Monitoring Wheat Growth Using a Portable Three-Band Instrument for Crop Growth Monitoring and Diagnosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design of Field Experiments
2.2. Equipment
2.2.1. Portable Three-Band CGMD Instrument
2.2.2. ASD FieldSpec HandHeld2 Spectroradiometer
2.2.3. LAI-2200C Plant Canopy Analyzer
2.3. Measurement Method
2.3.1. Measurement of Spectral Data
2.3.2. Determination of Growth Indices
2.4. Data Analysis Methods
2.4.1. Vegetation Index Calculation
2.4.2. Data Analysis
3. Results
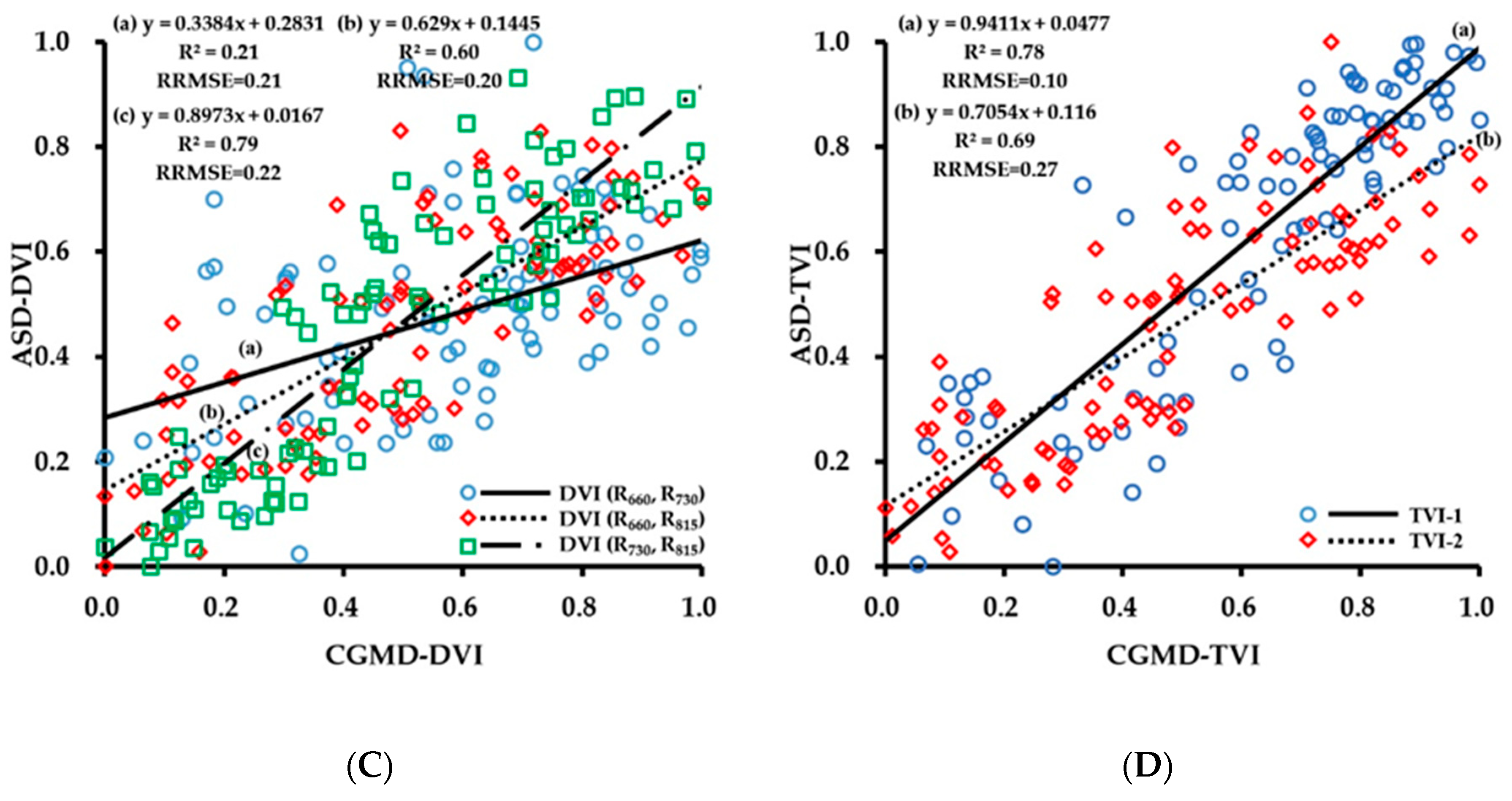
3.1. Evaluation of Data Acquisition Performance of the Portable Three-Band CGMD Instrument
3.2. Fitting Results of Vegetation Indices With Growth Indices
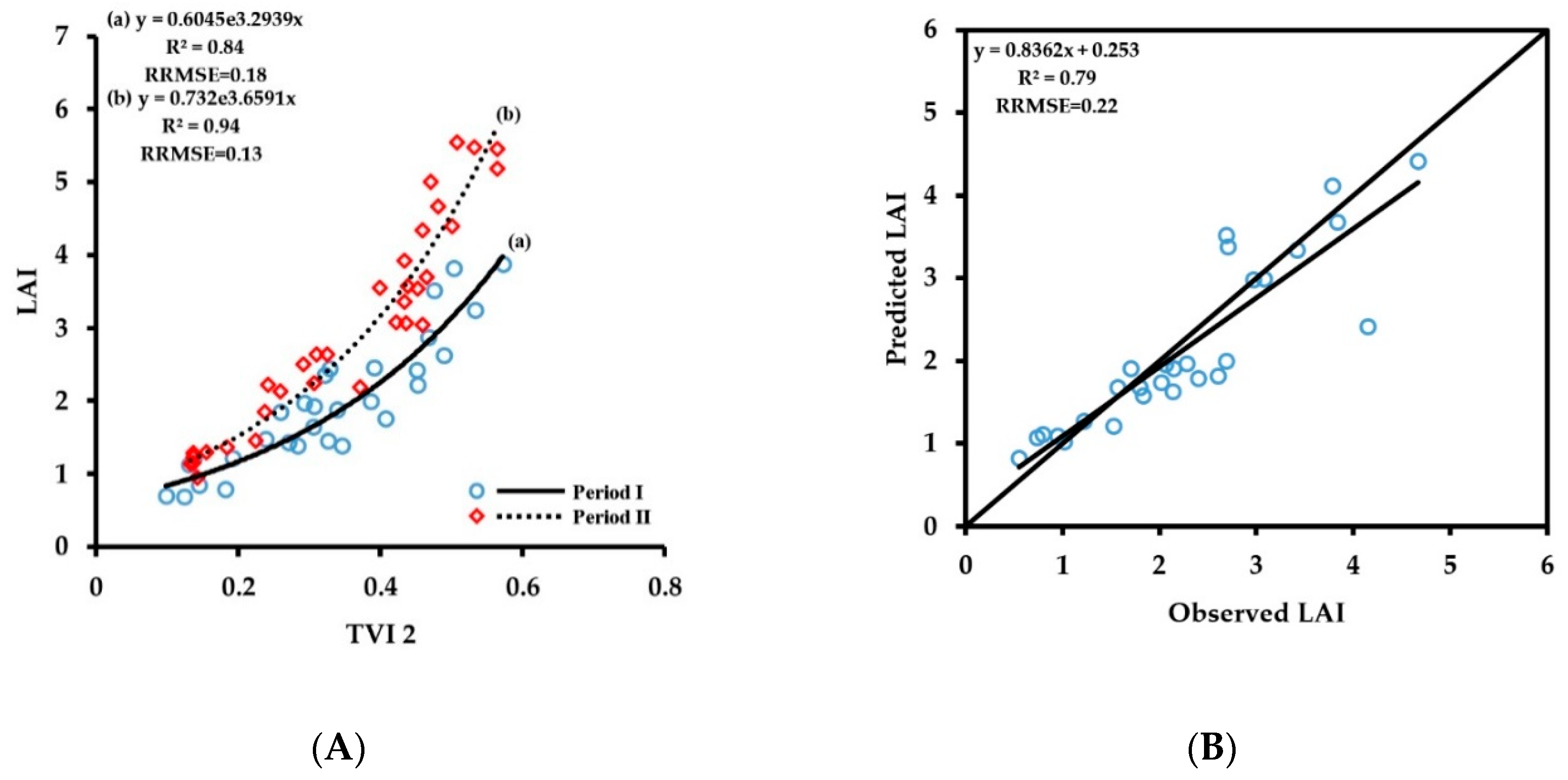
3.2.1. Fitting Results of Vegetation Indices with LAI
3.2.2. Fitting Results of Vegetation Indices with LDW
3.2.3. Fitting Results of Vegetation Indices with LNC
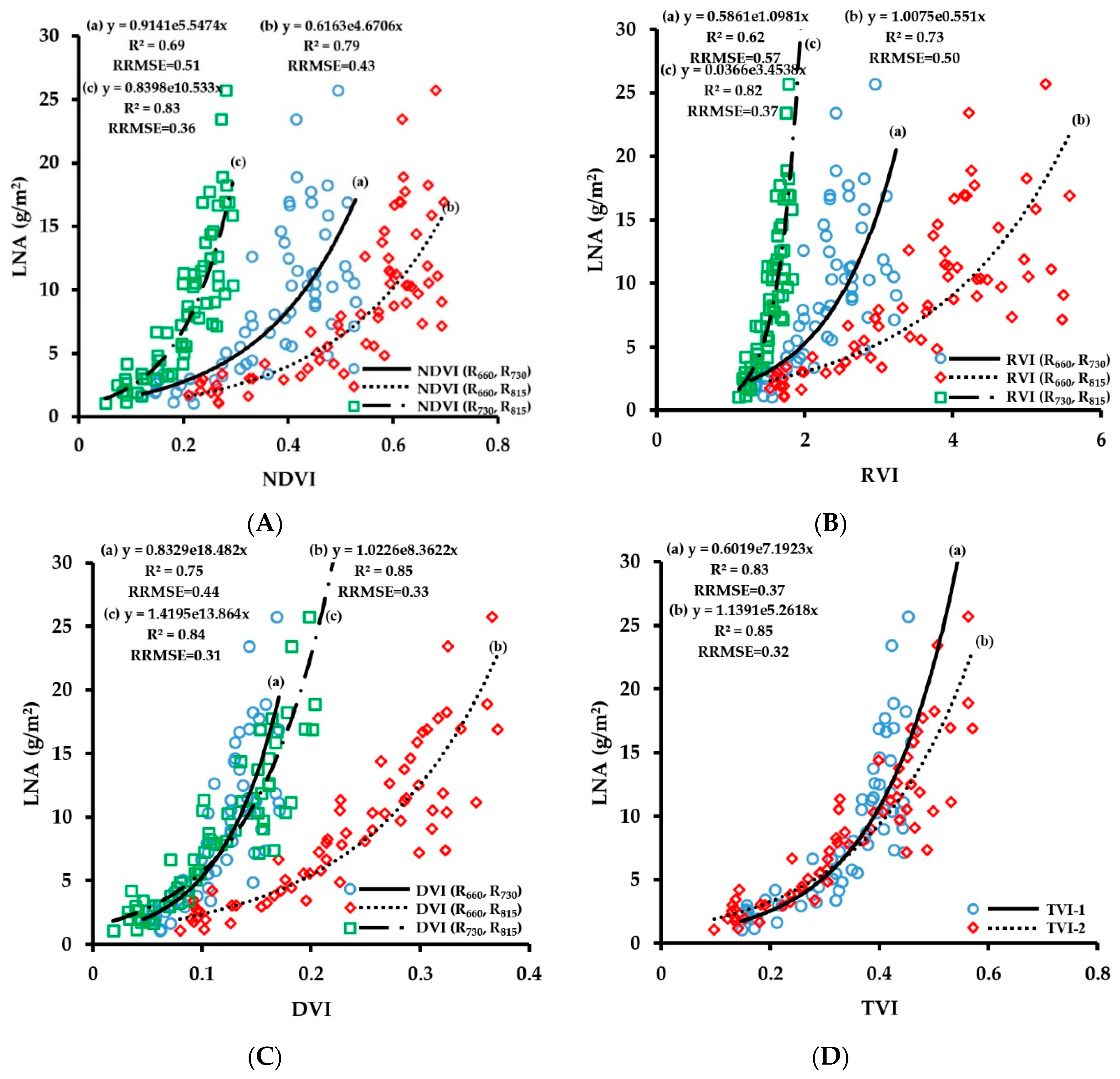
3.2.4. Fitting Results of Vegetation Indices with LNA
3.3. Spectral Monitoring Model of Wheat Growth
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Matthew, R.; David, B.; Chapman, S.C.; Furbank, R.T.; Yann, M.; Mather, D.E.; Parry, M.A.J. Raising yield potential of wheat. I. Overview of a consortium approach and breeding strategies. J. Exp. Bot. 2010, 62, 439–452. [Google Scholar]
- Cao, Q.; Cui, Z.; Chen, X.; Khosla, R.; Dao, T.H.; Miao, Y. Quantifying spatial variability of indigenous nitrogen supply for precision nitrogen management in small scale farming. Precis Agric. 2012, 13, 45–61. [Google Scholar] [CrossRef]
- Cao, W.X.; Zhu, Y.; Tian, Y.C.; Yao, X.; Tang, L.; Liu, X.J.; Ni, J. Development and Implementation of Crop Precision Cultivation Technology. Sci. Agric. Sin. 2011, 10, 583–595. [Google Scholar]
- Uddling, J.; Gelang-Alfredsson, J.; Piikki, K.; Pleijel, H. Evaluating the relationship between leaf chlorophyll concentration and SPAD-502 chlorophyll meter readings. Photosynth. Res. 2007, 91, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Cerovic, Z.G.; Masdoumier, G.; Ghozlen, N.B.; Latouche, G. A new optical leaf-clip meter for simultaneous non-destructive assessment of leaf chlorophyll and epidermal flavonoids. Physiol. Plant. 2012, 146, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Liu, X.; Liang, Y.; Cao, Q.; Tian, Y.C.; Zhu, Y.; Cao, W.X.; Liu, X.J. Using a Portable Active Sensor to Monitor Growth Parameters and Predict Grain Yield of Winter Wheat. Sensors 2019, 19, 1108. [Google Scholar] [CrossRef] [Green Version]
- Osborne, S.L. Utilization of Existing Technology to Evaluate Spring Wheat Growth and Nitrogen Nutrition in South Dakota. Commun. Soil Sci. Plan 2007, 38, 949–958. [Google Scholar] [CrossRef]
- Cao, Q.; Miao, Y.X.; Shen, J.N.; Yu, W.F.; Yuan, F.; Cheng, S.S.; Huang, S.Y.; Wang, H.Y.; Yang, W.; Liu, F.Y. Improving in-season estimation of rice yield potential and responsiveness to topdressing nitrogen application with Crop Circle active crop canopy sensor. Precis. Agric. 2016, 17, 136–154. [Google Scholar] [CrossRef]
- Cao, Q.; Miao, Y.; Li, F.; Gao, X.; Liu, B.; Lu, D.; Chen, X. Developing a new Crop Circle active canopy sensor-based precision nitrogen management strategy for winter wheat in North China Plain. Precis. Agric. 2017, 18, 2–18. [Google Scholar] [CrossRef]
- Feng, W.; Yao, X.; Zhu, Y.; Tian, Y.C.; Cao, W.X. Monitoring leaf nitrogen status with hyperspectral reflectance in wheat. Eur. J. Agron. 2008, 28, 394–404. [Google Scholar] [CrossRef]
- Ni, J.; Yao, X.; Tian, Y.C.; Cao, W.; Zhu, Y. Design and experiments of portable apparatus for plant growth monitoring and diagnosis. Trans. Chin. Soc. Agric. Eng. 2013, 29, 150–156. [Google Scholar]
- Ni, J.; Zhang, J.C.; Wu, R.S.; Pang, F.R.; Zhu, Y. Development of an Apparatus for Crop-Growth Monitoring and Diagnosis. Sensors 2018, 18, 3129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, X.; Guo, Y.J.; He, J.Y.; Yao, X.; Zhu, Y.; Cao, W.X.; Cheng, T.; Tian, Y.C. Comparison of Different Hyperspectral Vegetation Indices for Estimating Canopy Leaf Nitrogen Accumulation in Rice. Agron. J. 2014, 106, 1911–1920. [Google Scholar] [CrossRef]
- Yao, X.; Zhu, Y.; Tian, Y.C.; Feng, W.; Cao, W.X. Exploring hyperspectral bands and estimation indices for leaf nitrogen accumulation in wheat. Int. J. Appl. Earth Obs. 2010, 12, 89–100. [Google Scholar] [CrossRef]
- Li, Y.X.; Zhu, Y.; Dai, T.B.; Tian, Y.C.; Cao, W.X. Quantitative relationships between leaf area index and canopy reflectance spectra of wheat. Chin. J. Appl. Ecol. 2006, 17, 1443. [Google Scholar]
- Feng, W.; Zhu, Y.; Yao, X.; Tian, Y.C. Monitoring leaf dry weight and leaf area index in wheat with hyperspectral remote sensing. Chin. J. Plant Ecol. 2009, 33, 34–44. [Google Scholar]
- Tian, Y.C.; Zhu, Y.; Yao, X.; Liu, X.J.; Cao, W.X. Non-destructive monitoring of crop nitrogen nutrition based on spectral information. Chin. J. Ecol. 2007, 26, 1454–1463. [Google Scholar]
- Feng, W.; Yao, X.; Tian, Y.C.; Cao, W.X.; Zhu, Y. Monitoring leaf pigment status with hyperspectral remote sensing in wheat. Aust. J. Agric. Res. 2008, 59, 748. [Google Scholar] [CrossRef]
- Baret, F.; Champion, I.; Guyot, G.; Podaire, A. Monitoring wheat canopies with a high spectral resolution radiometer. Remote Sens. Environ. 1987, 22, 367–378. [Google Scholar] [CrossRef]
- Thenkabail, P.S.; Smith, R.B.; De Pauw, E. Hyperspectral Vegetation Indices and Their Relationships with Agricultural Crop Characteristics. Remote Sens. Environ. 2000, 71, 158–182. [Google Scholar] [CrossRef]
- Bunnik, N.J.J. The multispectral reflectance of shortwave radiation by agricultural crops in relation with their morphological and optical properties. Ph.D. Thesis, Landbouwhoge School Wageningen, Wageningen, The Netherlands, 1978. [Google Scholar]
- Zhu, Y.; Yao, X.; Tian, Y.; Liu, X.; Cao, W. Analysis of common canopy vegetation indices for indicating leaf nitrogen accumulations in wheat and rice. Int. J. Appl. Earth Obs. 2008, 10, 1–10. [Google Scholar] [CrossRef]
- Datt, B. A New Reflectance Index for Remote Sensing of Chlorophyll Content in Higher Plants: Tests using Eucalyptus Leaves. J. Plant Physiol. 1999, 154, 30–36. [Google Scholar] [CrossRef]
- Rodriguez, D.; Fitzgerald, G.J.; Belford, R.; Christensen, L.K. Detection of nitrogen deficiency in wheat from spectral reflectance indices and basic crop eco-physiological concepts. Aust. J. Agric. Res. 2006, 57, 781. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Yao, X.; Yao, X.; Tian, Y.; Liu, X.; Ni, J.; Cao, W.; Zhu, Y. Estimating leaf nitrogen concentration with three-band vegetation indices in rice and wheat. Field Crop. Res. 2012, 129, 90–98. [Google Scholar] [CrossRef]
- Zhao, C.J.; Wang, Z.J.; Wang, J.H.; Huang, W.J. Relationships of leaf nitrogen concentration and canopy nitrogen density with spectral features parameters and narrow-band spectral indices calculated from field winter wheat (Triticum aestivum L.) spectra. Int. J. Remote Sens. 2011, 33, 3472–3491. [Google Scholar] [CrossRef]
- Lennox, L.J.; Flanagan, M.J. An automated procedure for the determination of total Kjeldahl nitrogen. Water Res. 1982, 16, 1127–1133. [Google Scholar] [CrossRef]
- Rouse, J.W., Jr.; Haas, R.H.; Deering, D.W.; Schnell, J.A.; Harlan, J.C. Monitoring the Vernal Advancement and Retrogradation (Greenwave Effect) of Natural Vegetation. Technical Report; Texas A&M University, Remote Sensing Center: College Station, TX, USA, November 1974. [Google Scholar]
- Tan, Y.; Sun, J.; Zhang, B.; Chen, M.; Liu, Y.; Liu, X. Sensitivity of a Ratio Vegetation Index Derived from Hyperspectral Remote Sensing to the Brown Planthopper Stress on Rice Plants. Sensors 2019, 19, 375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, A.J.; Wiegand, C.L. Distinguishing Vegetation from Soil Background Information. Photogramm. Eng. Remote Sens. 1978, 43, 1541–1552. [Google Scholar]
- Myneni, R.B.; Hoffman, S.; Knyazikhin, Y.; Privette, J.L.; Glassy, J.; Tian, Y.; Wang, Y.; Song, X.; Zhang, Y.; Smith, G.R.; et al. Global products of vegetation leaf area and fraction absorbed PAR from year one of MODIS data. Remote Sens. Environ. 2002, 83, 214–231. [Google Scholar] [CrossRef] [Green Version]
- Erdle, K.; Mistele, B.; Schmidhalter, U. Comparison of active and passive spectral sensors in discriminating biomass parameters and nitrogen status in wheat cultivars. Field Crop Res 2011, 124, 74–84. [Google Scholar] [CrossRef]
- Hansen, P.M.; Schjoerring, J.K. Reflectance measurement of canopy biomass and nitrogen status in wheat crops using normalized difference vegetation indices and partial least squares regression. Remote Sens. Environ. 2003, 86, 542–553. [Google Scholar] [CrossRef]
- He, J.; Liu, B.F.; Guo, Y.; Wang, L.G.; Zheng, G.Q.; Li, J. Biomass estimation model of winter wheat (L.) using hyperspectral reflectances. J. Plant Nutr. Fertil. 2017, 23, 313–323. [Google Scholar]
- Tian, X.; Wu, W.B.; Zhou, Q.B.; Zhou, Y. Comparison of two inversion methods for winter wheat leaf area index based on hyperspectral remote sensing. Trans. Chin. Soc. Agric. Eng. 2013, 29, 139–147. [Google Scholar]
- Yao, Y.; Miao, Y.; Cao, Q.; Wang, H.; Gnyp, M.L.; Bareth, G.; Khosla, R.; Yang, W.; Liu, F.; Liu, C. In-Season Estimation of Rice Nitrogen Status with an Active Crop Canopy Sensor. IEEE J.-Stars 2014, 7, 4403–4413. [Google Scholar] [CrossRef]
- Wang, F.M.; Huang, J.F.; Tang, T.L.; Wang, X.Z. New Vegetation Index and Its Application in Estimating Leaf Area Index of Rice. Rice Sci. 2007, 14, 195–203. [Google Scholar] [CrossRef]
- Li, D.; Li, F.; Hu, Y.C.; Mistele, B.; Schmidhalter, U. Study on the Estimation of Nitrogen Content in Wheat and Maize Canopy Based on Band Optimization of Spectral Parameters. Spectrosc. Spect. Anal. 2016, 36, 1150–1157. [Google Scholar] [CrossRef]
- Li, F.; Mistele, B.; Hu, Y.; Chen, X.; Schmidhalter, U. Optimising three-band spectral indices to assess aerial N concentration, N uptake and aboveground biomass of winter wheat remotely in China and Germany. Isprs J. Photogramm. 2014, 92, 112–123. [Google Scholar] [CrossRef]
- Gitelson, A.A. Wide Dynamic Range Vegetation Index for Remote Quantification of Biophysical Characteristics of Vegetation. J. Plant Physiol. 2004, 161, 165–173. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Liu, B.F.; Li, J. Monitoring model of leaf area index of winter wheat based on hyperspectral reflectance at different growth stages. Trans. Chin. Soc. Agric. Eng. 2014, 30, 141–150. [Google Scholar]
- He, J.; Liu, B.F.; Li, S.M.; Guo, Y.; Wang, L.G.; Zhang, Y.; Li, J. Winter wheat grain protein content monitoring model driven by hyperspectral remote sensing images at different growth stages. Chin. J. Eco-Agric. 2017, 25, 865–875. [Google Scholar]
- Yao, Y.; Liu, Q.; Liu, Q.; Li, X. LAI retrieval and uncertainty evaluations for typical row-planted crops at different growth stages. Remote Sens. Environ. 2008, 112, 94–106. [Google Scholar] [CrossRef]
- Li, F.; Miao, Y.; Hennig, S.D.; Gnyp, M.L.; Chen, X.; Jia, L.; Bareth, G. Evaluating hyperspectral vegetation indices for estimating nitrogen concentration of winter wheat at different growth stages. Precis. Agric. 2010, 11, 335–357. [Google Scholar] [CrossRef]
- Yu, K.; Li, F.; Gnyp, M.L.; Miao, Y.; Bareth, G.; Chen, X. Remotely detecting canopy nitrogen concentration and uptake of paddy rice in the Northeast China Plain. Isprs J. Photogramm. 2013, 78, 102–115. [Google Scholar] [CrossRef]
- Zhao, J.; Huang, W.J.; Zhang, Y.H.; Jing, Y.S. Inversion of Leaf Area Index during Different Growth Stages in Winter Wheat. Spectrosc. Spect. Anal. 2013, 33, 2546–2552. [Google Scholar]
- Li, X.C.; Xu, X.G.; Bao, Y.S.; Huang, W.J.; Luo, J.H.; Dong, Y.Y.; Song, X.Y.; Wang, J.H. Retrieving LAI of Winter Wheat Based on Sensitive Vegetation Index by the Segmentation Method. Sci. Agric. Sin. 2012, 45, 3486–3496. [Google Scholar]
- Verstraete, M.M.; Pinty, B. Designing optimal spectral indexes for remote sensing applications. IEEE T Geosci. Remote 1996, 34, 1254–1265. [Google Scholar] [CrossRef]
- Xue, L.H.; Yang, L.Z.; Fan, X.H. Estimation of Nitrogen Content and C/N in Rice Leaves and Plant with Canopy Reflectance Spectra. Acta. Agronomica Sinica. 2006, 59, 289–302. [Google Scholar]
- Tian, Y.C.; Gu, K.J.; Chu, X.; Yao, X.; Cao, W.X.; Zhu, Y. Comparison of different hyperspectral vegetation indices for canopy leaf nitrogen concentration estimation in rice. Plant Soil. 2014, 376, 193–209. [Google Scholar] [CrossRef]
- Tian, Y.C.; Yao, X.; Yang, J.; Cao, W.X.; Hannaway, D.B.; Zhu, Y. Assessing newly developed and published vegetation indices for estimating rice leaf nitrogen concentration with ground- and space-based hyperspectral reflectance. Field Crop. Res. 2011, 120, 299–310. [Google Scholar] [CrossRef]















| Instrument | Sensor Size (mm) | Weight (kg) | Price (CNY) | Waveband (nm) | Light Source |
|---|---|---|---|---|---|
| CGMD | 54 × 38 × 38 | 1.6 | About 4000 | 660, 730, 815 | Sunlight |
| ASD Handheld 2 | 215 × 140 × 90 | 1.2 | Over 150,000 | 325–1075 | Sunlight |
| Crop Circle ACS-470 | 201 × 89 × 48 | 3.6 | Over 100,000 | 450, 550, 650, 670, 730, 760 | LED |
| GreenSeeker Handheld | 277 × 86 × 15 | 1.02 | Over 100,000 | 656, 774 | LED |
| SPAD-502 | 164 × 78 × 49 | 0.23 | Over 10,000 | 650, 940 | LED |
| Dualex 4 | 205 × 65 × 55 | 0.22 | Over 100,000 | 375, 655, 710, 850 | LED |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Lin, W.; Pang, F.; Jiang, X.; Cao, W.; Zhu, Y.; Ni, J. Monitoring Wheat Growth Using a Portable Three-Band Instrument for Crop Growth Monitoring and Diagnosis. Sensors 2020, 20, 2894. https://doi.org/10.3390/s20102894
Li H, Lin W, Pang F, Jiang X, Cao W, Zhu Y, Ni J. Monitoring Wheat Growth Using a Portable Three-Band Instrument for Crop Growth Monitoring and Diagnosis. Sensors. 2020; 20(10):2894. https://doi.org/10.3390/s20102894
Chicago/Turabian StyleLi, Huaimin, Weipan Lin, Fangrong Pang, Xiaoping Jiang, Weixing Cao, Yan Zhu, and Jun Ni. 2020. "Monitoring Wheat Growth Using a Portable Three-Band Instrument for Crop Growth Monitoring and Diagnosis" Sensors 20, no. 10: 2894. https://doi.org/10.3390/s20102894
APA StyleLi, H., Lin, W., Pang, F., Jiang, X., Cao, W., Zhu, Y., & Ni, J. (2020). Monitoring Wheat Growth Using a Portable Three-Band Instrument for Crop Growth Monitoring and Diagnosis. Sensors, 20(10), 2894. https://doi.org/10.3390/s20102894






