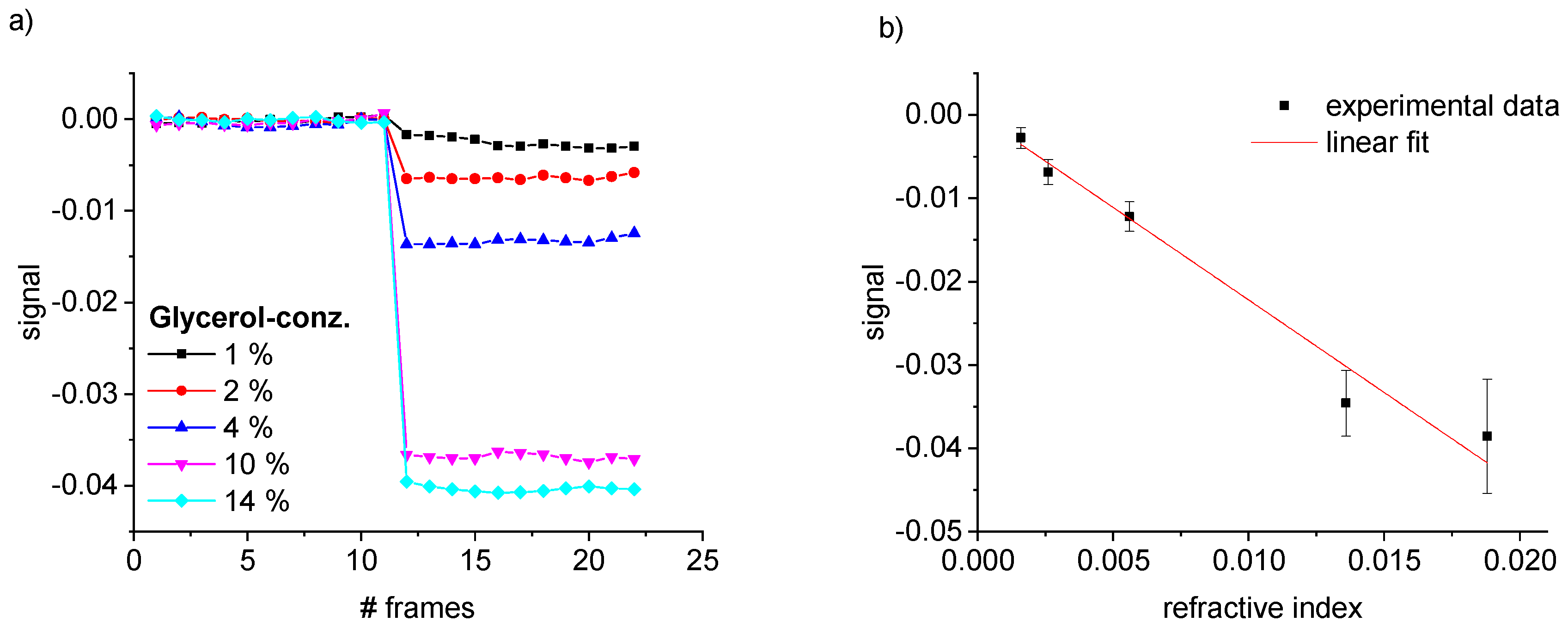
5.1. Characterization
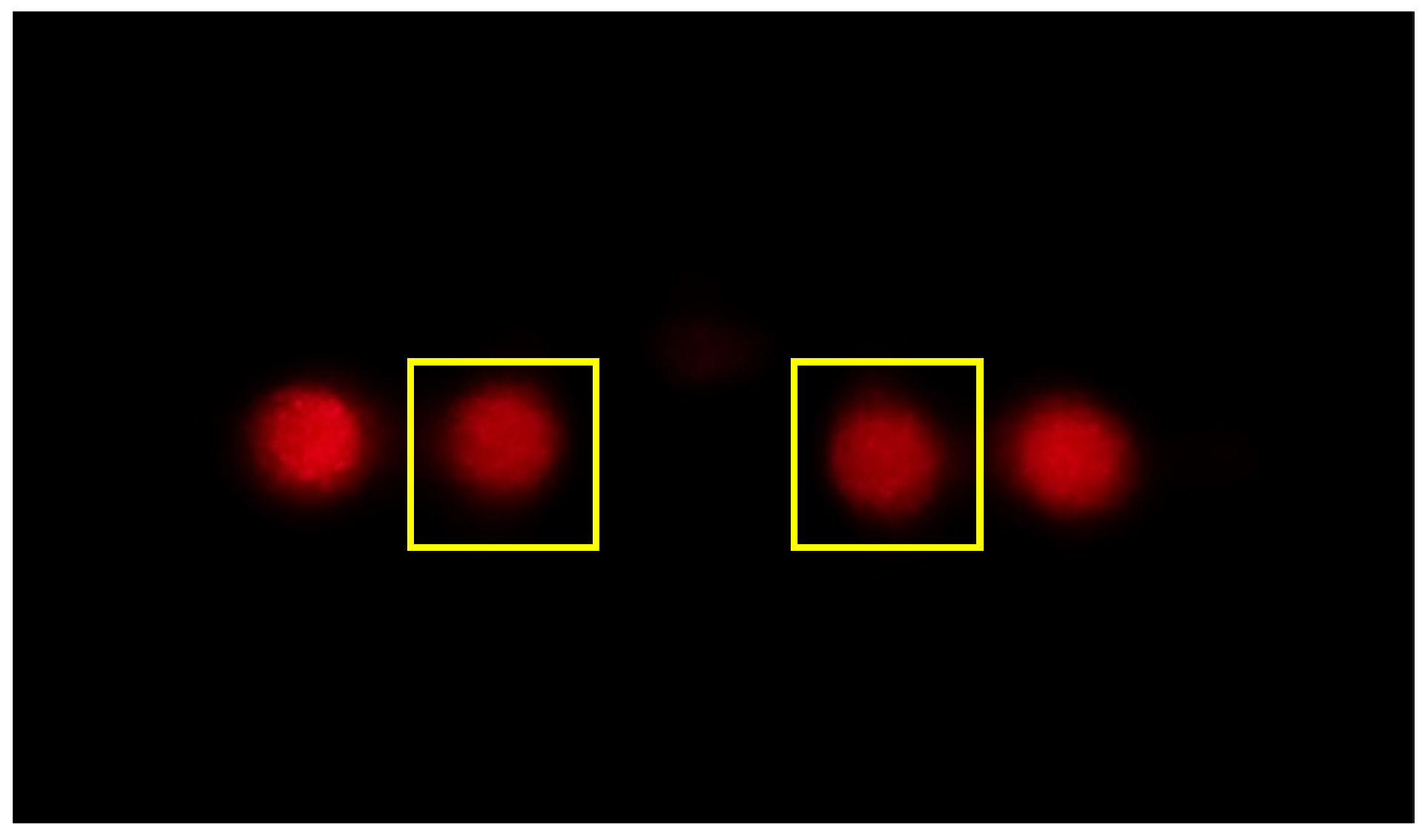
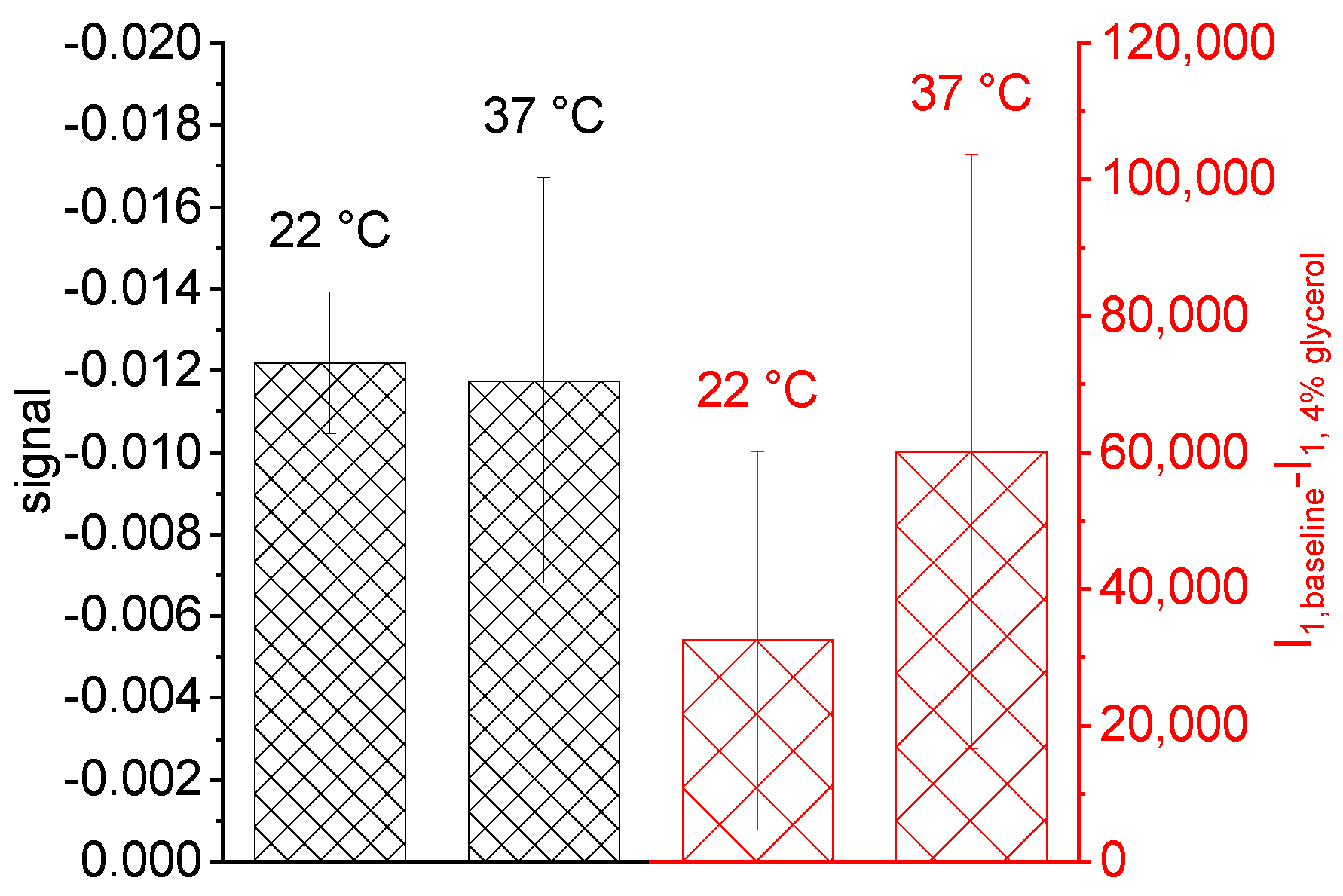
5.2. Common Mode Rejection
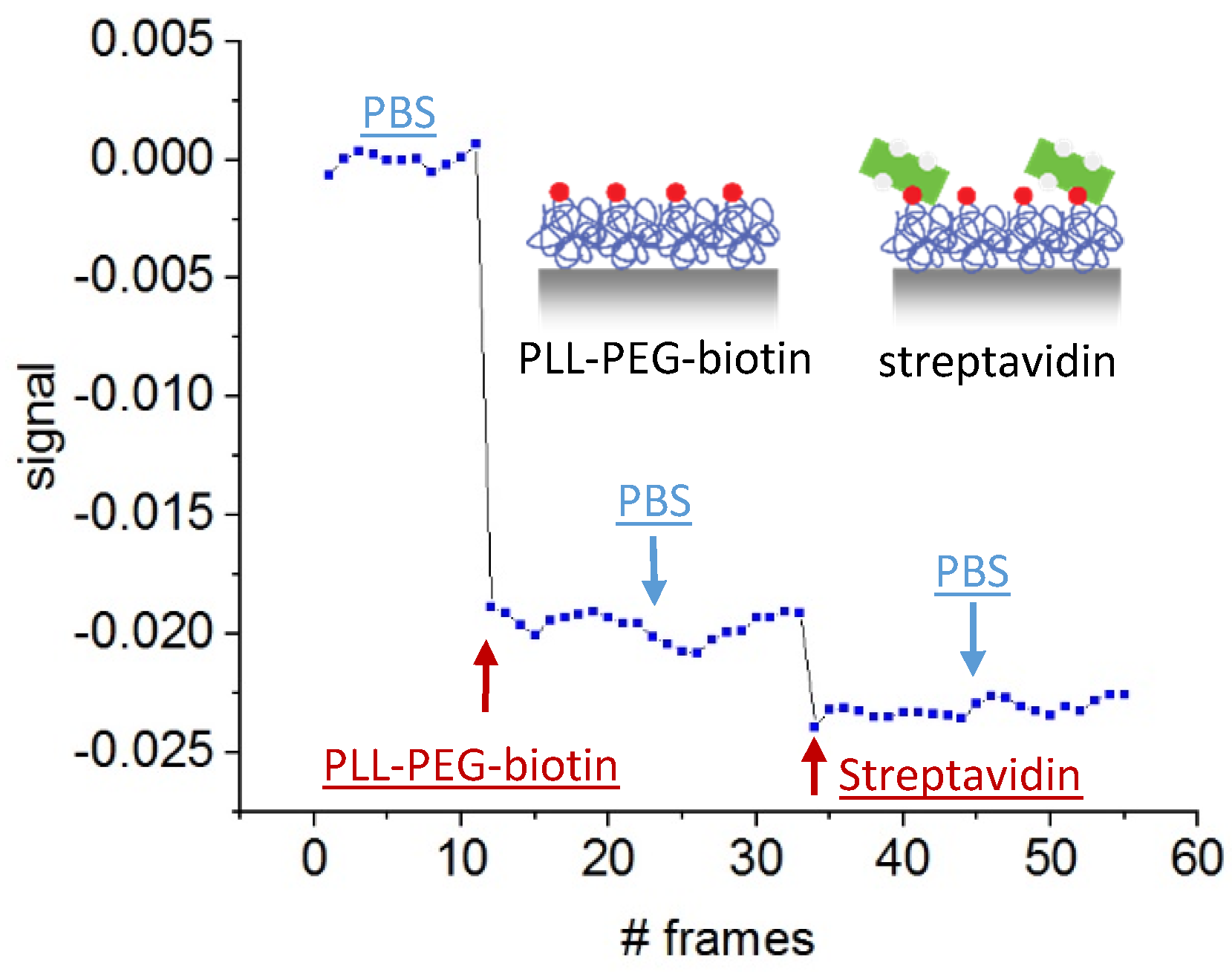
5.3. Protein Detection
5.4. Concept for POC Loading
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ABS | acrylonitrile butadiene styrene |
| CAD | computer-aided design |
| DI | deionized water |
| h | nanochannel depth |
| I | integrated intensity of a maximum |
| k | wave number |
| l | asymmetry |
| ISO | international organization for standardization |
| wavelength | |
| n | refractive index |
| m | maximum mode |
| P | period of the optical grating |
| PBS | phosphate buffered saline |
| optical phaseshift | |
| PEG | polyethylenglycol |
| PLL | poly-l-lysine |
| RIU | refractive index unit |
| S | signal |
| SPR | surface plasmon resonance |
| T | temperature |
| w | nanochannel width |
References
- Luppa, P.B.; Bietenbeck, A.; Beaudoin, C.; Giannetti, A. Clinically relevant analytical techniques, organizational concepts for application and future perspectives of point-of-care testing. Biotechnol. Adv. 2016, 34, 139–160. [Google Scholar] [CrossRef] [PubMed]
- Luppa, P.B.; Junker, R. POCT—Patientennahe Labordiagnostik; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar] [CrossRef]
- Business of Apps. Prognose zur Anzahl der Smartphone-Nutzer Weltweit von 2012 Bis 2021. Available online: https://de.statista.com/statistik/daten/studie/309656/umfrage/prognose-zur-anzahl-der-smartphone-nutzer-weltweit/ (accessed on 11 March 2019).
- Hernández-Neuta, I.; Neumann, F.; Brightmeyer, J.; Ba Tis, T.; Madaboosi, N.; Wei, Q.; Ozcan, A.; Nilsson, M. Smartphone-based clinical diagnostics: Towards democratization of evidence-based health care. J. Intern. Med. 2019, 285, 19–39. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Huang, X.; Guo, J.; Ma, X. Automatic smartphone-based microfluidic biosensor system at the point of care. Biosens. Bioelectron. 2018, 110, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Thies, J.W.; Thürmann, B.; Vierheller, A.; Dietzel, A. Particle-Based Microfluidic Quartz Crystal Microbalance (QCM) Biosensing Utilizing Mass Amplification and Magnetic Bead Convection. Micromachines 2018, 9, 194. [Google Scholar] [CrossRef] [PubMed]
- Pandey, C.M.; Augustine, S.; Kumar, S.; Kumar, S.; Nara, S.; Srivastava, S.; Malhotra, B.D. Microfluidics Based Point-of-Care Diagnostics. Biotechnol. J. 2018, 13. [Google Scholar] [CrossRef]
- Fan, X.; White, I.M.; Shopova, S.I.; Zhu, H.; Suter, J.D.; Sun, Y. Sensitive optical biosensors for unlabeled targets: A review. Anal. Chim. Acta 2008, 620, 8–26. [Google Scholar] [CrossRef] [PubMed]
- Myers, F.B.; Lee, L.P. Innovations in optical microfluidic technologies for point-of-care diagnostics. Lab Chip 2008, 8, 2015–2031. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Park, J.; Kang, S.; Kim, M. Surface plasmon resonance: A versatile technique for biosensor applications. Sensors 2015, 15, 10481–10510. [Google Scholar] [CrossRef] [PubMed]
- Slavík, R.; Homola, J. Ultrahigh resolution long range surface plasmon-based sensor. Sens. Actuators B Chem. 2007, 123, 10–12. [Google Scholar] [CrossRef]
- Metrohm. Autolab ESPRIT Data Acquisition 4.4 User Manual SPR. 2017. Available online: http://www.ecochemie.nl/download/Manuals/ESPRIT_user_manual_4.4.0-2.pdf (accessed on 27 June 2017).
- Ymeti, A.; Kanger, J.S.; Greve, J.; Lambeck, P.V.; Wijn, R.; Heideman, R.G. Realization and characterization of a four-channel integrated optical Young interferometer. In Proceedings of the 12th International Conference on Solid-State Sensors, Actuators and Microsystems, Digest of Technical Papers, Boston, MA, USA, 8–12 June 2003; pp. 1192–1196. [Google Scholar] [CrossRef]
- Purr, F.; Bassu, M.; Lowe, R.D.; Thürmann, B.; Dietzel, A.; Burg, T.P. Asymmetric nanofluidic grating detector for differential refractive index measurement and biosensing. Lab Chip 2017, 17, 4265–4272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Chang, T.W.; Lin, G.; Gartia, M.R.; Liu, G.L. Self-Referenced Smartphone-Based Nanoplasmonic Imaging Platform for Colorimetric Biochemical Sensing. Anal. Chem. 2017, 89, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Lertvachirapaiboon, C.; Baba, A.; Shinbo, K.; Kato, K. A smartphone-based surface plasmon resonance platform. Anal. Methods 2018, 10, 4732–4740. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Q.; Chen, S.; Cheng, F.; Wang, H.; Peng, W. Surface Plasmon Resonance Biosensor Based on Smart Phone Platforms. Sci. Rep. 2015, 5, 12864. [Google Scholar] [CrossRef] [Green Version]
- Park, J. An Optimized Colorimetric Readout Method for Lateral Flow Immunoassays. Sensors 2018, 18, 4084. [Google Scholar] [CrossRef]
- Zhu, H.; Mavandadi, S.; Coskun, A.F.; Yaglidere, O.; Ozcan, A. Optofluidic fluorescent imaging cytometry on a cell phone. Anal. Chem. 2011, 83, 6641–6647. [Google Scholar] [CrossRef] [PubMed]
- Coskun, A.F.; Topkaya, S.N.; Yetisen, A.K.; Cetin, A.E. Portable Multiplex Optical Assays. Adv. Opt. Mater. 2019, 7, 1801109. [Google Scholar] [CrossRef]
- Dutta, S. Point of care sensing and biosensing using ambient light sensor of smartphone: Critical review. TrAC Trends Anal. Chem. 2019, 110, 393–400. [Google Scholar] [CrossRef]
- Huang, X.; Xu, D.; Chen, J.; Liu, J.; Li, Y.; Song, J.; Ma, X.; Guo, J. Smartphone-based analytical biosensors. Analyst 2018, 143, 5339–5351. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.K.; Mudanyali, O.; Schneider, E.M.; Zengerle, R.; Ozcan, A. Cellphone-based devices for bioanalytical sciences. Anal. Bioanal. Chem. 2014, 406, 3263–3277. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Akay, A.; Wei, H.; Wang, S.; Pingguan-Murphy, B.; Erlandsson, B.E.; Li, X.; Lee, W.; Hu, J.; Wang, L.; et al. Advances in Smartphone-Based Point-of-Care Diagnostics. Proc. IEEE 2015, 103, 236–247. [Google Scholar] [CrossRef]
- Zarei, M. Portable biosensing devices for point-of-care diagnostics: Recent developments and applications. TrAC Trends Anal. Chem. 2017, 91, 26–41. [Google Scholar] [CrossRef]
- Geng, Z.; Zhang, X.; Fan, Z.; Lv, X.; Su, Y.; Chen, H. Recent Progress in Optical Biosensors Based on Smartphone Platforms. Sensors 2017, 17, 2449. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Nath, P. Design of a 3D printed compact interferometric system and required phone application for small angular measurements. Rev. Sci. Instrum. 2018, 89, 103111. [Google Scholar] [CrossRef]
- Long, K.D.; Woodburn, E.V.; Le, H.M.; Shah, U.K.; Lumetta, S.S.; Cunningham, B.T. Multimode smartphone biosensing: The transmission, reflection, and intensity spectral (TRI)-analyzer. Lab Chip 2017, 17, 3246–3257. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.J.; Chang, Y.C.; Ge, X.; Osmanson, A.T.; Du, D.; Lin, Y.; Li, L. Smartphone Optosensing Platform Using a DVD Grating to Detect Neurotoxins. ACS Sens. 2016, 1, 366–373. [Google Scholar] [CrossRef]
- Wang, L.J.; Chang, Y.C.; Sun, R.; Li, L. A multichannel smartphone optical biosensor for high-throughput point-of-care diagnostics. Biosens. Bioelectron. 2017, 87, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Gallegos, D.; Long, K.D.; Yu, H.; Clark, P.P.; Lin, Y.; George, S.; Nath, P.; Cunningham, B.T. Label-free biodetection using a smartphone. Lab Chip 2013, 13, 2124–2132. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Serrano, L.J.; P Torres, J.; Valencia, A. A 3D Printed Toolbox for Opto-Mechanical Components. PLoS ONE 2017, 12, e0169832. [Google Scholar] [CrossRef] [PubMed]
- Bashkatov, A.N.; Genina, E.A. Water refractive index in dependence on temperature and wavelength: A simple approximation. Proc. SPIE 2003, 5068, 393–395. [Google Scholar] [CrossRef]



| Step | Channels 1 and 2, in/out | Channel 3, in/out |
|---|---|---|
| wash out | 0.2/0.1 bar | 0.5/0.3 bar |
| sample | 0.5/0.3 bar | 0.2/0.1 bar |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purr, F.; Eckardt, M.-F.; Kieserling, J.; Gronwald, P.-L.; Burg, T.P.; Dietzel, A. Robust Smartphone Assisted Biosensing Based on Asymmetric Nanofluidic Grating Interferometry. Sensors 2019, 19, 2065. https://doi.org/10.3390/s19092065
Purr F, Eckardt M-F, Kieserling J, Gronwald P-L, Burg TP, Dietzel A. Robust Smartphone Assisted Biosensing Based on Asymmetric Nanofluidic Grating Interferometry. Sensors. 2019; 19(9):2065. https://doi.org/10.3390/s19092065
Chicago/Turabian StylePurr, Foelke, Max-Frederik Eckardt, Jonas Kieserling, Paul-Luis Gronwald, Thomas P. Burg, and Andreas Dietzel. 2019. "Robust Smartphone Assisted Biosensing Based on Asymmetric Nanofluidic Grating Interferometry" Sensors 19, no. 9: 2065. https://doi.org/10.3390/s19092065
APA StylePurr, F., Eckardt, M.-F., Kieserling, J., Gronwald, P.-L., Burg, T. P., & Dietzel, A. (2019). Robust Smartphone Assisted Biosensing Based on Asymmetric Nanofluidic Grating Interferometry. Sensors, 19(9), 2065. https://doi.org/10.3390/s19092065







