Ammonium Pyrrolidine Dithiocarbamate-Modified CdTe/CdS Quantum Dots as a Turn-on Fluorescent Sensor for Detection of Trace Cadmium Ions
Abstract
1. Introduction
2. Experimental
2.1. Instruments and Reagents
2.2. Fluorescence Intensity Measurements
3. Results and Discussion
3.1. The Stability of the Fluorescence Intensity of the QD Solution
3.2. Optimization of the Concentration of APDC in the Detection Cuvette
3.3. Optimization of the pH of the Tris-HCl Buffer Solution
3.4. Fluorescence Quenching Caused by the Etching Effect of APDC in the CdTe/CdS QD Solution
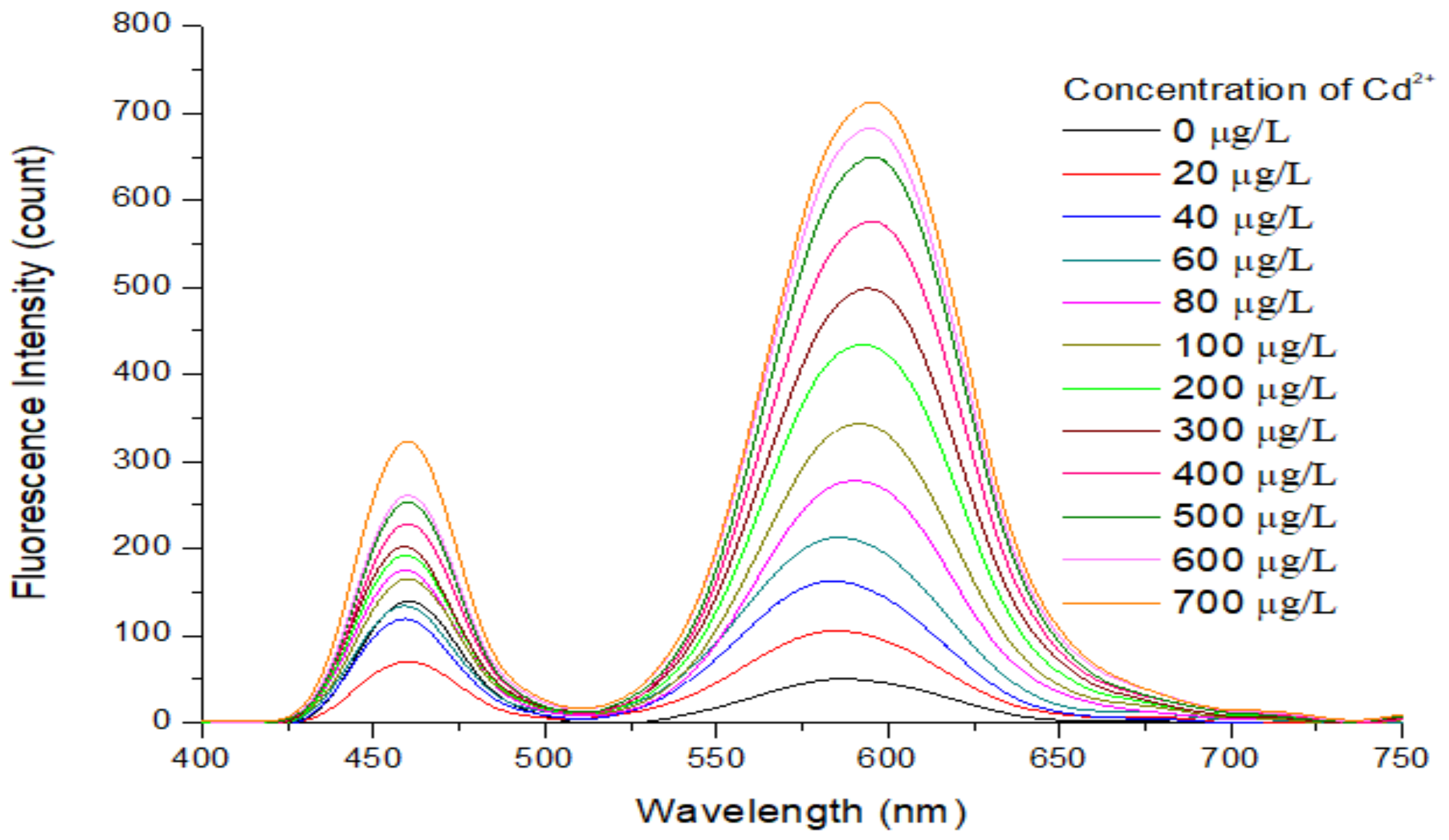
3.5. Fluorescence Recovery Caused by Addition of Cd2+ to the APDC/CdTe/CdS QD Solution
3.6. Analytical Performance of the APDC/CdTe/CdS QD Solution
3.7. Interference Study
3.8. Sample Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fatima, G.; Raza, A.M.; Hadi, N.; Nigam, N.; Mahdi, A.A. Cadmium in Human Diseases: It’ More than Just a Mere Metal. Indian. J. Biochem. Biol. 2019, 34, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Sunli, K.; Anupama, S. Cadmium toxicity: Effects on human reproduction and fertility. Rev. Environ. Health. 2019, 34, 327–338. [Google Scholar]
- Rebecca, M.N.; Caterina, V.S.; Carmen, M.; Bruce, D.; Jaymie, M.R. Expression of Genes Involved in Stress, Toxicity, Inflammation, and Autoimmunity in Relation to Cadmium, Mercury, and Lead in Human Blood: A Pilot Study. Toxics 2018, 6, 35–43. [Google Scholar]
- Zhao, J.; Han, P.Y.; Tian, S.N.; Shi, H.T.; He, J.H.; Xiao, C.F. Polypyrrole/cadmium sulfide hollow fiber with high performance contaminant removal and photocatalytic activity fabricated by layer-by-layer deposition and fiber-sacrifice template approach. J. Colloid. Interf. Sci. 2019, 557, 94–102. [Google Scholar] [CrossRef]
- Chen, J.Q.; Xu, Y.M.; Han, Q.; Yao, Y.C.; Xing, H.J.; Teng, X.H. Immunosuppression, oxidative stress, and glycometabolism disorder caused by cadmium in common carp (Cyprinus carpio L.): Application of transcriptome analysis in risk assessment of environmental contaminant cadmium. J. Hazard. Mater. 2019, 366, 386–394. [Google Scholar] [CrossRef]
- Jaehong, S.; Manish, K.; Santanu, M.; Ritusmita, G. Sustainable removal of pernicious arsenic and cadmium by a novel composite of MnO2 impregnated alginate beads: A cost-effective approach for wastewater treatment. J. Environ. Manage. 2019, 234, 8–20. [Google Scholar]
- Roy, S.; Mondal, I.; Harms, K.; Chattopadhyay, S. Synthesis, structure, catechol oxidase and phenoxazinone synthase mimicking activity of a manganese(III) Schiff base complex [Mn(HL)2(CH3OH)2][Mn(HL)2(N3)2]. Polyhedron 2018, 141, 198–207. [Google Scholar]
- Lu, Y.Y.; Liang, X.Q.; Niyungeko, C.; Zhou, J.J.; Xu, J.M.; Tian, G.M. A review of the identification and detection of heavy metal ions in the environment by voltammetry. Talanta 2018, 178, 324–338. [Google Scholar] [CrossRef]
- Ruangyuttikarn, W.; Panyamoon, A.; Nambunmee, K.; Honda, R.; Swaddiwudhipong, W.; Nishijo, M. Use of the kidney injury molecule-1 as a biomarker for early detection of renal tubular dysfunction in a population chronically exposed to cadmium in the environment. Springerplus 2013, 2, 533–540. [Google Scholar] [CrossRef]
- Kasa, N.A.; Sel, S.; Chormey, D.S.; Bakirdere, S. Determination of cadmium at trace levels in parsley samples by slotted quartz tube-flame atomic absorption spectrometry after preconcentration with cloud point extraction. Measurement 2019, 147, 106841. [Google Scholar] [CrossRef]
- Zvěřina, O.; Kuta, J.; Coufalík, P.; Kosečková, P.; Komárek, J. Simultaneous determination of cadmium and iron in different kinds of cereal flakes using high-resolution continuum source atomic absorption spectrometry. Food. Chem. 2019, 298, 125084. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.J.; Wang, Z.M.; Wang, Z.L. A Facile Acidic Digestion Method for Cosmetic Lead and Cadmium Determination by an Inductively Coupled Plasma Atomic Emission Spectrometer. J. Appl. Spectrosc. 2018, 85, 659–664. [Google Scholar]
- Azimi, S.; Es’haghi, Z. A Magnetized Nanoparticle Based Solid-Phase Extraction Procedure Followed by Inductively Coupled Plasma Atomic Emission Spectrometry to Determine Arsenic, Lead and Cadmium in Water, Milk, Indian Rice and Red Tea. Bull. B. Environ. Contam. Tox. 2017, 98, 830–836. [Google Scholar] [CrossRef]
- Krata, A.A.; Wojciechowski, M.; Kalabun, M.; Bulska, E. Reference measurements of cadmium and lead contents in candidates for new environmental certified materials by isotope dilution inductively coupled plasma mass spectrometry. Microchem. J. 2018, 142, 36–42. [Google Scholar] [CrossRef]
- Gray, P.J.; Cunningham, W. Inductively Coupled Plasma Collision Cell Quadrupole Mass Spectrometric Determination of Extractible Arsenic, Cadmium, Chromium, Lead, Mercury, and Other Elements in Food Using Microwave-Assisted Digestion: Results from an FDA Interlaboratory Study. J. AOAC Int. 2019, 102, 590–604. [Google Scholar] [CrossRef] [PubMed]
- Iammarino, M.; Taranto, A.D.; Centonze, D. Determination of Sulphiting Agents in Raw and Processed Meat: Comparison between a Modified Monier-Williams Method and the Direct Analysis by Ion Chromatography with Conductometric Detection. Food. Anal. Method 2017, 10, 3956–3963. [Google Scholar] [CrossRef]
- Griffin, M.J.; Kabir, K.M.; Coyle, V.E.; Kandjani, A.E.; Sabri, Y.M.; Ippolito, S.J.; Bhargava, S.K. A Nanoengineered Conductometric Device for Accurate Analysis of Elemental Mercury Vapor. Environ. Sci. Technol. 2016, 50, 1384–1392. [Google Scholar] [CrossRef]
- Lisboa, T.P.; Faria, L.V.; Matos, M.A.C.; Matos, R.C.; Sousa, R.A. Simultaneous determination of cadmium, lead, and copper in the constituent parts of the illegal cigarettes by Square Wave Anodic Stripping Voltammetry. Microchem. J. 2019, 150, 104183. [Google Scholar] [CrossRef]
- Dali, M.; Zinoubi, K.; Chrouda, A.; Abderrahmane, S.; Cherrad, S.; Renault, N.J. A biosensor based on fungal soil biomass for electrochemical detection of lead (II) and cadmium (II) by differential pulse anodic stripping voltammetry. J. Electroanal. Chem. 2018, 813, 9–19. [Google Scholar] [CrossRef]
- Dahaghin, Z.; Kilmartin, P.A.; Mousavi, H.Z. Simultaneous determination of lead(II) and cadmium(II) at a glassy carbon electrode modified with GO@Fe3O4@benzothiazole-2-carboxaldehyde using square wave anodic stripping voltammetry. J. Mol. Liq. 2018, 249, 1125–1132. [Google Scholar] [CrossRef]
- Rosolina, S.M.; Chambers, J.Q.; Lee, C.W.; Xue, Z.L. Direct determination of cadmium and lead in pharmaceutical ingredients using anodic stripping voltammetry in aqueous and DMSO/water solutions. Anal. Chim. Acta 2015, 893, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Tra-ngan, S.; Siripornadulsil, S.; Thanwisai, L.; Siripornadulsil, W. Potential application of a recombinant bacterial strain carrying a groEL promoter as a whole-cell microbial biosensor for detecting bioavailable cadmium. Environ. Technol. Innov. 2019, 15, 100375. [Google Scholar] [CrossRef]
- Ngamdee, K.; Ngeontae, W. Circular dichroism glucose biosensor based on chiral cadmium sulfide quantum dots. Sens. Actuators B Chem. 2018, 274, 402–411. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Raoof, J.B.; Ojani, R. Design of an electrochemical DNA-based biosensor for selective determination of cadmium ions using a DNA hybridization indicator. Int. J. Biol. Macromol. 2018, 108, 1237–1241. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.W.; Gong, J.Y.; Rong, P.S.; Liu, C.S.; Chen, J.H. A portable and quantitative biosensor for cadmium detection using glucometer as the point-of-use device. Talanta 2019, 198, 412–416. [Google Scholar] [CrossRef]
- Ghenaatian, H.R.; Fard, M.S.; Moghadam, M.R.; Kamath, G.; Rahmanian, M. Tailoring of graphene quantum dots for toxic heavy metals detection. Appl. Phys. A Mater. 2019, 125, 754–765. [Google Scholar] [CrossRef]
- Liu, J.Q.; Zhang, Q.R.; Xue, W.T.; Zhang, H.P.; Bai, Y.; Wu, L.T.; Zhai, Z.X.; Jin, G.H. Fluorescence Characteristics of Aqueous Synthesized Tin Oxide Quantum Dots for the Detection of Heavy Metal Ions in Contaminated Water. Nanonaterials 2019, 9, 1294. [Google Scholar] [CrossRef]
- Zhang, D.W.; Xu, Y.; Liu, Q.L.; Xia, Z.G. Encapsulation of CH3NH3PbBr3 Perovskite Quantum Dots in MOF-5 Microcrystals as a Stable Platform for Temperature and Aqueous Heavy Metal Ion Detection. Inorg. Chem. 2018, 57, 4613–4619. [Google Scholar] [CrossRef]
- Siong, L.T.; Shu, J.E.; Arundithi, A.; Kam, C.L.; Peng, C. Graphene quantum dots functionalized gold nanoparticles for sensitive electrochemical detection of heavy metal ions. Electrochim. Acta 2015, 172, 7–11. [Google Scholar]
- Pourghobadi, Z.; Mirahmadpour, P.; Zare, H. Fluorescent biosensor for the selective determination of dopamine by TGA-capped CdTe quantum dots in human plasma samples. Opt. Mater. 2018, 84, 757–762. [Google Scholar] [CrossRef]
- Zare, H.; Ghalkhani, M.; Akhavan, O.; Taghavinia, N.; Marandi, M. Highly sensitive selective sensing of nickel ions using repeatable fluorescence quenching-emerging of the CdTe quantum dots. Mater. Res. Bull. 2017, 95, 532–538. [Google Scholar] [CrossRef]
- Boonmee, C.; Noipa, T.; Tuntulani, T.; Ngeontae, W. Cysteamine capped CdS quantum dots as a fluorescence sensor for the determination of copper ion exploiting fluorescence enhancement and long-wave spectral shifts. Spectrochim. Acta A 2016, 169, 161–168. [Google Scholar] [CrossRef]
- Wu, Q.; Zhou, M.; Shi, J.; Li, Q.J.; Yang, M.Y.; Zhang, Z.X. Synthesis of Water-Soluble Ag2S Quantum Dots with Fluorescence in the Second Near-Infrared Window for Turn-On Detection of Zn(II) and Cd(II). Anal. Chem. 2017, 89, 6616–6623. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Fu, Q.Q.; Shen, H.C.; Chen, Y.; Xiao, W.; Yan, D.G.; Tang, X.J.; Zhong, Z.Y.; Tang, Y. A turn-on competitive immunochromatographic strips integrated with quantum dots and gold nano-stars for cadmium ion detection. Talanta 2018, 178, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.Y.; Zhu, K.; Guo, Q.S.; Liu, Y.Q.; Ye, M.F.; Sun, Q.J. Ligand displacement-induced fluorescence switch of quantum dots for ultrasensitive detection of cadmium ions. Anal. Chim. Acta 2014, 812, 191–198. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, X.; Wang, L.; Shang, Z.; Chao, J.; Jin, W. A new acridine derivative as a highly selective “off-on” fluorescence chemosensor for Cd2+ in aqueous media. Sens. Actuators B Chem. 2011, 156, 126–131. [Google Scholar] [CrossRef]
- Xue, Y.; Zhao, H.; Wu, Z.; Li, X.; He, Y.; Yuan, Z. Colorimetric detection of Cd2+ using gold nanoparticles cofunctionalized with 6-mercaptonicotinic acid and l-cysteine. Analyst 2011, 136, 3725–3730. [Google Scholar] [CrossRef]













| QD Sensor/Fluorescent Probe | Linear Range (μmol/L) | LOD (μmol/L) | Reference |
|---|---|---|---|
| Ag2S QD | 1–40 | 0.5460 | [33] |
| competitive immunochromatographic strips/gold nanoparticles QD | 0.0022–0.0712 | 0.0016 | [34] |
| 4,5-bis (N, N-di (2- hydroxyethyl) iminomethyl) acridine fluorescent probe | 1–30 | 0.1300 | [36] |
| 6-mercaptonicotinic acid/L-Cys/gold nanoparticles fluorescent probe | 0.2–1.7 | 0.1000 | [37] |
| CdTe/CdS QD | 0.0784–5.338 | 0.0235 | This work |
| Ion | Number | Detected by the QD Solution (μg/L) a | Detected by AAS (μg/L) a | Relative Error (%) | Standard Deviation (SD) (μg/L) | Relative Standard Deviation (RSD) (%) |
|---|---|---|---|---|---|---|
| Cd2+ | Sample 1 | 28.75 | 28.17 | 2.01 | 0.56 | 1.94 |
| Sample 2 | 14.39 | 14.85 | 3.10 | 0.41 | 2.87 | |
| Sample 3 | 32.81 | 33.59 | 2.32 | 0.77 | 2.35 |
| Ion | Number | Added (μg/L) | Detected by the QD Solution (μg/L) a. | Recovery (%) | Standard Deviation (SD) (μg/L) | Relative Standard Deviation (RSD) (%) |
|---|---|---|---|---|---|---|
| Cd2+ | Sample 4 | 40 | 39.10 | 97.75 | 0.62 | 1.77 |
| Sample 5 | 80 | 78.43 | 98.04 | 2.08 | 2.65 | |
| Sample 6 | 200 | 202.59 | 101.3 | 4.01 | 1.98 | |
| Sample 7 | 400 | 391.73 | 97.93 | 9.44 | 2.41 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, Y.; Yang, Q.; Liu, G. Ammonium Pyrrolidine Dithiocarbamate-Modified CdTe/CdS Quantum Dots as a Turn-on Fluorescent Sensor for Detection of Trace Cadmium Ions. Sensors 2020, 20, 312. https://doi.org/10.3390/s20010312
Yin Y, Yang Q, Liu G. Ammonium Pyrrolidine Dithiocarbamate-Modified CdTe/CdS Quantum Dots as a Turn-on Fluorescent Sensor for Detection of Trace Cadmium Ions. Sensors. 2020; 20(1):312. https://doi.org/10.3390/s20010312
Chicago/Turabian StyleYin, Yuan, Qingliang Yang, and Gang Liu. 2020. "Ammonium Pyrrolidine Dithiocarbamate-Modified CdTe/CdS Quantum Dots as a Turn-on Fluorescent Sensor for Detection of Trace Cadmium Ions" Sensors 20, no. 1: 312. https://doi.org/10.3390/s20010312
APA StyleYin, Y., Yang, Q., & Liu, G. (2020). Ammonium Pyrrolidine Dithiocarbamate-Modified CdTe/CdS Quantum Dots as a Turn-on Fluorescent Sensor for Detection of Trace Cadmium Ions. Sensors, 20(1), 312. https://doi.org/10.3390/s20010312





