Fecal Malodor Detection Using Low-Cost Electrochemical Sensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Gas Sensors Selection and Characteristics
2.2. Sensor Assembly Device
2.3. Odor Generation and Collection
2.4. Experimental Setup and Protocol for Odor Sensing Experiments
2.5. Data Analysis
2.6. Analytical Methods
3. Results
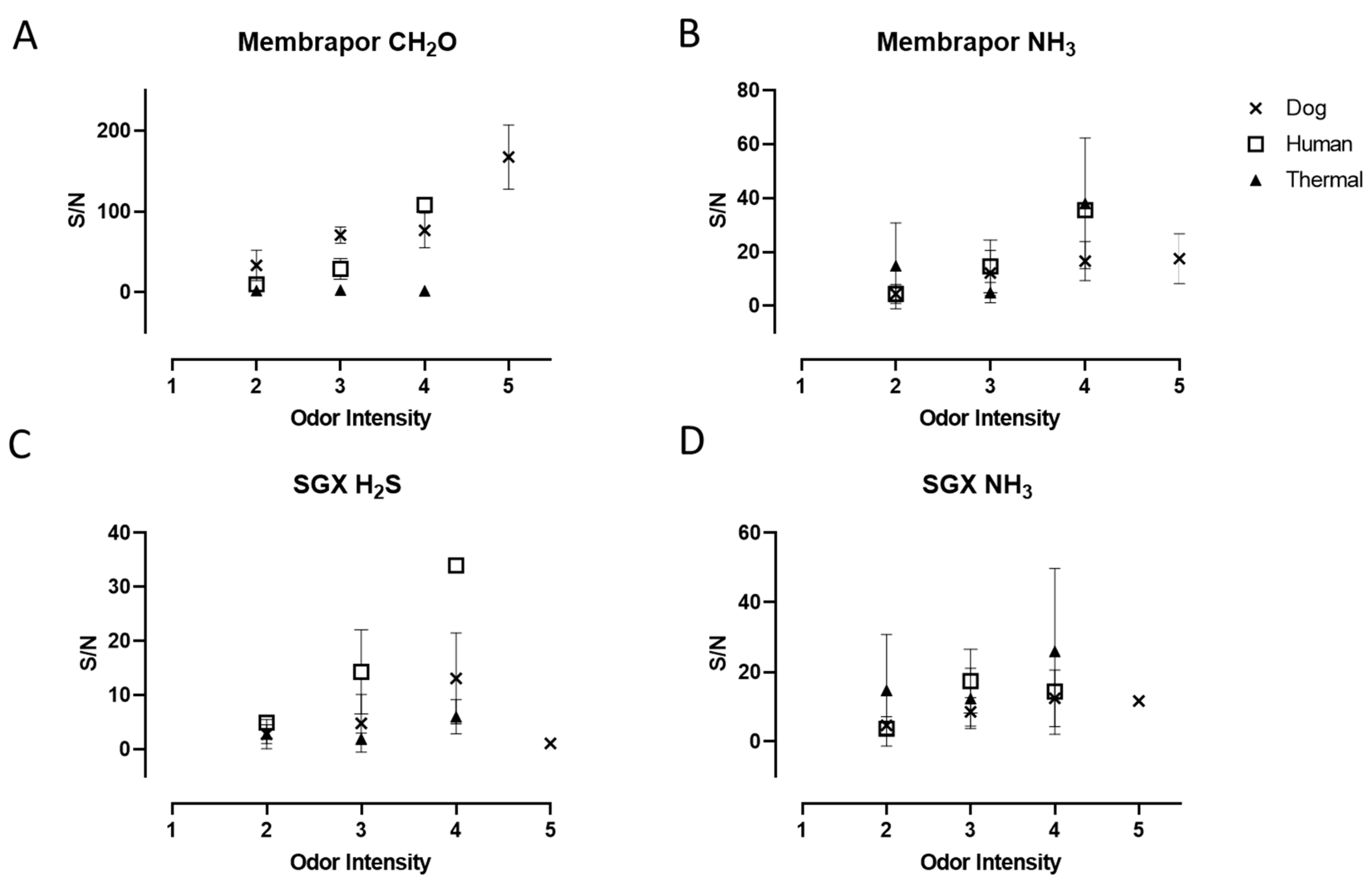
3.1. Electrochemical Sensors Response
3.2. Olfactometry Results
3.3. Relationship of Sensor Responses and Olfactometry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Progress on Household Drinking Water, Sanitation and Hygiene I 2000-2017 Special Focus on Inequalities. Available online: https://www.who.int/water_sanitation_health/publications/jmp-report-2019/en/ (accessed on 10 December 2019).
- Target 6.2 –Sanitation and Hygiene -sdg6monitoring. Available online: https://www.sdg6monitoring.org/indicators/target-6-2 (accessed on 10 December 2019).
- Reinvent the Toilet Challenge & Expo-Bill & Melinda Gates Foundation. Available online: https://www.gatesfoundation.org/what-we-do/global-growth-and-opportunity/water-sanitation-and-hygiene/reinvent-the-toilet-challenge-and-expo (accessed on 10 December 2019).
- Parkinson, J.; Tayler, K.; Colin, J.; Nema, A. A Guide to Decisionmaking Technology Options for Urban Sanitation in India. Water Sanit. Program. South. Asia World Bank New Delhi 2008, Part C, 55. Available online: http://mohua.gov.in/upload/uploadfiles/files/Urban_Sanitation.pdf (accessed on 19 May 2020).
- Bekchanov, M.; Evia, P. Working Paper 168 Resources Recovery in South and Southeast Asia. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3293535 (accessed on 19 May 2020).
- Lin, J.; Aoll, J.; Niclass, Y.; Velazco, M.I.; Wünsche, L.; Pika, J.; Starkenmann, C. Qualitative and quantitative analysis of volatile constituents from latrines. Environ. Sci. Technol. 2013, 47, 7876–7882. [Google Scholar] [CrossRef] [PubMed]
- Chappuis, C.J.F.; Niclass, Y.; Vuilleumier, C.; Starkenmann, C. Quantitative headspace analysis of selected odorants from latrines in africa and India. Environ. Sci. Technol. 2015, 49, 6134–6140. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Hirose, T.; Kimura, T.; Moriyama, Y.; Nakashima, Y. Analysis of malodorous volatile substances of human waste: Feces and urine. J. Health Sci. 2001, 47, 483–490. [Google Scholar] [CrossRef] [Green Version]
- DIN EN 13725-European Standards. Available online: https://www.en-standard.eu/din-en-13725-air-quality-determination-of-odour-concentration-by-dynamic-olfactometry/ (accessed on 10 December 2019).
- McGinley, C.M.; St, P.; McGinley, M.A.; McGinley, D.L. “Odor Basics”, Understanding and Using Odor Testing. In Proceedings of the the 22nd Annual Hawaii Water Environment Association Conference, Honolulu, HI, USA, 6–7 June 2000. [Google Scholar]
- Wu, C.; Liu, J.; Zhao, P.; Piringer, M.; Schauberger, G. Conversion of the chemical concentration of odorous mixtures into odour concentration and odour intensity: A comparison of methods. Atmos. Environ. 2016. [Google Scholar] [CrossRef]
- Akdeniz, N.; Jacobson, L.D.; Hetchler, B.P.; Bereznicki, S.D.; Heber, A.J.; Koziel, J.A.; Cai, L.; Zhang, S.; Parker, D.B. Odor and Odorous Chemical Emissions from Animal Buildings: Part 4. Correlations Between Sensory and Chemical Measurements. Trans. ASABE 2012, 55, 2347–2356. [Google Scholar] [CrossRef]
- Chappuis, C.J.-F.; Huber, R.; Niclass, Y.; Starkenmann, C. Simulating latrine conditions to assess perfume performance against malodour. Flavour Fragr. J. 2018, 33, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Capelli, L.; Sironi, S.; Céntola, P.; Del Rosso, R.; Il Grande, M. Electronic noses for the continuous monitoring of odours from a wastewater treatment plant at specific receptors: Focus on training methods. Sens. Actuators B Chem. 2008, 131, 53–62. [Google Scholar] [CrossRef]
- Nicolas, J.; Romain, A.C.; Ledent, C. The electronic nose as a warning device of the odour emergence in a compost hall. Sens. Actuators B Chem. 2006, 116, 95–99. [Google Scholar] [CrossRef]
- Kim, H.; Konnanath, B.; Sattigeri, P.; Wang, J.; Mulchandani, A.; Myung, N.; Deshusses, M.A.; Spanias, A.; Bakkaloglu, B. Electronic-nose for detecting environmental pollutants: Signal processing and analog front-end design. Analog. Integr. Circuits Signal. Process. 2012, 70, 15–32. [Google Scholar] [CrossRef]
- Falasconi, M.; Concina, I.; Gobbi, E.; Sberveglieri, V.; Pulvirenti, A.; Sberveglieri, G. Electronic Nose for Microbiological Quality Control of Food Products. Int. J. Electrochem. 2012, 2012, 1–12. [Google Scholar] [CrossRef]
- Lorwongtragool, P.; Wongchoosuk, C.; Kerdcharoen, T. Portable artificial nose system for assessing air quality in swine buildings. In Proceedings of the 2010 International Conference on Electrical Engineering/Electronics Computer Telecommunications and Information Technology (ECTI-CON), Chiang Mai, Thailand, 19–21 May 2010; pp. 2–5. [Google Scholar]
- Teo, A.W.; Garg, H.K.; Puthusserypady, S. Detection of humans buried in rubble: An electronic nose to detect human body odor. In Proceedings of the Second Joint 24th Annual Conference and the Annual Fall Meeting of the Biomedical Engineering Society] [Engineering in Medicine and Biology, Houston, TX, USA, 23–26 October 2002; pp. 1811–1812. [Google Scholar] [CrossRef]
- Hirano, S.H.; Hayes, G.R.; Truong, K.N. uSmell: Exploring the potential for gas sensors to classify odors in ubicomp applications relative to airflow and distance. Pers. Ubiquitous Comput. 2015, 19, 189–202. [Google Scholar] [CrossRef]
- Giungato, P.; de Gennaro, G.; Barbieri, P.; Briguglio, S.; Amodio, M.; de Gennaro, L.; Lasigna, F. Improving recognition of odors in a waste management plant by using electronic noses with different technologies, gas chromatography–mass spectrometry/olfactometry and dynamic olfactometry. J. Clean. Prod. 2016, 133, 1395–1402. [Google Scholar] [CrossRef]
- Milan, B.J.B.; Bootsma, S.S.K.; Bilsen, I. ENoses as a tool to measure odour nuisance caused by restaurants. Chem. Eng. Trans. 2016, 54, 61–66. [Google Scholar] [CrossRef]
- eNose-Comon Invent. Available online: https://www.comon-invent.com/enose/ (accessed on 10 December 2019).
- Zhou, J.; Welling, C.M.; Kawadiya, S.; Deshusses, M.A.; Grego, S.; Chakrabarty, K. Sensor-Array Optimization Based on Mutual Information for Sanitation-Related Malodor Alerts. In Proceedings of the 2019 IEEE Biomedical Circuits and Systems Conference (BioCAS), Nara, Japan, 17–19 October 2019; pp. 1–4. [Google Scholar]
- Turk, A.; Switala, E.D.; Thomas, S.H. Dynamic Olfactometry: Principles and Practice. J. Air Pollut. Control. Assoc. 2012, 30, 1289–1294. [Google Scholar] [CrossRef]
- Invernizzi, M.; Capelli, L.; Sironi, S. Proposal of Odor Nuisance Index as Urban Planning Tool. Chem. Senses 2017, 42, 105–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huerta, R.; Mosqueiro, T.S.; Fonollosa, J.; Rulkov, N.F.; Rodriguez-Lujan, I. Online Decorrelation of Humidity and Temperature in Chemical Sensors for Continuous Monitoring. Chemom. Intell. Lab. Syst. 2016. [Google Scholar] [CrossRef] [Green Version]
- Popoola, O.A.M.; Carruthers, D.; Lad, C.; Bright, V.B.; Mead, M.I.; Stettler, M.E.J.; Saffell, J.R.; Jones, R.L. Use of networks of low cost air quality sensors to quantify air quality in urban settings. Atmos. Environ. 2018, 194, 58–70. [Google Scholar] [CrossRef]
- Hirano, S.H.; Brubaker, J.R.; Patterson, D.J.; Hayes, G.R. Detecting cooking state with gas sensors during dry cooking. In Proceedings of the 2013 ACM International Joint Conference on Pervasive and Ubiquitous Computing, Zurich, Switzerland, 8–12 September 2013; pp. 411–414. [Google Scholar] [CrossRef]





| Compound | Concentration in Odorous Latrines (ppbv) | Odor Character | Odor Threshold (ppbv) |
|---|---|---|---|
| Hydrogen sulfide | 25–55 [7,8] | Rotten egg | 0.5–2 |
| Ammonia | 50–60 [8] | Sharp, pungent | 3000–20,000 |
| Butyric acid | 36 [7] | Rancid, vomit | 10–500 |
| Methyl mercaptan | 2–15 [8] | Rotten egg, fermented cabbage | 1–20 |
| Indole | 0.31 [7] | Fecal, musty | 5–20 |
| p-cresol | 1.2 [7] | Animal barn, medicinal | 0.05–9 |
| Acetic acid | 3–10 [8] | Sour, vinegar | 400–1000 |
| Propionaldehyde | 10 [8] | Sweet, ester | 50–200 |
| Trimethylamine | 10–100 [7] | Stale urine | 50–200 |
| Brand | Sensor | Retail Price (USD) | Gas | LDL 1 (ppmv) | Range (ppmv) | Sensitivity (nA/ppmv) |
|---|---|---|---|---|---|---|
| SGX | SGX-7H2S | $45 | H2S | <0.1 | 0–50 | 1700 ± 400 |
| Membrapor | H2S/C-10 | $87 | H2S | 0.003 | 0–10 | 4500 ± 1000 |
| SGX | EC4-20-SO2 | $98 | SO2 | 0.1 | 0–20 | 400–600 |
| SGX | SGX-4NH3 | $88 | NH3 | 1 | 0–100 | 100 ± 30 |
| Membrapor | NH3/CR-200 | $140 | NH3 | 0.06 | 0–200 | 90 ± 18 |
| Membrapor | Alc/C-100 | $130 | MeOH/EtOH | 0.03 | 0–100 | 1600 ± 600 |
| Membrapor | CH2O/C-10 | $105 | CH2O | 0.003 | 0–10 | 4600 ± 1200 |
| Membrapor | ETO/C-20 | $105 | Ethylene Oxide | 0.006 | 0–20 | 2500 ± 600 |
| CityTech | Sensoric TBM 2E 50 | $379 | Mercaptan | <0.14 | 0–14 | 40–100 2 |
| CityTech | Sensoric THT 3E 50 | $554 | Tetrahydrothiophene | <0.42 | 0–14 | 500 ± 180 |
| Odorant Sample | Number of Runs |
|---|---|
| Dog feces | 3 |
| Human feces | 2 |
| Thermally dried feces | 3 |
| Popcorn | 2 |
| Citrus odor control product | 2 |
| Water vapor (effect of relative humidity) | 1 |
| Odor Intensity Score | Description | Details |
|---|---|---|
| 0 | No odor | - |
| 1 | Very faint | Can detect presence of an odor, but unable to assign a character to the odor |
| 2 | Faint | Can detect an odor and assign character to the odor after some thought |
| 3 | Easily detectable | Can detect odor and assign character to the odor instantaneously |
| 4 | Strong | Unpleasant odor. Can withstand it, but would prefer to move away from the odor source |
| 5 | Very strong | Unacceptable odor. Would move away from the odor source as soon as possible |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawadiya, S.; Welling, C.; Grego, S.; Deshusses, M.A. Fecal Malodor Detection Using Low-Cost Electrochemical Sensors. Sensors 2020, 20, 2888. https://doi.org/10.3390/s20102888
Kawadiya S, Welling C, Grego S, Deshusses MA. Fecal Malodor Detection Using Low-Cost Electrochemical Sensors. Sensors. 2020; 20(10):2888. https://doi.org/10.3390/s20102888
Chicago/Turabian StyleKawadiya, Siddharth, Claire Welling, Sonia Grego, and Marc A. Deshusses. 2020. "Fecal Malodor Detection Using Low-Cost Electrochemical Sensors" Sensors 20, no. 10: 2888. https://doi.org/10.3390/s20102888
APA StyleKawadiya, S., Welling, C., Grego, S., & Deshusses, M. A. (2020). Fecal Malodor Detection Using Low-Cost Electrochemical Sensors. Sensors, 20(10), 2888. https://doi.org/10.3390/s20102888






