Research on Key Techniques of Insect Flapping Onset Control Based on Electrical Stimulation
Abstract
:1. Introduction
2. Principle and Method
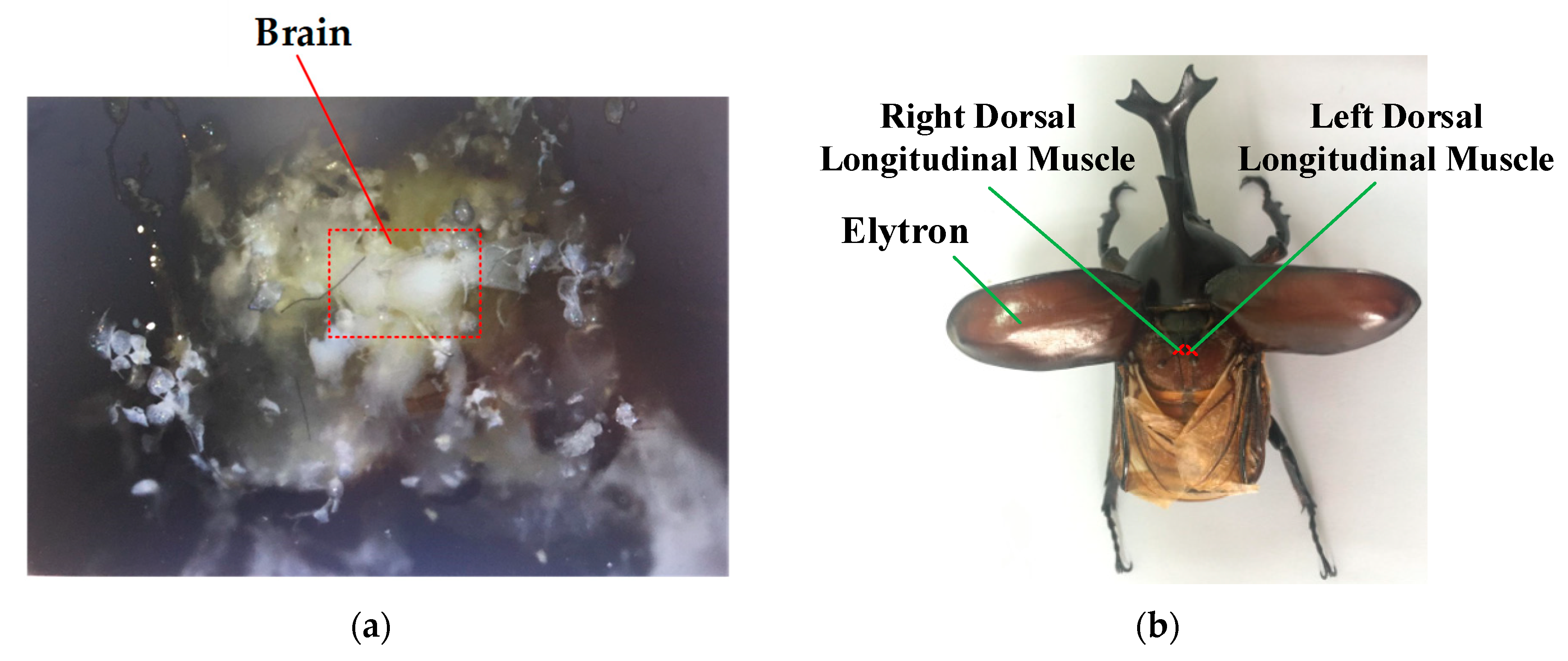
2.1. Structure Design
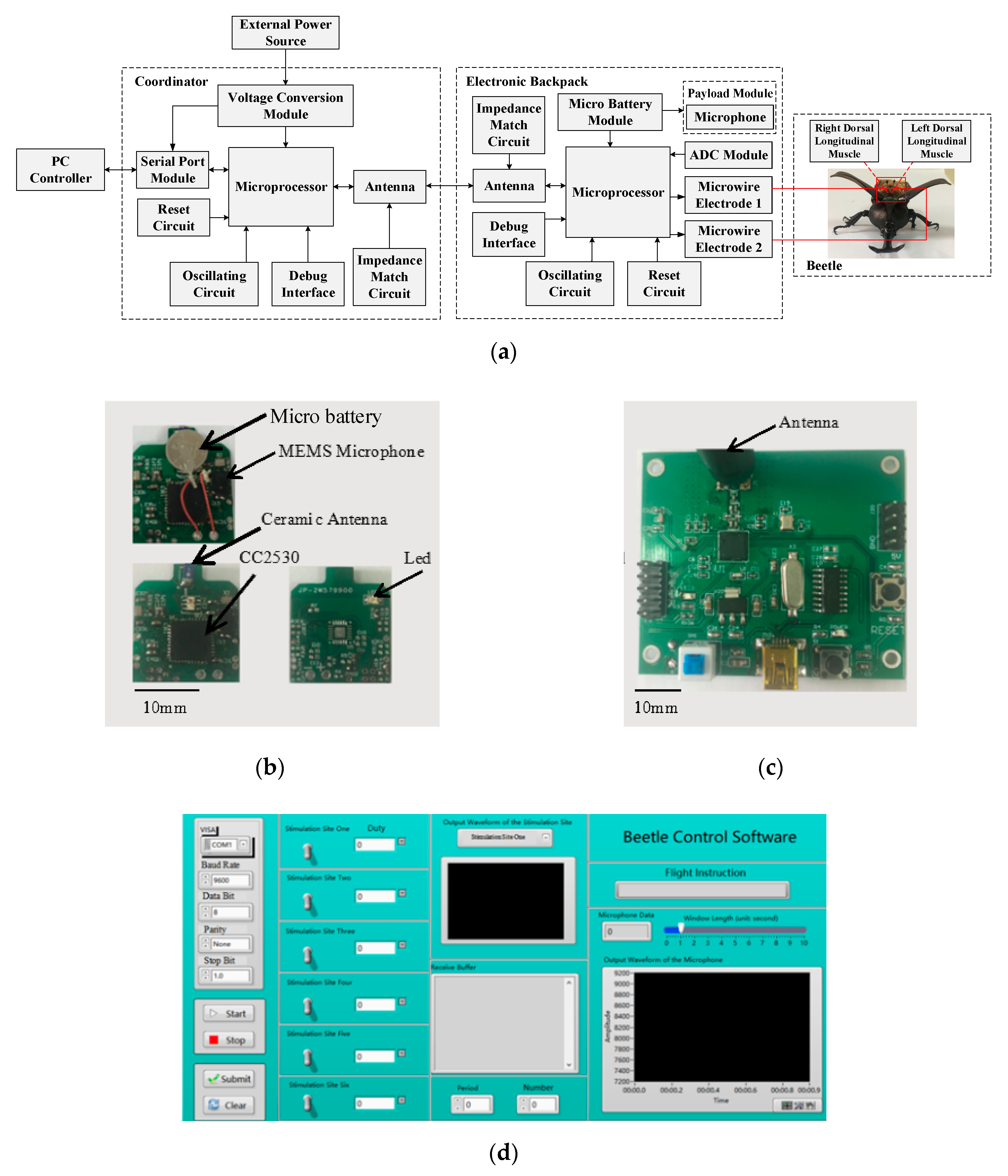
2.2. Design of Micro-Control System
2.2.1. Hardware Design of Micro-Control System
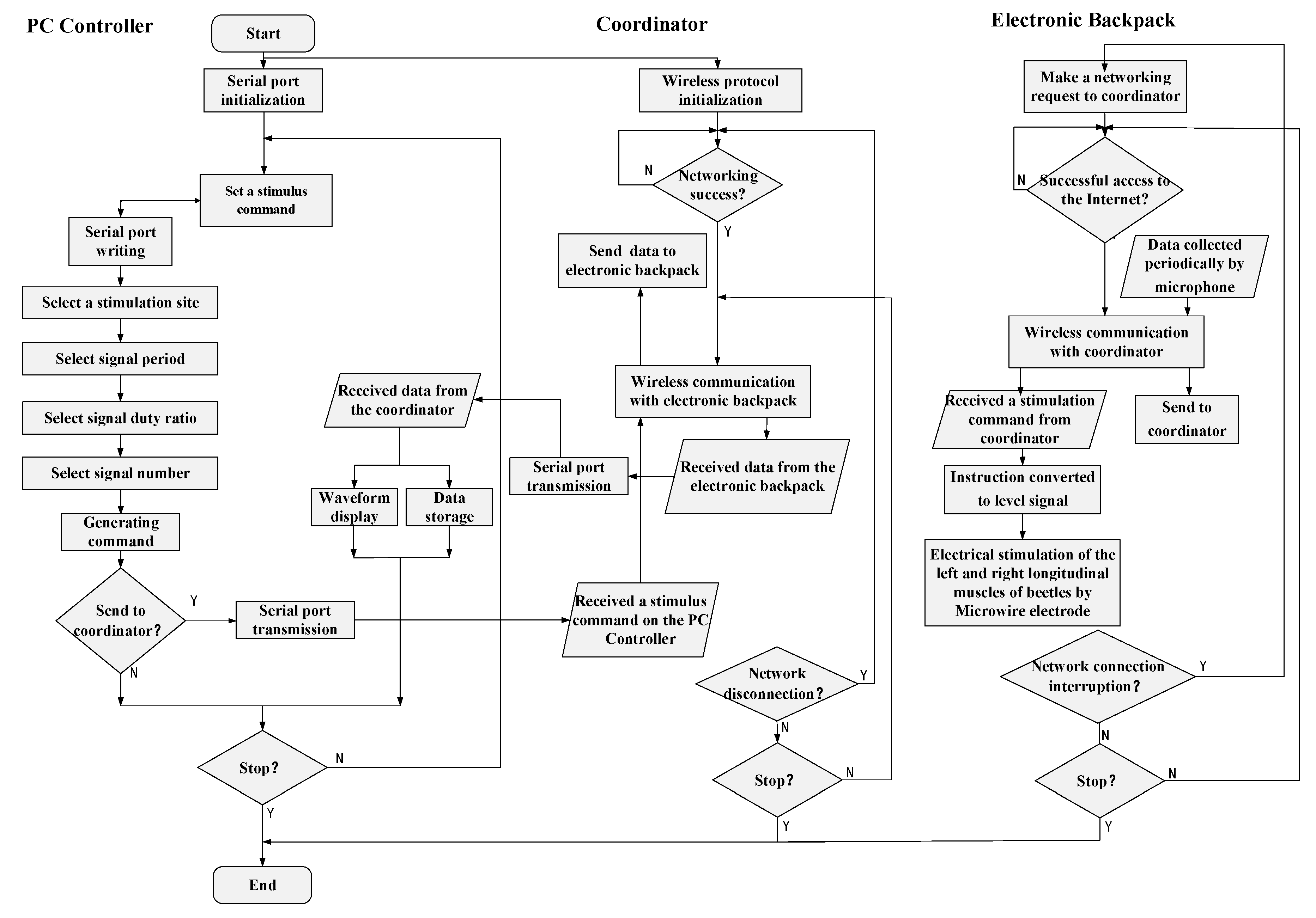
2.2.2. Control Algorithm for Micro-Control System
3. Experiments and Results
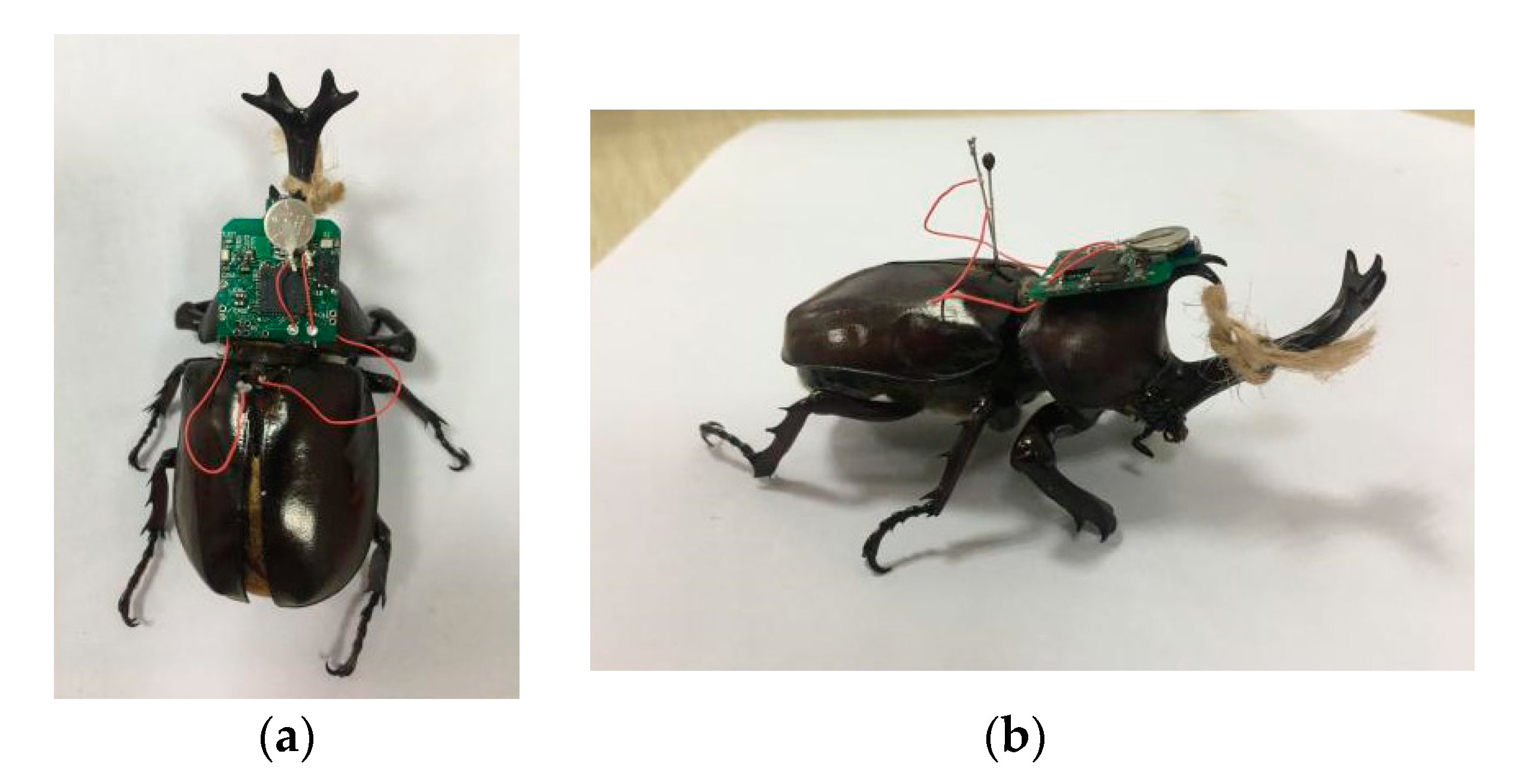
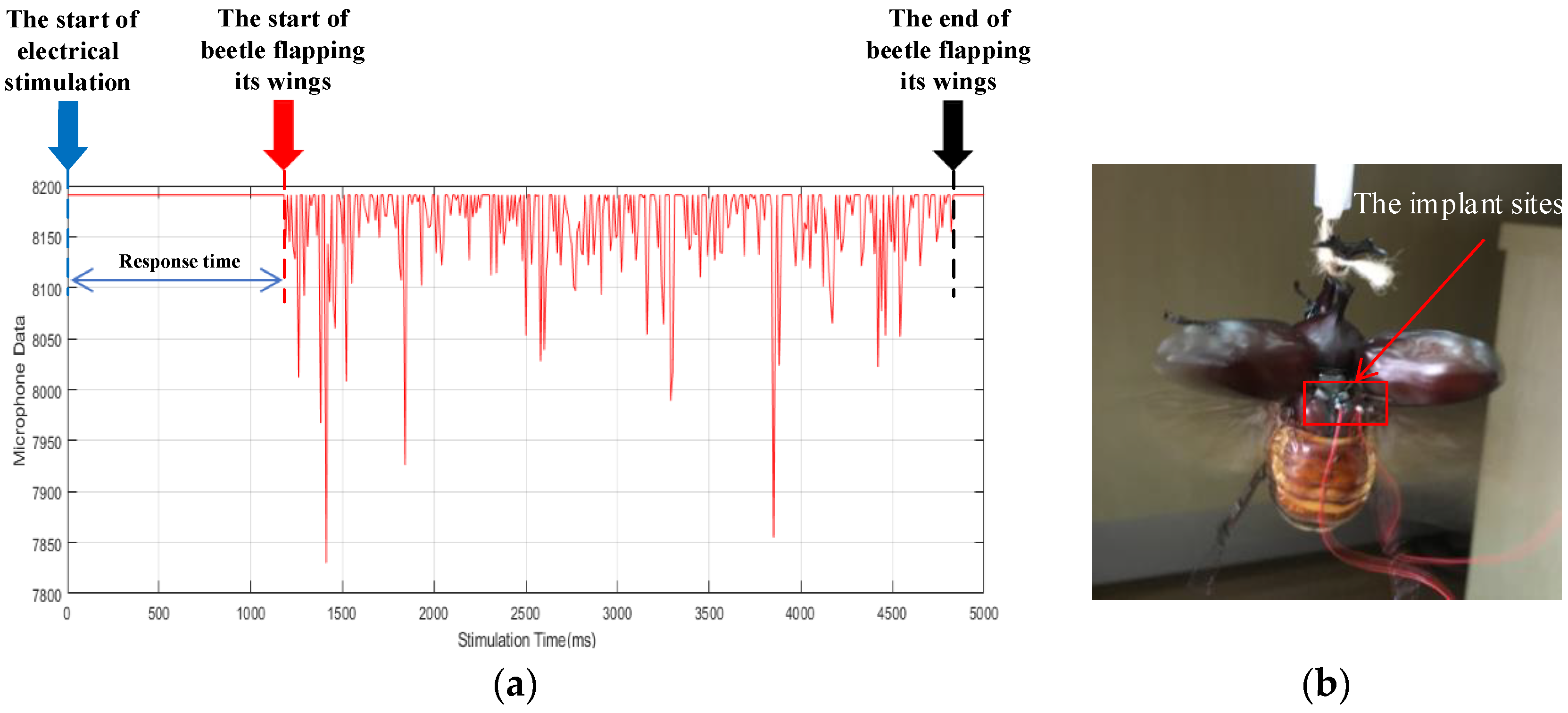
3.1. Experimental Preparation
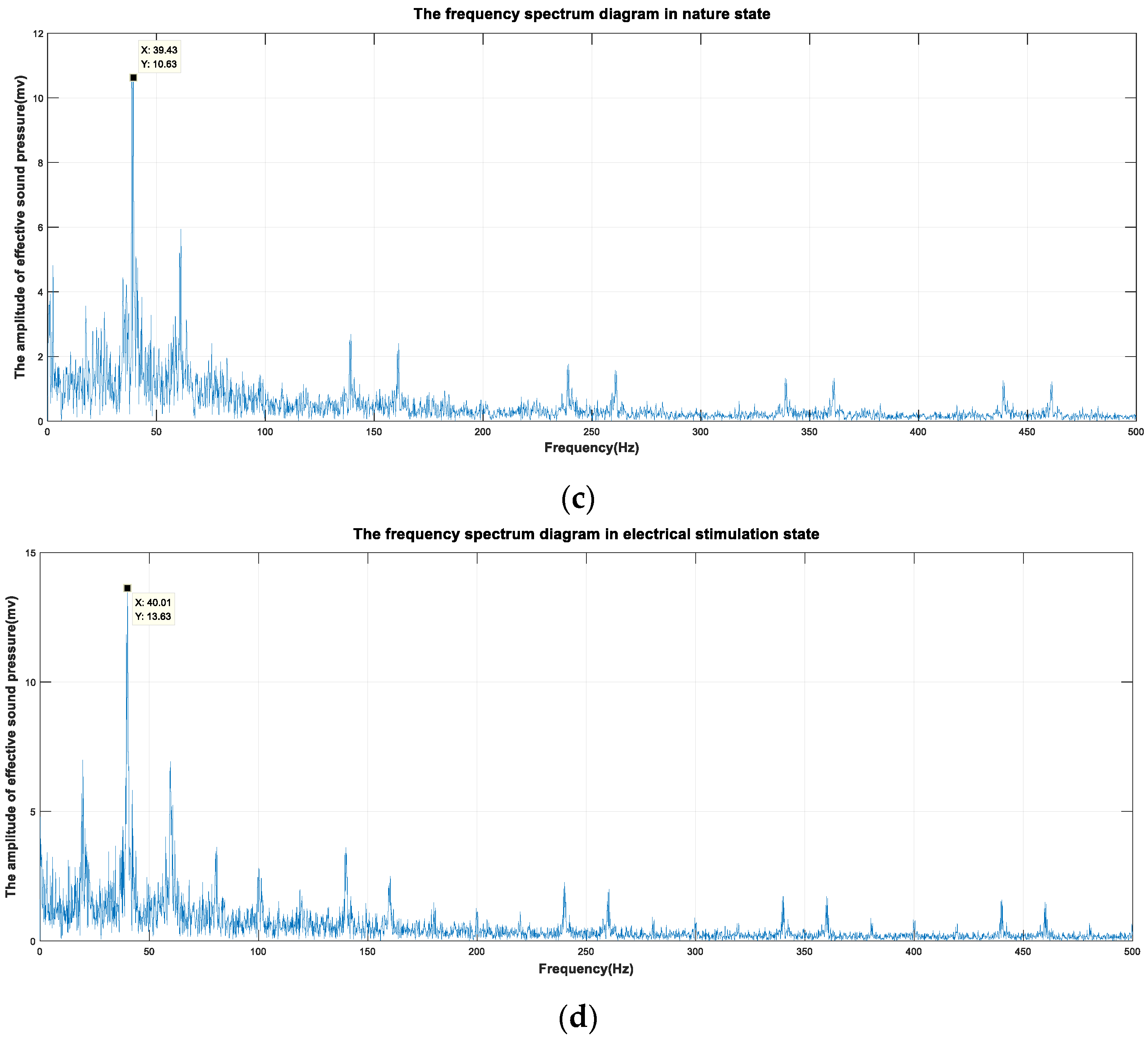
3.2. Electrical Stimulation Signals
3.3. Validation Experiment
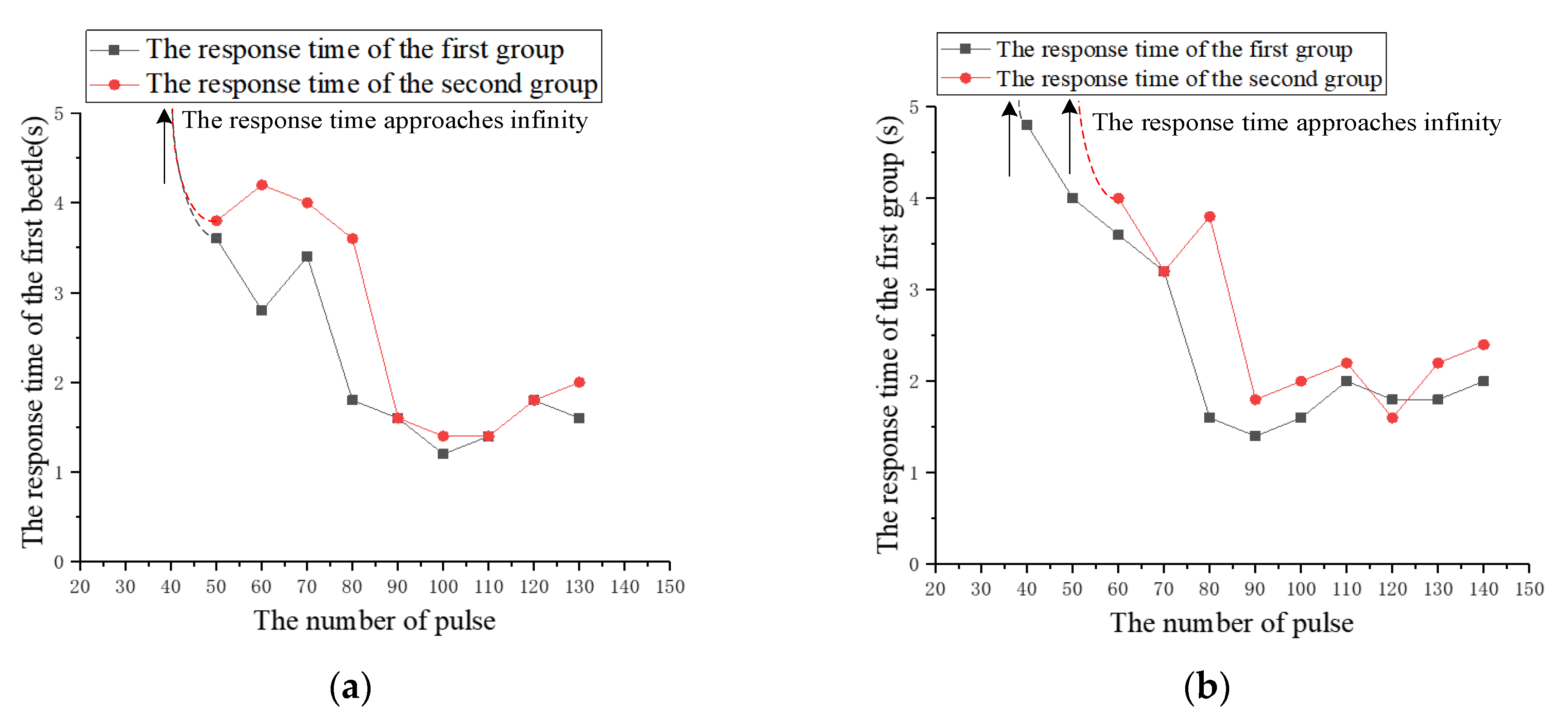
3.4. Comparative Experiment
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sato, H.; Maharbiz, M.M. Recent developments in the remote radio control of insect flight. Front. Neurosci. 2010, 4, 199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maharbiz, M.M.; Sato, H. Cyborg beetles. Sci. Am. 2010, 303, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sato, H. Insect-computer hybrid robot. Mol. Front. J. 2018, 2, 30–42. [Google Scholar] [CrossRef]
- Sanchez, C.J.; Chiu, C.W.; Zhou, Y.; González, J.M.; Vinson, S.B.; Liang, H. Locomotion control of hybrid cockroach robots. J. R. Soc. Interface 2015, 12, 20141363. [Google Scholar] [CrossRef] [PubMed]
- Register, J.; Callahan, D.M.; Segura, C.; LeBlanc, J.; Lissandrello, C.; Kumar, P.; Salthouse, C.; Wheeler, J. Advances in flexible optrode hardware for use in cybernetic insects. In Proceedings of the SPIE NANOSCIENCE + ENGINEERING, San Diego, CA, USA, 6–10 August 2017. [Google Scholar]
- Bozkurt, A.; Gilmour, R.F., Jr.; Lal, A. Balloon-assisted flight of radio-controlled insect biobots. IEEE Trans. Biomed. Eng. 2009, 56, 2304–2307. [Google Scholar] [CrossRef] [PubMed]
- Tsang, W.M.; Stone, A.L.; Aldworth, Z.N.; Hildebrand, J.G.; Daniel, T.L.; Akinwande, A.I.; Voldman, J. Flexible split-ring electrode for insect flight biasing using multisite neural stimulation. IEEE Trans. Biomed. Eng. 2010, 57, 1757–1764. [Google Scholar] [CrossRef] [PubMed]
- Tsang, W.M.; Stone, A.L.; Otten, D.; Aldworth, Z.N.; Daniel, T.L.; Hildebrand, J.G.; Levine, R.B.; Voldman, J. Insect-machine interface: A carbon nanotube-enhanced flexible neural probe. J. Neurosci. Methods 2012, 204, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Mann, K.; Massey, T.L.; Guha, S.; Van Kleef, J.P.; Maharbiz, M.M. A wearable wireless platform for visually stimulating small flying insects. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014. [Google Scholar]
- Sato, H.; Berry, C.W.; Maharbiz, M.M. Flight control of 10gram insects by implanted neural stimulators. In Proceedings of the Solid-State Sensors, Actuators, and Microsystems Workshop, Hilton Head Island, SC, USA, 1–5 June 2008; pp. 90–91. [Google Scholar]
- Sato, H.; Peeri, Y.; Baghoomian, E.; Berry, C.W.; Maharbiz, M.M. Radio-controlled cyborg beetles: A radio-frequency system for insect neural flight control. In Proceedings of the 2009 IEEE 22nd International Conference on Micro Electro Mechanical Systems, Sorrento, Italy, 25–29 January 2009; IEEE: New York, NY, USA, 2009. [Google Scholar]
- Sato, H.; Berry, C.W.; Peeri, Y.; Baghoomian, E.; Casey, B.E.; Lavella, G.; Vandenbrooks, J.M.; Harrison, J.F.; Maharbiz, M.M. Remote radio control of insect flight. Front. Integr. Neurosci. 2009, 3, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, H.; Vo Doan, T.T.; Kolev, S.; Huynh, N.A.; Zhang, C.; Massey, T.L.; Van Kleef, J.; Ikeda, K.; Abbeel, P.; Maharbiz, M.M. Deciphering the role of a coleopteran steering muscle via free flight stimulation. Curr. Biol. 2015, 25, 798–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Wu, J.; Sato, H. Feedback control-based navigation of a flying insect-machine hybrid robot. Soft Robot. 2018, 5, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Z. Insect Physiology; China Agriculture Press: Beijing, China, 2004. [Google Scholar]
- Choo, H.Y.; Li, Y.; Cao, F.; Sato, H. Electrical stimulation of coleopteran muscle for initiating flight. PLoS ONE 2016, 11, e0151808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roland, T.; Amsuess, S.; Russold, M.F.; Baumgartner, W. Ultra-low-power digital filtering for insulated EMG sensing. Sensors 2019, 19, 959. [Google Scholar] [CrossRef] [PubMed] [Green Version]


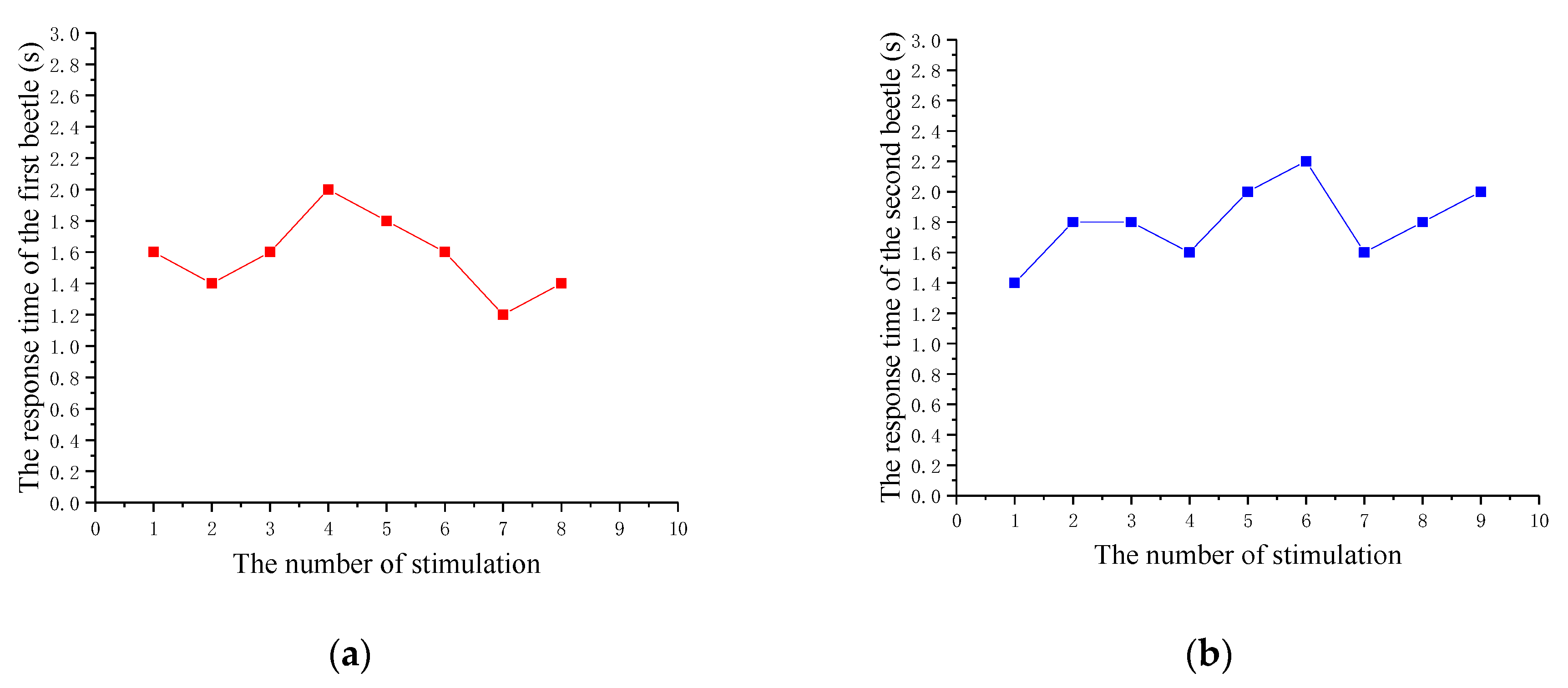
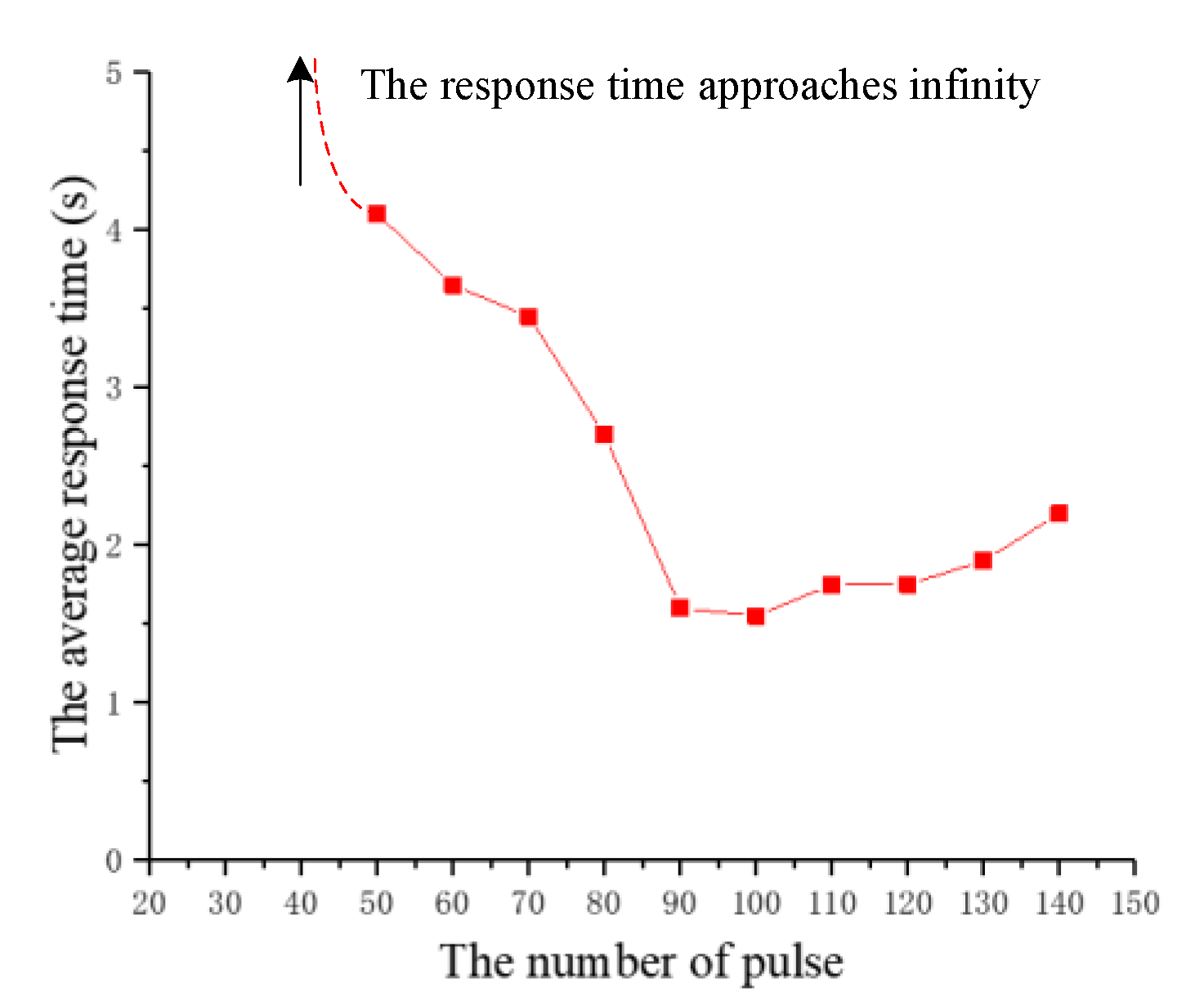
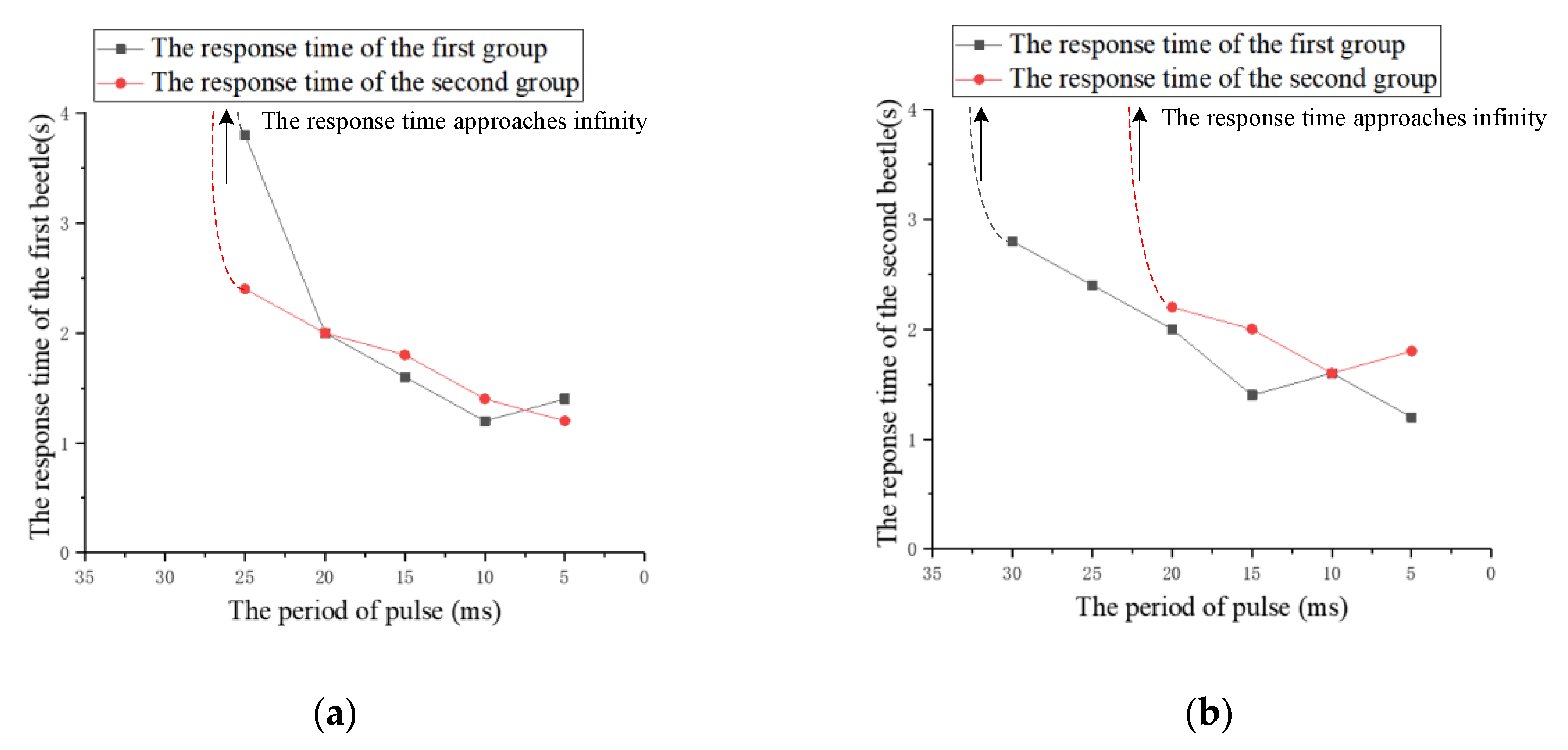


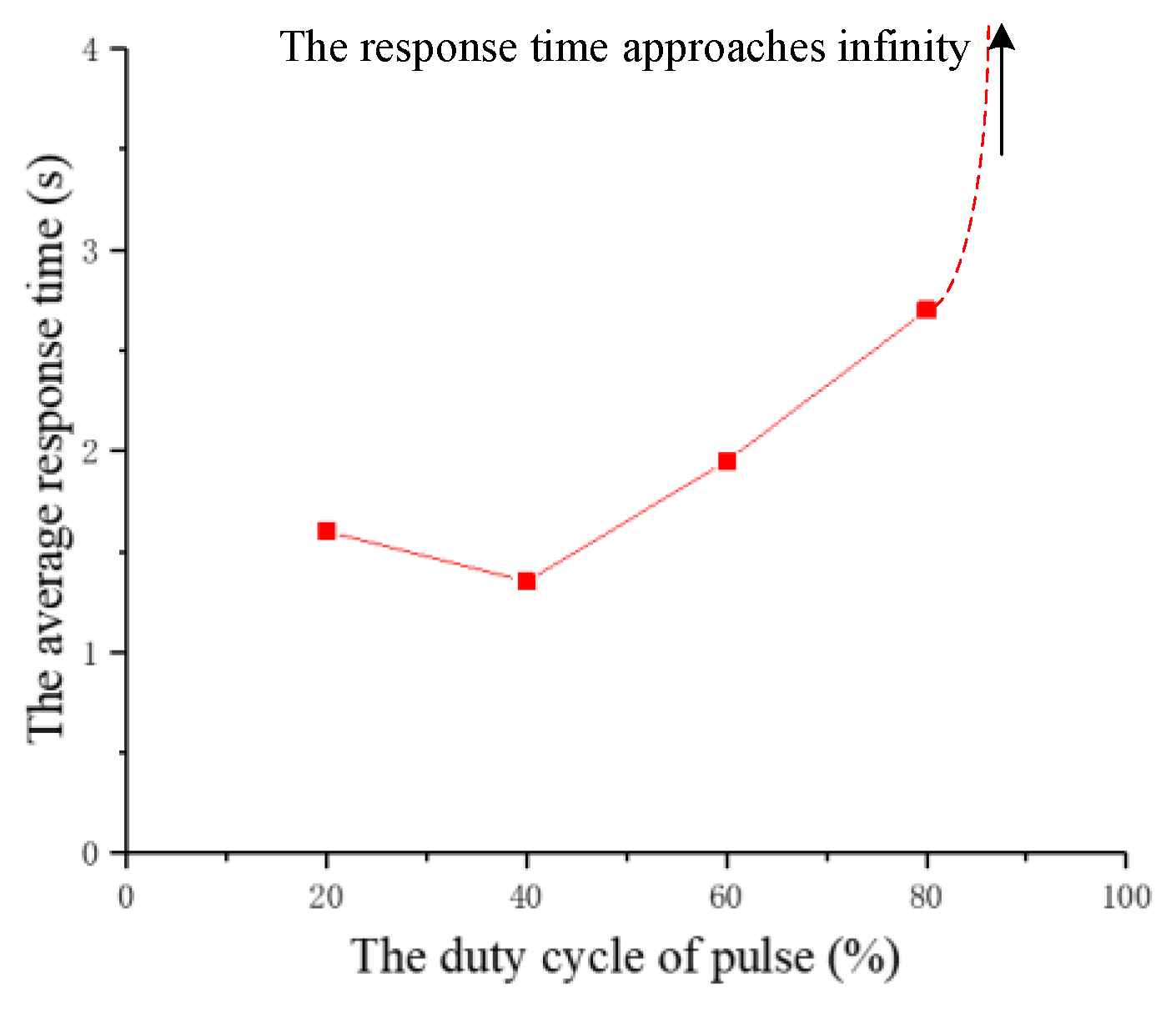
| The Number of Experiments | The Response Time of the First Beetle (s) | The Response Time of the Second Beetle (s) |
|---|---|---|
| 1 | 1.6 | 1.4 |
| 2 | 1.4 | 1.8 |
| 3 | 1.6 | 1.8 |
| 4 | - | 1.6 |
| 5 | 2 | 2 |
| 6 | 1.8 | - |
| 7 | - | 2.2 |
| 8 | 1.6 | 1.6 |
| 9 | 1.2 | 1.8 |
| 10 | 1.4 | 2 |
| The average response time | 1.58 | 1.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Yang, B.; Jiang, Y.; Zheng, X. Research on Key Techniques of Insect Flapping Onset Control Based on Electrical Stimulation. Sensors 2020, 20, 239. https://doi.org/10.3390/s20010239
Feng Y, Yang B, Jiang Y, Zheng X. Research on Key Techniques of Insect Flapping Onset Control Based on Electrical Stimulation. Sensors. 2020; 20(1):239. https://doi.org/10.3390/s20010239
Chicago/Turabian StyleFeng, Yu, Bo Yang, Yongchang Jiang, and Xiang Zheng. 2020. "Research on Key Techniques of Insect Flapping Onset Control Based on Electrical Stimulation" Sensors 20, no. 1: 239. https://doi.org/10.3390/s20010239




