Development of SPR Imaging-Impedance Sensor for Multi-Parametric Living Cell Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cells Lines
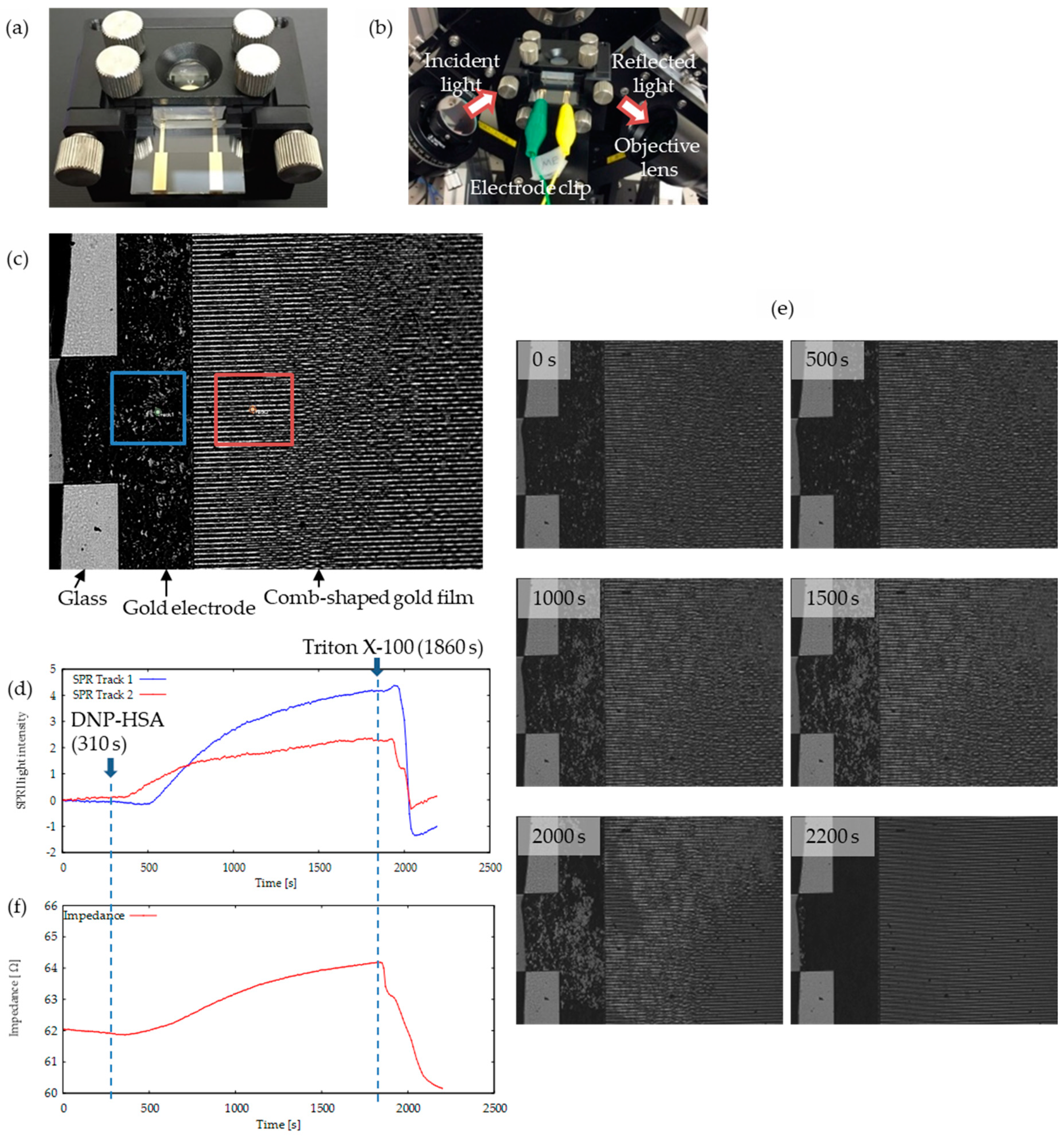
2.3. Instrument for SPR Imaging (SPRI)-Impedance Sensor
2.4. Measurement of Impedance
2.5. Staining of Actin Cytoskeleton in RBL-2H3 Cells
2.6. Assays of RBL-2H3 Cell Degranulation (Release of β-Hexosaminidase and Histamine)
3. Results
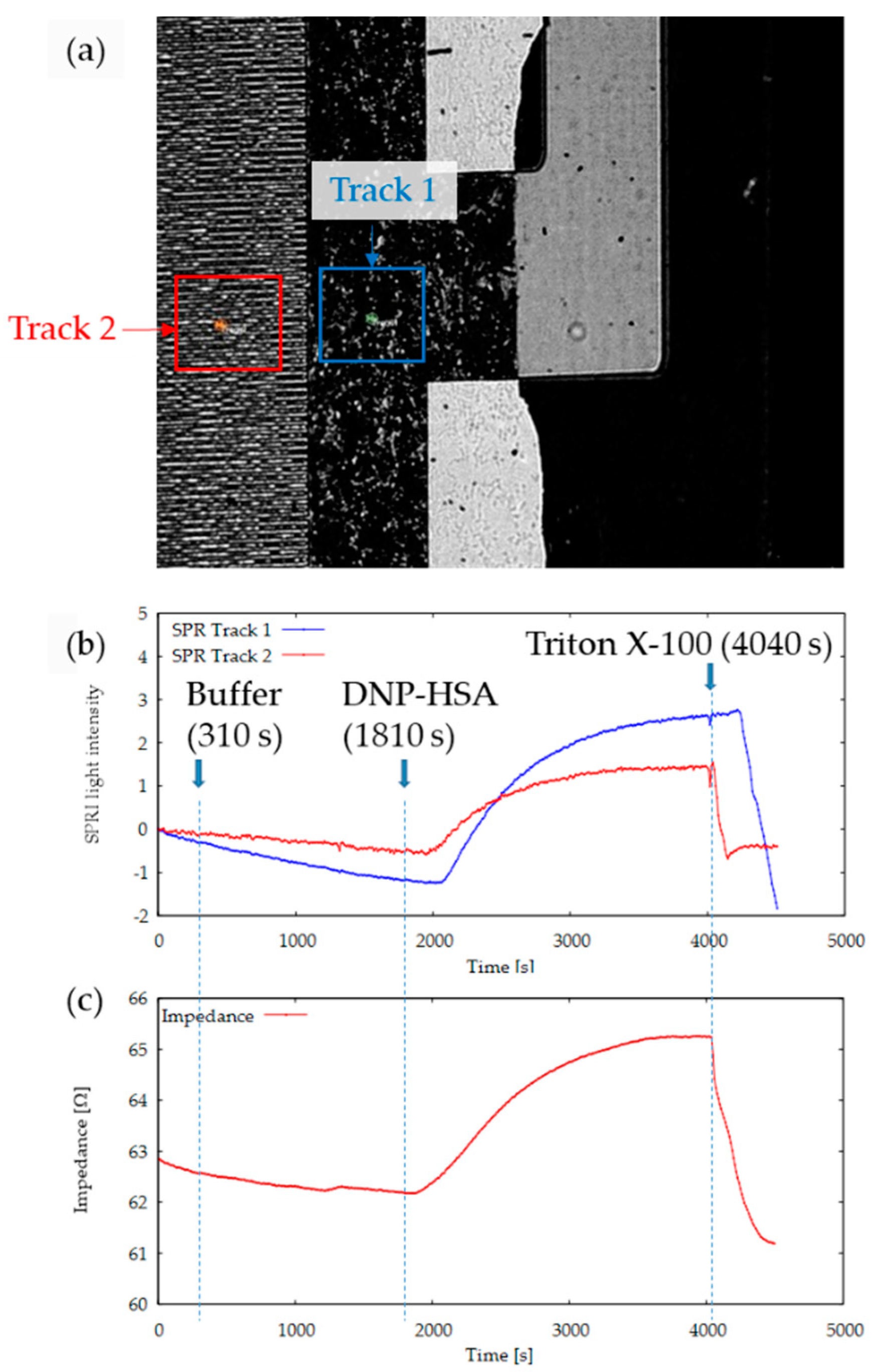
3.1. Development of SPRI-IMP Sensor and Sensor Chip
3.2. Activation of RBL-2H3 Cells Cultured on Comb-Shaped Electrodes
3.3. Frequency Characteristics of Impedance of RBL-2H3 with or without Stimulation
3.4. Simultaneous Monitoring of Living Cell-Derived RI and Impedance on an SPRI-Impedance Chip
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

References
- Marx, K.A.; Zouh, T.; Montrone, A.; Schulze, H.; Braunhut, S.J. A quartz crystal microbalance cell biosensor: Detection of microtubule alterations in living cells at nM nocodazole concentrations. Biosens. Bioelectron. 2001, 16, 773–782. [Google Scholar] [CrossRef]
- Saitakis, M.; Gizeli, E. Acoutic sensors as a biophysical tool for probing cell attachment and cell/surface interactions. Cell Mol. Life Sci. 2012, 69, 357–371. [Google Scholar] [CrossRef]
- Yang, H.; Honda, M.; Saito, A.; Kajisa, T.; Yanase, Y.; Sakata, T. Nonoptical Detection of Allergic Response with a Cell-Coupled Gate Field-Effect Transistor. Anal. Chem. 2017, 89, 12918–12923. [Google Scholar] [CrossRef] [PubMed]
- Hide, M.; Tsutsui, T.; Sato, H.; Nishimura, T.; Morimoto, K.; Yamamoto, S.; Yoshizato, K. Real-Time Analysis of Ligand-Induced Cell Surface and Intracellular Reactions of Living Mast Cells Using a Surface Plasmon Resonance-Based Biosensor. Anal. Biochem. 2002, 302, 28–37. [Google Scholar] [CrossRef]
- Fang, Y.; Ferrie, A.M.; Fontaine, N.H.; Yuen, P.K. Characteristics of Dynamic Mass Redistribution of Epidermal Growth Factor Receptor Signaling in Living Cells Measured with Label-Free Optical Biosensors. Anal. Chem. 2005, 77, 5720–5725. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.L.; Nam, J.O.; Jean, C.; Lawson, C.; Walsh, C.T.; Goka, E.; Lim, S.T.; Tomar, A.; Tancioni, I.; Uryu, S.; et al. VEGF-induced vascular permeability is mediated by FAK. Dev. Cell. 2012, 22, 146–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urcan, E.; Haertel, U.; Styllou, M.; Hickel, R.; Scherthan, H.; Reichl, F.X. Real-time xCELLigence impedance analysis of the cytotoxity of dental composite components on human gingival fibroblasts. Dent. Mater. 2010, 26, 51–58. [Google Scholar] [CrossRef]
- Guan, N.; Deng, J.; Li, T.; Xu, X.; Irelan, J.T.; Wang, M.W. Label-free monitoring of T cell activation by the impedance-based xCELLigence system. Mol. Biosyst. 2013, 9, 1035–1043. [Google Scholar] [CrossRef]
- Yanase, Y.; Suzuki, H.; Tsutsui, T.; Hiragun, T.; Kameyoshi, Y.; Hide, M. The SPR signal in living cells reflects changes other than the area of adhesion and the formation of cell constructions. Biosens. Bioelectron. 2007, 22, 1081–1086. [Google Scholar] [CrossRef]
- Yanase, Y.; Suzuki, H.; Tsutsui, T.; Uechi, I.; Hiragun, T.; Mihara, S.; Hide, M. Living cell positioning on the surface of gold film for SPR analysis. Biosens. Bioelectron. 2007, 23, 562–567. [Google Scholar] [CrossRef]
- Suzuki, H.; Yanase, Y.; Tsutsui, T.; Ishii, K.; Hiragun, T.; Hide, M. Applying surface plasmon resonance to monitor the IgE-mediated activation of human basophils. Allergol. Int. 2008, 57, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Hiragun, T.; Tsutsui, T.; Yanase, Y.; Suzuki, H.; Hide, M. Surface plasmon resonance biosensor detects the downstream events of active PKCbeta in antigen-stimulated mast cells. Biosens. Bioelectron. 2008, 23, 1652–1658. [Google Scholar] [CrossRef] [PubMed]
- Hiragun, T.; Yanase, Y.; Kose, K.; Kawaguchi, T.; Uchida, K.; Tanaka, S.; Hide, M. Surface plasmon resonance-biosensor detects the diversity of responses against epidermal growth factor in various carcinoma cell lines. Biosens. Bioelectron. 2012, 32, 202–207. [Google Scholar] [CrossRef]
- Yanase, Y.; Araki, A.; Suzuki, H.; Tsutsui, T.; Kimura, T.; Okamoto, K.; Nakatani, T.; Hiragun, T.; Hide, M. Development of an optical fiber SPR sensor for living cell activation. Biosens. Bioelectron. 2010, 25, 1244–1247. [Google Scholar] [CrossRef]
- Yanase, Y.; Hiragun, T.; Kaneko, S.; Gould, H.J.; Greaves, M.W.; Hide, M. Detection of refractive index changes in individual living cells by means of surface plasmon resonance imaging. Biosens. Bioelectron. 2010, 26, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Yanase, Y.; Hiragun, T.; Yanase, T.; Kawaguchi, T.; Ishii, K.; Hide, M. Evaluation of peripheral blood basophil activation by means of surface plasmon resonance imaging. Biosens. Bioelectron. 2012, 32, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Yanase, Y.; Hiragun, T.; Ishii, K.; Kawaguchi, T.; Yanase, T.; Kawai, M.; Sakamoto, K.; Hide, M. Surface Plasmon Resonance for Cell-Based Clinical Diagnosis. Sensors 2014, 14, 4948–4959. [Google Scholar] [CrossRef] [Green Version]
- Yanase, Y.; Hiragun, T.; Yanase, T.; Kawaguchi, T.; Ishii, K.; Kumazaki, N.; Obara, T.; Hide, M. Clinical diagnosis of type I allergy by means of SPR imaging with less than a microliter of peripheral blood. Sens. Bio-Sens. Res. 2014, 2, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Yanase, Y.; Sakamoto, K.; Kobayashi, K.; Hide, M. Diagnosis of immediate-type allergy using surface plasmon resonance. Opt. Mater. Express. 2016, 6, 1339–1348. [Google Scholar] [CrossRef]
- Irifuku, R.; Yanase, Y.; Kawaguchi, T.; Ishii, K.; Takahagi, S.; Hide, M. Impedance-Based Living Cell Analysis for Clinical Diagnosis of Type I Allergy. Sensors 2017, 17, 2503. [Google Scholar] [CrossRef]
- Yanase, Y.; Morioke, S.; Iwamoto, K.; Takahagi, S.; Uchida, K.; Kawaguchi, T.; Ishii, K.; Hide, I.; Hide, M. Histamine and Toll-like receptor ligands synergistically induce endothelial cell gap formation by the extrinsic coagulating pathway. J. Allergy Clin. Immunol. 2018, 141, 1115–1118. [Google Scholar] [CrossRef] [PubMed]
- Michaelis, S.; Wegener, J.; Robelek, R. Label-free monitoring of cell-based assays: Combining impedance analysis with SPR for multiparametric cell profiling. Biosens. Bioelectron. 2013, 49, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Chen, X.; Wei, H.T.; Li, H.; Sun, J.H.; Cai, H.Y.; Chen, J.L.; Cui, D.F. An electrochemical surface plasmon resonance imaging system targeting cell analysis. Rev. SciInstrum. 2013, 84. [Google Scholar] [CrossRef]
- Peng, Z.; Lu, J.; Zhang, L.; Liu, Y.; Li, J. Label-free imaging of epidermal growth factor receptor-induced response in single living cells. Analyst 2018, 143, 5264–5270. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, H.; Sakai, Y.; Mir, T.A. Real-time monitoring of intracellular signal transduction in PC12 cells by two-dimensional surface plasmon resonance imager. Anal. Biochem. 2013, 441, 185–189. [Google Scholar] [CrossRef]
- Peterson, A.W.; Halter, M.; Tona, A.; Bhadriraju, K.; Plant, A.L. Surface plasmon resonance imaging of cells and surface-associated fibronectin. BMC Cell Biol. 2009. [Google Scholar] [CrossRef] [PubMed]
- El Hasni, A.; Schmitz, C.; Bui-Gobbels, K.; Braunig, P.; Jahnen-Dechent, W.; Schnakenberg, U. Electrical impedance spectroscopy of single cells in hydrodynamic traps. Sens. Actuators B Chem. 2017, 248, 419–429. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Zanganeh, S.; Alcbarnejad, E.; Salehi, F.; Abdolahad, M. Microfluidic device for label-free quantitation and distinction of bladder cancer cells from the blood cells using micro machined silicon based electrical approach; suitable in urinalysis assays. J. Pharm. Biomed. Anal. 2017, 134, 36–42. [Google Scholar] [CrossRef]
- Mamouni, J.; Yang, L.J. Interdigitated microelectrode-based microchip for electrical impedance spectroscopic study of oral cancer cells. Biomed. Microdevices 2011, 13, 1075–1088. [Google Scholar] [CrossRef]
- Evander, M.; Ricco, A.J.; Morser, J.; Kovacs, G.T.A.; Leung, L.L.K.; Giovangrandi, L. Microfluidic impedance cytometer for platelet analysis. Lab Chip 2013, 13, 722–729. [Google Scholar] [CrossRef]
- Chung, Y.-K.; Reboud, J.; Lee, K.C.; Lim, H.M.; Lim, P.Y.; Wang, K.Y.; Tang, K.C.; Ji, H.; Chen, Y. An electrical biosensor for the detection of circulating tumor cells. Biosens. Bioelectron. 2011, 26, 2520–2526. [Google Scholar] [CrossRef] [PubMed]
- Ribaut, C.; Reybier, K.; Reynes, O.; Launay, J.; Valentin, A.; Fabre, P.L.; Nepveu, F. Electrochemical impedance spectroscopy to study physiological changes affecting the red blood cell after invasion by malaria parasites. Biosens. Bioelectron. 2009, 24, 2721–2725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Xie, X.; Duan, Y.; Wang, L.; Cheng, Z.; Cheng, J. A review of impedance measurements of whole cells. Biosens. Bioelectron. 2016, 77, 824–836. [Google Scholar] [CrossRef]
- Cheung, K.C.; Di Berardino, M.; Schade-Kampmann, G.; Hebeisen, M.; Pierzchalski, A.; Bocsi, J.; Mittag, A.; Tarnok, A. Microfluidic impedance-based flow cytometry. Cytometry A 2010, 77, 648–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chabot, V.; Miron, Y.; Grandbois, M.; Charette, P.G. Long range surface plasmon resonance for increased sensitivity in living cell biosensing through greater probing depth. Sens. Actuators B Chem. 2012, 174, 94–101. [Google Scholar] [CrossRef]
- Vala, M.; Robelek, R.; Bocková, M.; Wegener, J.; Homola, J. Real-time label-free monitoring of the cellular response to osmotic stress using conventional and long-range surface plasmons. Biosens. Bioelectron. 2013, 40, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.W.; Halter, M.; Tona, A.; Plant, A.L. High resolution surface plasmon resonance imaging for single cells. BMC Cell Biol. 2014, 15, 35. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yanase, Y.; Yoshizaki, K.; Kimura, K.; Kawaguchi, T.; Hide, M.; Uno, S. Development of SPR Imaging-Impedance Sensor for Multi-Parametric Living Cell Analysis. Sensors 2019, 19, 2067. https://doi.org/10.3390/s19092067
Yanase Y, Yoshizaki K, Kimura K, Kawaguchi T, Hide M, Uno S. Development of SPR Imaging-Impedance Sensor for Multi-Parametric Living Cell Analysis. Sensors. 2019; 19(9):2067. https://doi.org/10.3390/s19092067
Chicago/Turabian StyleYanase, Yuhki, Kyohei Yoshizaki, Kaiken Kimura, Tomoko Kawaguchi, Michihiro Hide, and Shigeyasu Uno. 2019. "Development of SPR Imaging-Impedance Sensor for Multi-Parametric Living Cell Analysis" Sensors 19, no. 9: 2067. https://doi.org/10.3390/s19092067
APA StyleYanase, Y., Yoshizaki, K., Kimura, K., Kawaguchi, T., Hide, M., & Uno, S. (2019). Development of SPR Imaging-Impedance Sensor for Multi-Parametric Living Cell Analysis. Sensors, 19(9), 2067. https://doi.org/10.3390/s19092067




