Salt Priming Protects Photosynthetic Electron Transport against Low-Temperature-Induced Damage in Wheat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design and Materials
2.2. Chlorophyll a Fluorescence
= [(RC/CS)treatment/(RC/CS)control]·[(ABS/CS)treatment/(ABS/CS)control],
2.3. Staining for Membrane Damage and Measurement of Malondialdehyde (MDA)
2.4. Statistical Analysis
3. Results
3.1. Chl a Fluorescence Transient
3.2. Electron Transport and Quantum Yield of PS II
3.3. Technical Fluorescence Parameters in PS II
3.4. Reaction Centers in PS II
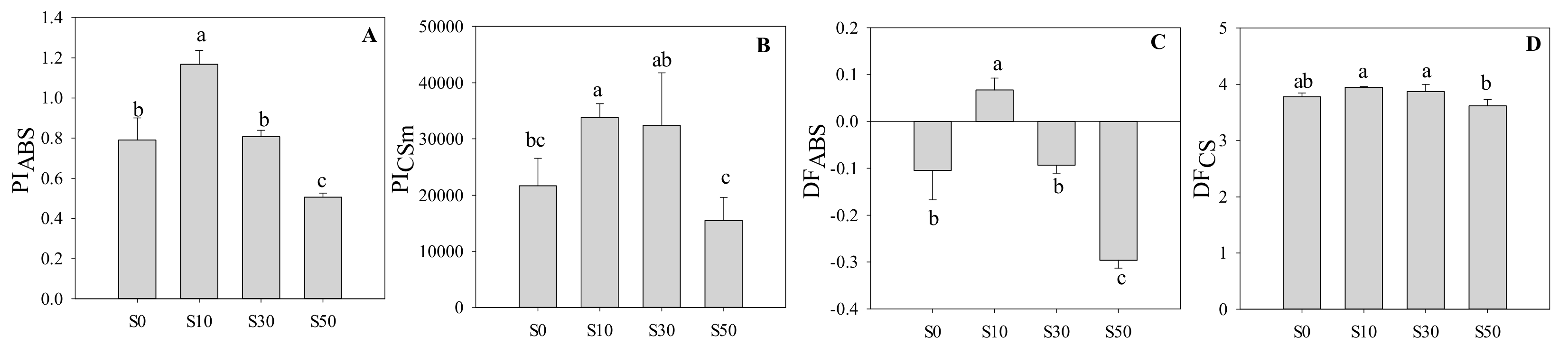
3.5. Performance Index and Driving Force in PS II
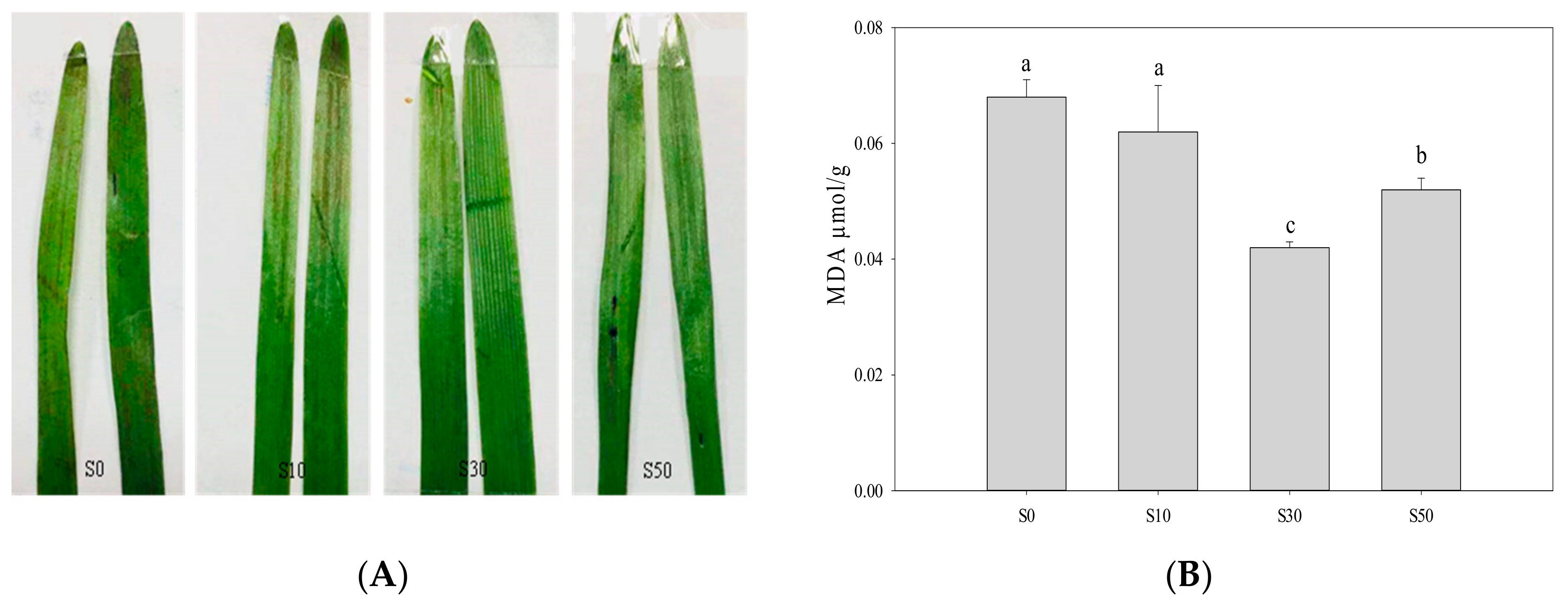
3.6. Membrane Damage and Malondialdehyde of Leave
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, X.; Jiang, H.; Liu, F.; Cai, J.; Dai, T.; Cao, W.; Jiang, D. Induction of chilling tolerance in wheat during germination by pre-soaking seed with nitric oxide and gibberellin. Plant Growth Regul. 2013, 71, 31–40. [Google Scholar] [CrossRef]
- Li, X.; Cai, J.; Liu, F.; Dai, T.; Cao, W.; Jiang, D. Cold priming drives the sub-cellular antioxidant systems to protect photosynthetic electron transport against subsequent low temperature stress in winter wheat. Plant Physiol. Biochem. 2014, 82, 34–43. [Google Scholar] [CrossRef]
- Zivcak, M.; Kalaji, H.M.; Shao, H.; Olsovska, K.; Brestic, M. Photosynthetic proton and electron transport in wheat leaves under prolonged moderate drought stress. J. Photochem. Photobiol. B Biol. 2014, 137, 107–115. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Rastogi, A.; Živčák, M.; Brestic, M.; Daszkowska-Golec, A.; Sitko, K.; Alsharafa, K.Y.; Lotfi, R.; Stypiński, P.; Samborska, I.A.; et al. Prompt chlorophyll fluorescence as a tool for crop phenotyping: An example of barley landraces exposed to various abiotic stress factors. Photosynthetica 2018, 56, 953–961. [Google Scholar] [CrossRef] [Green Version]
- Stirbet, A.; Strasser, B.J.; Strasser, R.J. Chlorophyll a fluorescence induction in higher plants: Modelling and numerical simulation. J. Theor. Biol. 1998, 193, 131–151. [Google Scholar] [CrossRef]
- Suzuki, K.; Ohmori, Y.; Ratel, E. High root temperature blocks both linear and cyclic electron transport in the dark during chilling of the leaves of rice seedlings. Plant Cell Physiol. 2011, 52, 1697–1707. [Google Scholar] [CrossRef]
- Öquist, G.; Huner, N.P.A. Photosynthesis of overwintering evergreen plants. Annu. Rev. Plant Biol. 2003, 54, 329–355. [Google Scholar] [CrossRef]
- Li, X.; Brestic, M.; Tan, D.; Zivcak, M.; Zhu, X.; Liu, S.; Song, F.; Reiter, R.J.; Liu, F. Melatonin alleviates low PS I limited carbon assimilation under elevated CO2 and enhances the cold tolerance of offspring in chlorophyll b-deficient mutant wheat. J. Pineal Res. 2017, 64, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Baker, N.R.; Rosenqvist, E. Applications of chlorophyll fluorescence can improve crop production strategies: An examination of future possibilities. J. Exp. Bot. 2004, 55, 1607–1621. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Kong, Q.; Xiang, C. Effects of low night temperature on pigments, chl a fluorescence and energy allocation in two bitter gourd (Momordica charantia L.) genotypes. Acta Physiol. Plant 2009, 31, 285–293. [Google Scholar] [CrossRef]
- Muller, P.; Li, X.; Niyogi, K. Non-photochemical quenching. A response to excess light energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campos, P.S.; nia Quartin, V.; chicho Ramalho, J.C.; Nunes, M.A. Electrolyte leakage and lipid degradation account for cold sensitivity in leaves of Coffea sp. plants. J. Plant Physiol. 2003, 160, 283–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atreya, A.; Vartak, V.; Bhargava, S. Salt priming improves tolerance to dessication stress and to extreme salt stress in Bruguiera cylindrica. Int. J. Integr. Biol. 2009, 6, 68–73. [Google Scholar]
- Janda, T.; Darko, É.; Shehata, S.; Kovacs, V.; Pal, M.; Szalai, G. Salt acclimation processes in wheat. Plant Physiol. Biochem. 2016, 101, 68–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koyro, H.W.; Hussain, T.; Huchzermeyer, B.; Khan, M.A. Photosynthetic and growth responses of a perennial halophytic grass Panicum turgidum to increasing NaCl concentrations. Environ. Exp. Bot. 2013, 91, 22–29. [Google Scholar] [CrossRef]
- Aloui, H.; Souguir, M.; Latique, S.; Hannachi, C. Germination and growth in control and primed seeds of pepper as affected by salt stress. Agron. Res. Moldavia 2014, 47, 407–414. [Google Scholar] [CrossRef] [Green Version]
- Qiu, N.; Lu, C. Enhanced tolerance of photosynthesis against high temperature damage in salt-adapted halophyte Atriplex centralasiatica plants. Plant Cell Environ. 2003, 26, 1137–1145. [Google Scholar] [CrossRef]
- Cheng, S.; Yang, Z.; Wang, M.; Song, J.; Sui, N. Salinity improves chilling resistance in Suaeda salsa. Acta Physiol. Plant. 2014, 36, 1823–1830. [Google Scholar] [CrossRef]
- Meng, C.; Wang, M.; Chen, M.; Sui, N. Suaeda salsa is adaptive to chilling stress under salinity at stages of seed germination and seedling establishment. J. Agric. Sci. 2015, 7, 217. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Strasser, R.J.; Qiang, S. In vivo assessment of effect of phytotoxin tenuazonic acid on PS II reaction centers. Plant Physiol. Biochem. 2014, 84, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Lazár, D.; Brokeš, M.; Nauš, J.; Dvořák, L. Mathematical modeling of 3-(3′,4′dichlorophenyl)-1,1-dimethylurea action in plant leaves. J. Theor. Biol. 1998, 191, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Appenroth, K.J.; Stöckel, J.; Srivastava, A.; Strasser, R.J. Multiple effects of chromate on the photosynthetic apparatus of Spirodela polyrhiza as probed by OJIP chlorophyll a fluorescence measurements. Environ. Pollut. 2001, 115, 49–64. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Brestic, M.; Bussotti, M.; Calatayud, A.; Ferroni, L.; Goltsev, V.; Guidi, L.; Jajoo, A.; Li, P.; et al. Frequently asked questions about chlorophyll fluorescence, the sequel. Photosynth. Res. 2017, 132, 13–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vijayaraghavareddy, P.; Adhinarayanreddy, V.; Vemanna, R.S.; Sreeman, S.; Makarla, U. Quantification of membrane damage/cell death using evan’s blue staining technique. Bio-Protoc 2017, 7, e2519. [Google Scholar]
- Chen, T.; Zhang, B. Measurements of proline and malondialdehyde contents and antioxidant enzyme activities in leaves of drought stressed cotton. Bio-Protoc 2016, 6, 17. [Google Scholar] [CrossRef] [Green Version]
- Hillier, W.; Messinger, J. Mechanism of photosynthetic oxygen production. In Photosystem II: The Light-Driven Water: Plastoquinone Oxidordructase, 2nd ed.; Wydrzynski, T., Satoh, K., Eds.; Springer: Dordrecht, The Netherlands, 2005; Volume 22, pp. 567–608. [Google Scholar]
- McEvoy, J.P.; Brudvig, G.W. Water-splitting chemistry of photosystem II. Chem. Rev. 2006, 106, 4455–4483. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 102–112. [Google Scholar] [CrossRef] [Green Version]
- Jajoo, A. Changes in photosystem II in response to salt stress. In Ecophysiology and Responses of Plants under Salt Stress; Ahmad, P., Azooz, M.M., Prasad, M.N.V., Eds.; Springer: Berlin, Germany, 2013; pp. 149–168. [Google Scholar]
- Brestic, M.; Zivcak, M.; Olsovska, K.; Shao, H.; Kalaji, H.M.; Allakhvediev, S.I. Reduced glutamine synthetase activity plays a role in control of photosynthetic responses to high light in barley leaves. Plant Physiol. Biochem. 2014, 81, 74–83. [Google Scholar] [CrossRef]
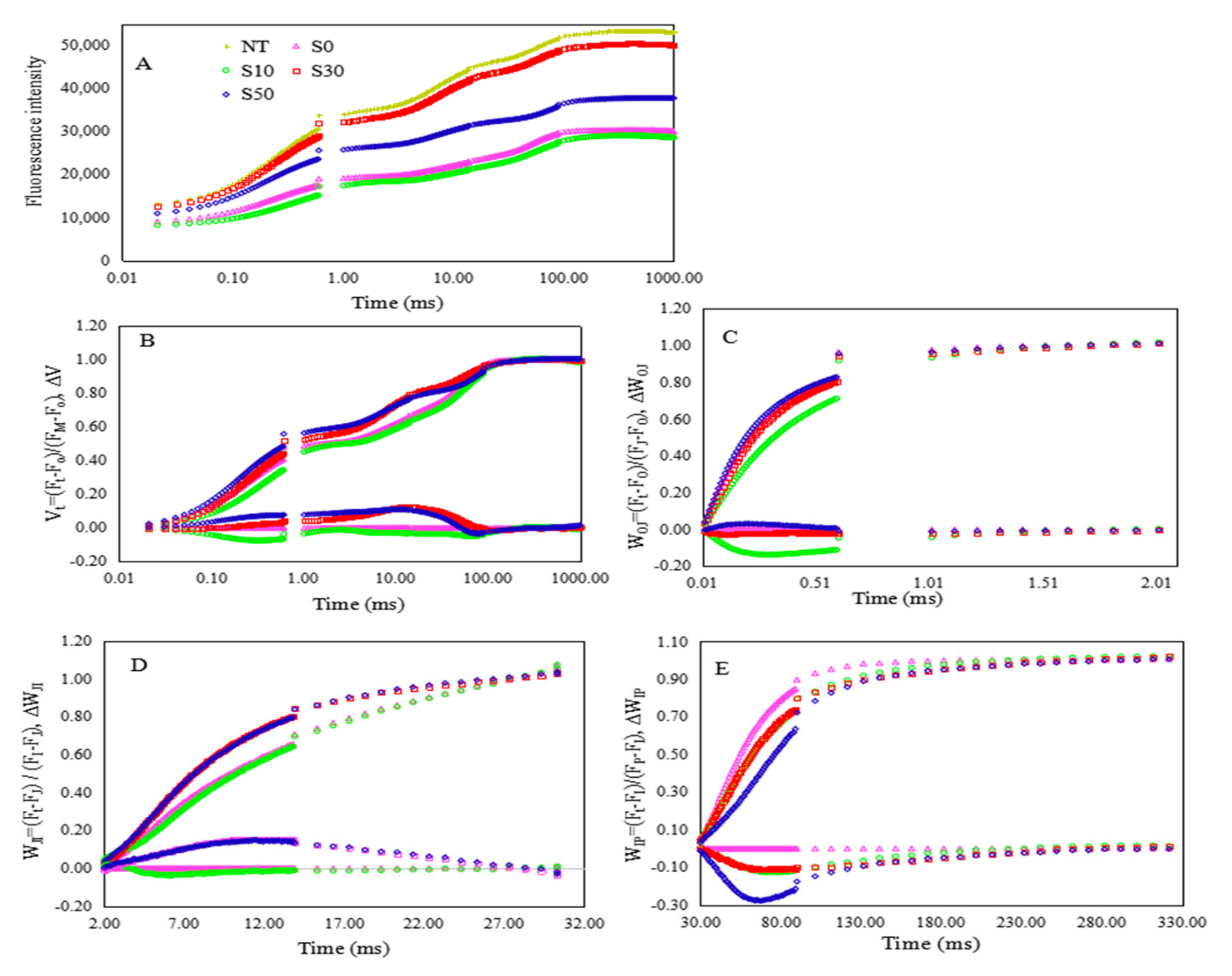
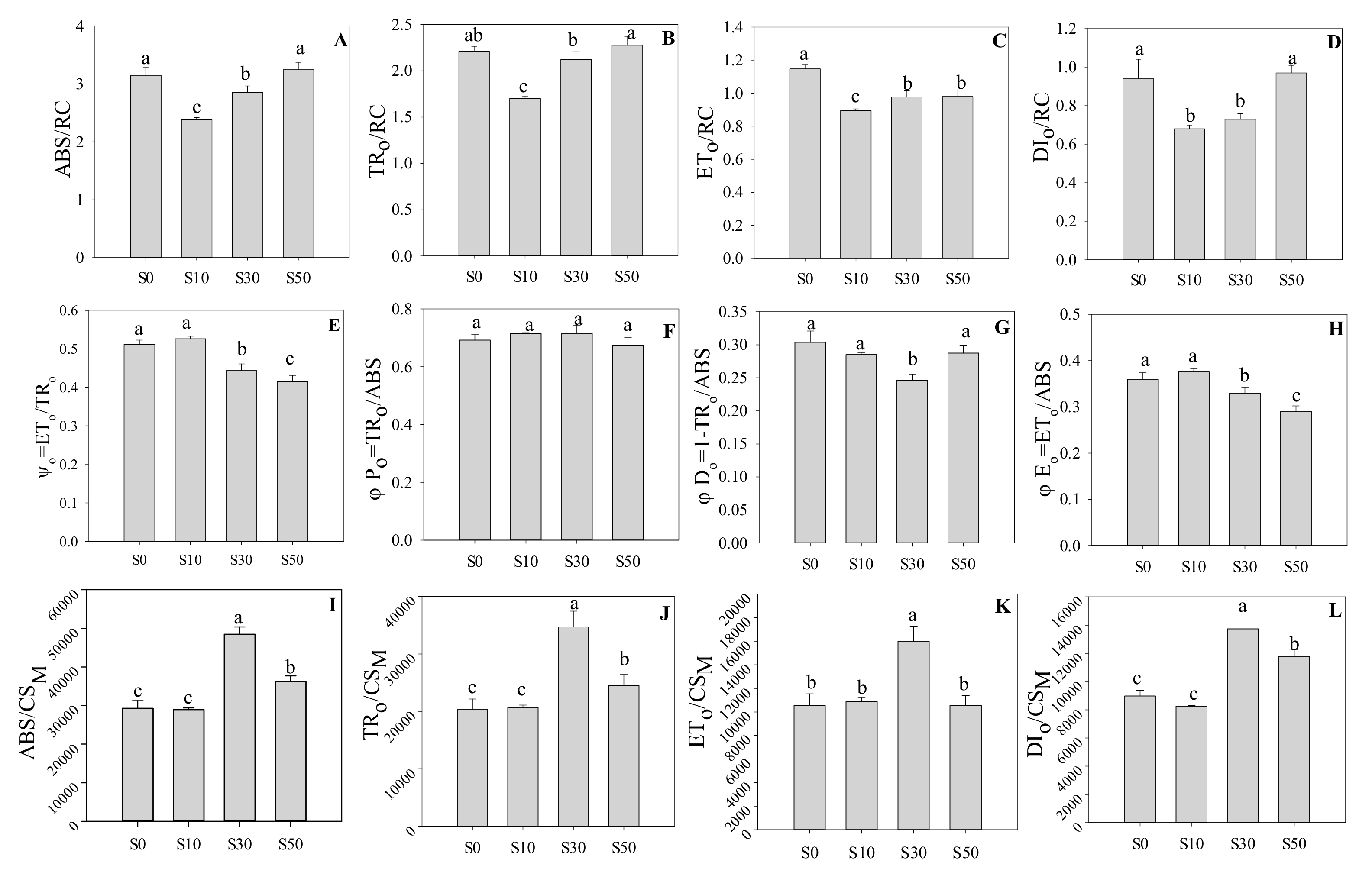
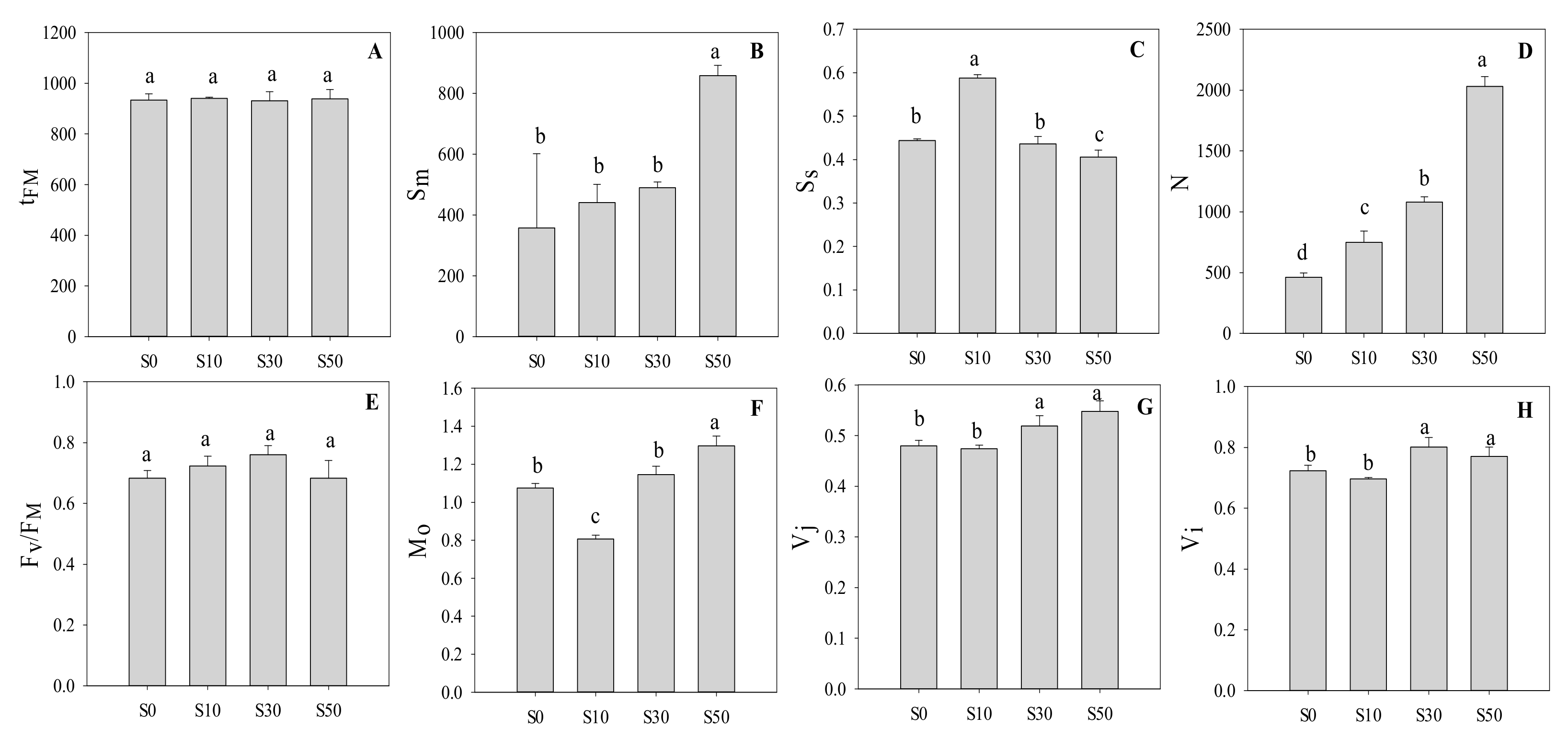
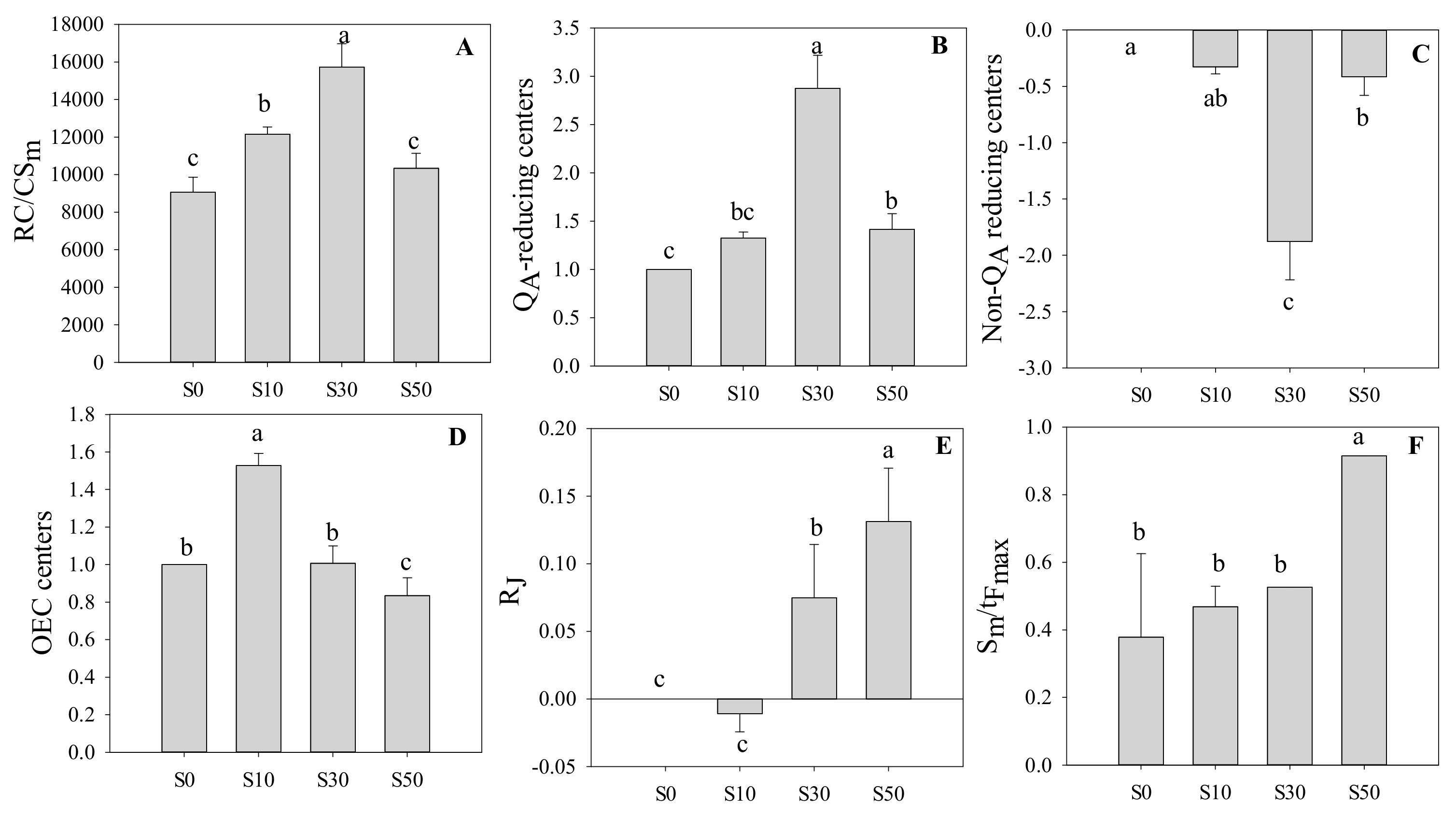
| Technical Fluorescence Parameters. | Illustrations |
| Fo ≌ F20μs | Minimal fluorescence, when all PSII RCs are open |
| FK ≡ F300μs | Fluorescence intensity at the K-step (300 μs) of OJIP |
| FJ = F2ms | Fluorescence intensity at the J-step (2 ms) of OJIP |
| FI = F30ms | Fluorescence intensity at the I-step (30 ms) of OJIP |
| Fp(= FM) | Maximal recorded fluorescence intensity, at the peak P of OJIP |
| tFM | Time (in ms) to reach the maximal fluorescence intensity FM |
| Area | Total complimentary area between the fluorescence induction curve and F = FM |
| Vj = (FJ − Fo)/(FM − Fo) | Relative variable fluorescence at the J-step |
| Mo = 4·(F270μs − Fo)/(FM − Fo) | Approximated initial slope (in m·s−1) of the fluorescence transient normalized on the maximal variable fluorescence FV |
| Sm = Area/(FM − Fo) | Normalized total complementary area above the O-J-I-P transient, reflecting multiple-turnover QA reduction events |
| SS = VJ/Mo | Normalized total complementary area corresponding only to the O-J phase, reflecting single-turnover QA reduction events |
| N = Sm/SS = Sm·Mo·(1 − VJ) | Frequency of QA reduction from t = 0 to t = t FM |
| Quantum Efficiencies or Flux Ratios | Illustrations |
| ψo = PS Io = ETo/TRo = (1 − VJ) | Probability that a trapped exciton moves an electron into the electron transport chain beyond QA at t = 0 |
| φPo = TRo/ABS = 1 − Fo/FM = FV/FM | Maximum quantum yield for PS II primary photochemistry at t = 0 |
| φDo = 1 − φPo = Fo/FM | Quantum yield for dissipation at t = 0 |
| φEo = ETo/ABS =(1 − Fo/FM)·(1 − VJ) | Quantum yield for PS II electron transport at t = 0 (ET) |
| Specific Energy Fluxes (per QA-reducing PS II reaction center-RC) | Illustrations |
| ABS/RC = Mo·(1/VJ)·(1/φPo) | Absorption flux per reaction center (RC) |
| TRo/RC = Mo·(1/VJ) | Trapped energy flux per RC at t = 0 |
| ETo/RC = Mo·(1/VJ)·ψo | Electron transport flux per RC at t = 0 |
| DIo/RC = ABS/RC − TRo/RC | Dissipated energy flux per RC at t = 0 |
| Phenomenological Energy Fluxes (per excited cross section-CS) | Illustrations |
| ABS/CSM≈ FM | Absorption flux per cross section (CS) at t = tFM |
| TRo/CSM = φPo·(ABS/CSM) | Trapped energy flux per CS at t = tFM |
| ETo/CSM = φEo·(ABS/CSM) | Electron transport flux per CS at t = tFM |
| DIo/CSM = ABS/CSM − TRo/CSM | Dissipated energy flux per CS at t = tFM |
| Density of RCs | Illustrations |
| RC/CSM = φPo·(VJ/Mo)·(ABS/CSM) | QA- reducing RCs per CS, reflecting density of RCs at t = tFM |
| QA-reducing centers = (RC/RCreference)·(ABS/ABSreference) = ((RC/CS)treatment/(RC/CS)control) ·((ABS/CS) treatment/(ABS/CS)control) | The fraction of QA-reducing reaction centers |
| Non-QA reducing centers = 1-QA-reducing centers | The fraction of non-QA reducing reaction centers |
| OEC centers = (1 − (VK/VJ) treatment)/(1 − (VK/VJ) control) | The fraction of oxygen-evolving complexes (OEC) |
| RJ = (ψEo (control) − ψEo (treatment))/ψEo (control) = (VJ (treatment) − VJ (control))/(1 − VJ (control)) | Number of PS II RCs with QB-site filled by PS II inhibitor |
| Sm/tFmax = [RCopen/(RCclose+RCopen)]av = [QA/QA(total)]av | Average fraction of open RCs of PS II in the time span between 0 and tFM |
| Performance Index and Driving Force | Illustrations |
| PIABS = (RC/ABS)·[φPo/(1 − φPo)]·[ψo/(1 − ψo)] | Performance index (potential) for energy conservation from photons absorbed by PS II to the reduction of intersystem electron acceptors |
| PICSm = (RC/CSM)·[φPo/(1 − φPo)]·[ψo/(1 − ψo)] | Performance index based on cross section at t = tFM |
| DFABS = log(PIABS) | Driving force based on absorption of light energy |
| DFCS = log(PICS) | Driving force based on cross section |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Li, H.; Lv, Y.; Wang, Y.; Wang, Z.; Xin, C.; Liu, S.; Zhu, X.; Song, F.; Li, X. Salt Priming Protects Photosynthetic Electron Transport against Low-Temperature-Induced Damage in Wheat. Sensors 2020, 20, 62. https://doi.org/10.3390/s20010062
Li H, Li H, Lv Y, Wang Y, Wang Z, Xin C, Liu S, Zhu X, Song F, Li X. Salt Priming Protects Photosynthetic Electron Transport against Low-Temperature-Induced Damage in Wheat. Sensors. 2020; 20(1):62. https://doi.org/10.3390/s20010062
Chicago/Turabian StyleLi, Hui, Huawei Li, Yanjie Lv, Yongjun Wang, Zongshuai Wang, Caiyun Xin, Shengqun Liu, Xiancan Zhu, Fengbin Song, and Xiangnan Li. 2020. "Salt Priming Protects Photosynthetic Electron Transport against Low-Temperature-Induced Damage in Wheat" Sensors 20, no. 1: 62. https://doi.org/10.3390/s20010062
APA StyleLi, H., Li, H., Lv, Y., Wang, Y., Wang, Z., Xin, C., Liu, S., Zhu, X., Song, F., & Li, X. (2020). Salt Priming Protects Photosynthetic Electron Transport against Low-Temperature-Induced Damage in Wheat. Sensors, 20(1), 62. https://doi.org/10.3390/s20010062






