Exploring the Effects of Various Polymeric Backbones on the Performance of a Hydroxyaromatic 1,2,3-Triazole Anion Sensor
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of Precursors (PTP Derivatives)
2.1.1. Synthesis of 2-(4-(4-N-phenylacrylamide)-1H-1,2,3-triazole-1-yl)Phenol or P03
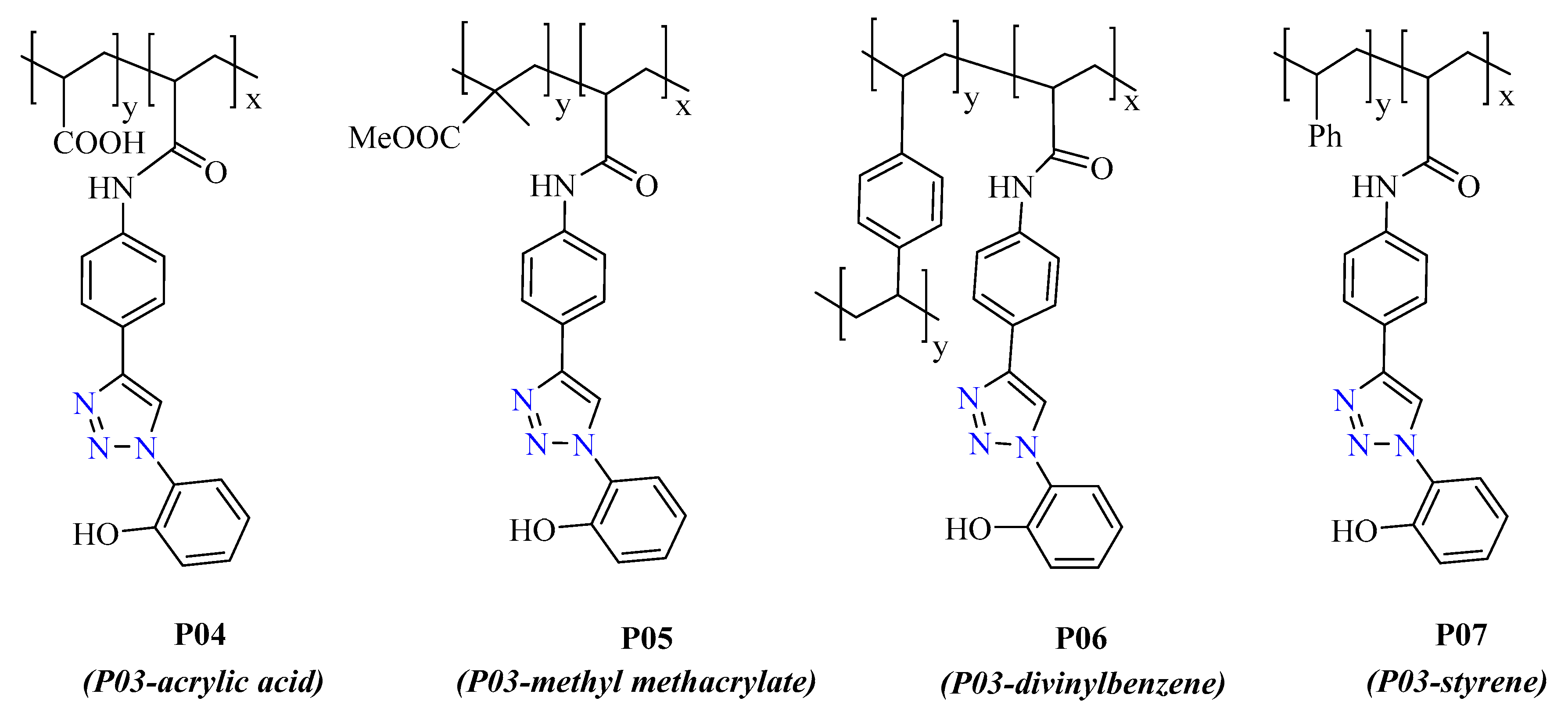
2.1.2. Synthesis of Polymeric Chemosensors: P04, P05, P06, and P07
3. Results and Discussion
3.1. Synthetic Approach
3.1.1. Synthesis of the Monomeric Sensor
3.1.2. Synthesis of Polymers
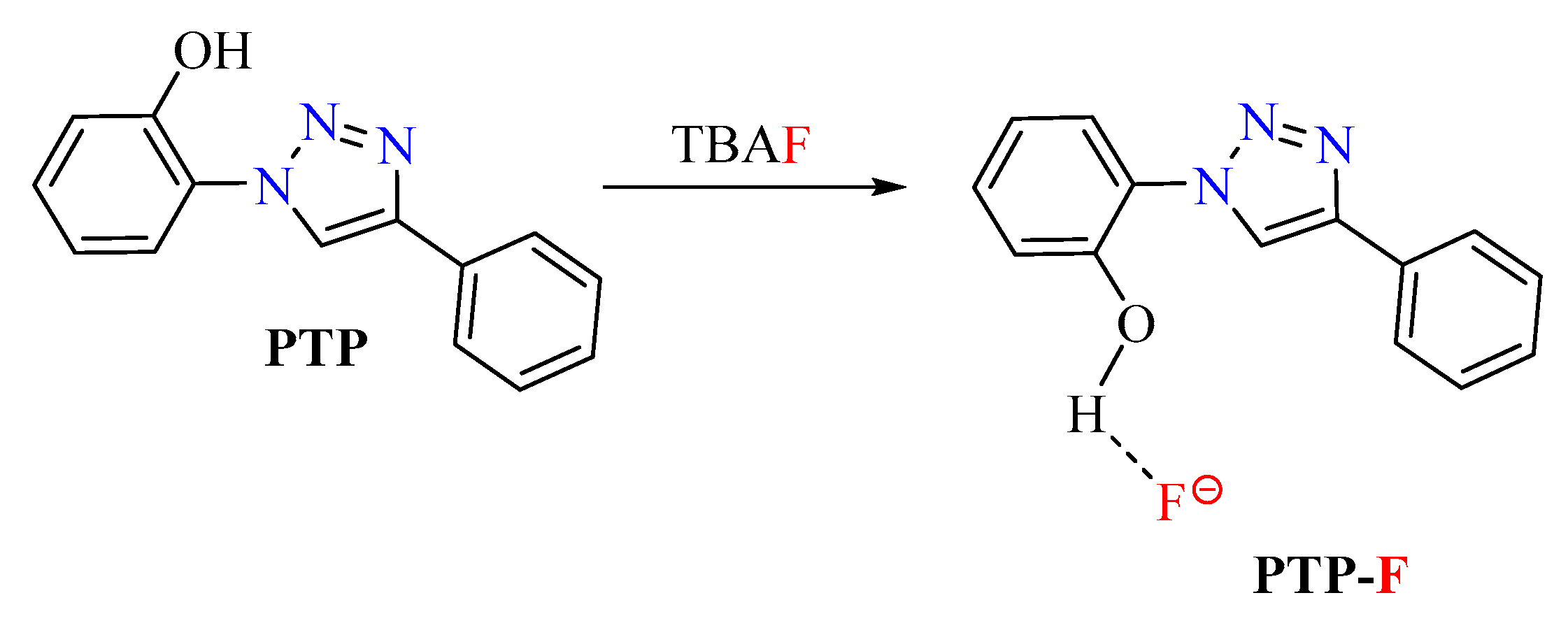
3.2. Anion Detection
3.2.1. Response with Synthetic Precursors
3.2.2. Response with Copolymers P04, P05, P06, and P07
3.2.3. Absorbance Spectroscopy
3.2.4. Fluorescence Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guo, A.; Zhu, R.; Ren, Y.; Dong, J.; Feng, L. A “turn-on” fluorescent chemosensor for aluminum ion and cell imaging application. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 153, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Kaur, G.; Fegade, U.A.; Singh, A.; Sahoo, S.K.; Kuwar, A.S.; Singh, N. Anion sensing with chemosensors having multiple NH recognition units. TrAC Trends Anal. Chem. 2017, 95, 86–109. [Google Scholar] [CrossRef]
- Balan Pillai, A.; Varghese, B.; Madhusoodanan, K.N. Design and Development of Novel Sensors for the Determination of Fluoride in Water. Environ. Sci. Technol. 2012, 46, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Tao, F.; Wang, H.; Li, Y.; Wang, L. A novel reversible colorimetric chemosensor for rapid naked-eye detection of Cu2+ in pure aqueous solution. Sens. Actuators B 2015, 211, 325–331. [Google Scholar] [CrossRef]
- Madhusudhana Reddy, P.; Hsieh, S.-R.; Chen, J.-K.; Chang, C.-J.; Kang, J.-Y.; Chen, C.-H. Robust, sensitive and facile method for detection of F−, CN− and Ac− anions. Spectrochim. Acta Part A 2017, 186, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Sakai, R.; Barasa, E.B.; Sakai, N.; Sato, S.-I.; Satoh, T.; Kakuchi, T. Colorimetric Detection of Anions in Aqueous Solution Using Poly(phenylacetylene) with Sulfonamide Receptors Activated by Electron Withdrawing Group. Macromolecules 2012, 45, 8221–8227. [Google Scholar] [CrossRef]
- Kaewtong, C.; Kampaengsri, S.; Singhana, B.; Pulpoka, B. Highly selective detection of Au3+ using rhodamine-based modified polyacrylic acid (PAA)-coated ITO. Dye. Pigment. 2017, 141, 277–285. [Google Scholar] [CrossRef]
- Chua, H.M.; Shah, W.K.; Zhou, H.; Xu, J. Recent Advances in Aggregation-Induced Emission Chemosensors for Anion Sensing. Molecules 2019, 24, 2711. [Google Scholar] [CrossRef]
- Wu, D.; Sedgwick, A.C.; Gunnlaugsson, T.; Akkaya, E.U.; Yoon, J.; James, T.D. Fluorescent chemosensors: The past, present and future. Chem. Soc. Rev. 2017, 46, 7105–7123. [Google Scholar] [CrossRef]
- Huang, X.; Meng, J.; Dong, Y.; Cheng, Y.; Zhu, C. Polymer-based fluorescence sensor incorporating triazole moieties for Hg2+ detection via click reaction. Polymer 2010, 51, 3064–3067. [Google Scholar] [CrossRef]
- Giri, D.; Islam, S.N.; Patra, S.K. Synthesis and characterization of 1,2,3-triazole appended polythiophene based reusable fluorescent probe for the efficient detection of trace nitroaromatics. Polymer 2018, 134, 242–253. [Google Scholar] [CrossRef]
- Xie, Z.; Kong, X.; Feng, L.; Ma, J.; Li, Y.; Wang, X.; Bao, W.; Shi, W.; Hui, Y. A novel highly selective probe with both aggregation-induced emission enhancement and intramolecular charge transfer characteristics for CN− detection. Sens. Actuators B 2018, 257, 154–165. [Google Scholar] [CrossRef]
- Johansson, J.R.; Beke-Somfai, T.; Said Stålsmeden, A.; Kann, N. Ruthenium-Catalyzed Azide Alkyne Cycloaddition Reaction: Scope, Mechanism, and Applications. Chem. Rev. 2016, 116, 14726. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Rhodes, S.; Winder, D.; Atkinson, A.; Gibson, J.; Ming, W.; Padgett, C.; Landge, S.; Aiken, K. Spectroscopic investigation of bis-appended 1,2,3-triazole probe for the detection of Cu(II) ion. J. Mol. Struct. 2017, 1134, 638–648. [Google Scholar] [CrossRef]
- Lauko, J.; Kouwer, P.H.J.; Kasak, P.; Rowan, A.E. Tunable properties based on regioselectivity of 1,2,3-triazole units in axially chiral 2,2′-linked 1,1′-binaphthyl-based copolymers for ions and acid responsiveness. Eur. Polym. J. 2018, 108, 191–198. [Google Scholar] [CrossRef]
- Park, S.Y.; Yoon, J.H.; Hong, C.S.; Souane, R.; Kim, J.S.; Matthews, S.E.; Vicens, J. A Pyrenyl-Appended Triazole-Based Calix[4]arene as a Fluorescent Sensor for Cd2+ and Zn2+. J. Org. Chem. 2008, 73, 8212–8218. [Google Scholar] [CrossRef]
- Juwarker, H.; Lenhardt, J.M.; Pham, D.M.; Craig, S.L. 1,2,3-Triazole CH⋅⋅⋅Cl− Contacts Guide Anion Binding and Concomitant Folding in 1,4-Diaryl Triazole Oligomers. Angew. Chem. Int. Ed. 2008, 47, 3740–3743. [Google Scholar] [CrossRef]
- Li, Y.; Griend, D.A.V.; Flood, A.H. Modelling triazolophane–halide binding equilibria using Sivvu analysis of UV–vis titration data recorded under medium binding conditions. Supramol. Chem. 2009, 21, 111–117. [Google Scholar] [CrossRef]
- Li, Y.; Flood, A.H. Strong, Size-Selective, and Electronically Tunable C−H···Halide Binding with Steric Control over Aggregation from Synthetically Modular, Shape-Persistent [34]Triazolophanes. J. Am. Chem. Soc. 2008, 130, 12111–12122. [Google Scholar] [CrossRef]
- Hein, J.E.; Fokin, V.V. Copper-catalyzed azide–alkyne cycloaddition (CuAAC) and beyond: New reactivity of copper(I) acetylides. Chem. Soc. Rev. 2010, 39, 1302–1315. [Google Scholar] [CrossRef]
- Ghosh, D.; Rhodes, S.; Hawkins, K.; Winder, D.; Atkinson, A.; Ming, W.; Padgett, C.; Orvis, J.; Aiken, K.; Landge, S. A simple and effective 1,2,3-triazole based “turn-on” fluorescence sensor for the detection of anions. New J. Chem. 2015, 39, 295–303. [Google Scholar] [CrossRef]
- Meisner, Q.J.; Accardo, J.V.; Hu, G.; Clark, R.J.; Jiang, D.-E.; Zhu, L. Fluorescence of Hydroxyphenyl-Substituted “Click” Triazoles. J. Phys. Chem. A 2018, 122, 2956–2973. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Landge, S.; Zhu, L.; Ofulue, U.; Akinsoji, O.A.; Govan, R.D.; Ugboya, A.; Hernandez, V.; Yates, K.; Henderson, A.; et al. The influence of amino substituents on the signal-output, selectivity, and sensitivity of a hydroxyaromatic 1,2,3-triazolyl chemosensor for anions—A structure–property relationship investigation. J. Phys. Org. Chem. 2020, e4078. [Google Scholar] [CrossRef]
- Landge, S.M.; Lazare, D.Y.; Freeman, C.; Bunn, J.; Cruz, J.I.; Winder, D.; Padgett, C.; Aiken, K.S.; Ghosh, D. Rationally designed phenanthrene derivatized triazole as a dual chemosensor for fluoride and copper recognition. Spectrochim. Acta Part A 2020, 228, 117758. [Google Scholar] [CrossRef] [PubMed]
- Aiken, K.; Bunn, J.; Sutton, S.; Christianson, M.; Winder, D.; Freeman, C.; Padgett, C.; McMillen, C.; Ghosh, D.; Landge, S. Nuclear Magnetic Resonance Spectroscopy Investigations of Naphthalene-Based 1,2,3-Triazole Systems for Anion Sensing. Magnetochemistry 2018, 4, 15. [Google Scholar] [CrossRef]
- Orita, R.; Franckevičius, M.; Vyšniauskas, A.; Gulbinas, V.; Sugiyama, H.; Uekusa, H.; Kanosue, K.; Ishige, R.; Ando, S. Enhanced fluorescence of phthalimide compounds induced by the incorporation of electron-donating alicyclic amino groups. Phys. Chem. Chem. Phys. 2018, 20, 16033–16044. [Google Scholar] [CrossRef]
- Sutradhar, T.; Misra, A. Role of Electron-Donating and Electron-Withdrawing Groups in Tuning the Optoelectronic Properties of Difluoroboron–Napthyridine Analogues. J. Phys. Chem. A 2018, 122, 4111–4120. [Google Scholar] [CrossRef]
- Abou-Hatab, S.; Spata, V.A.; Matsika, S. Substituent Effects on the Absorption and Fluorescence Properties of Anthracene. J. Phys. Chem. A 2017, 121, 1213–1222. [Google Scholar] [CrossRef]
- Williams, R.T.; Bridges, J.W. Fluorescence of solutions: A review. J. Clin. Pathol. 1964, 17, 371–394. [Google Scholar] [CrossRef]
- Seely, G.R. Quenching of pyrochlorophyll fluorescence by nitro compounds. J. Phys. Chem. 1969, 73, 125–129. [Google Scholar] [CrossRef]
- Das, B.; Hossain, S.M.; Pakhira, B.; Pramanick, A.K.; Das, R.; Ray, M. Fluorescence quenching based detection of p-nitrophenol using luminescent silicon nanocrystals and insights into the quenching mechanism. Semicond. Sci. Technol. 2020, 35, 035003. [Google Scholar] [CrossRef]
- Ooyama, Y.; Aoyama, S.; Furue, K.; Uenaka, K.; Ohshita, J. Fluorescence sensor for water based on PET (photo-induced electron transfer): Anthracene-bis(aminomethyl)phenylboronic acid ester. Dyes Pigm. 2015, 123, 248–253. [Google Scholar] [CrossRef]
- Ooyama, Y.; Uenaka, K.; Matsugasako, A.; Harima, Y.; Ohshita, J. Molecular design and synthesis of fluorescence PET (photo-induced electron transfer) sensors for detection of water in organic solvents. RSC Adv. 2013, 3, 23255–23263. [Google Scholar] [CrossRef]
- Ooyama, Y.; Furue, K.; Uenaka, K.; Ohshita, J. Development of highly-sensitive fluorescence PET (photo-induced electron transfer) sensor for water: Anthracene–boronic acid ester. RSC Adv. 2014, 4, 25330–25333. [Google Scholar] [CrossRef]
- Wu, T.-Y.; Chen, Y. Synthesis and characterization of luminescent copolymers containing iminodibenzyl and divinylbenzene chromophores. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 3847–3857. [Google Scholar] [CrossRef]
- Gao, W.; Yan, M.; Ge, S.; Liu, X.; Yu, J. Fluorescent sensor based on a novel conjugated polyfluorene derivative. Spectrochim. Acta Part A 2012, 95, 218–223. [Google Scholar] [CrossRef]
- Swager, T.M. The Molecular Wire Approach to Sensory Signal Amplification. Acc. Chem. Res. 1998, 31, 201–207. [Google Scholar] [CrossRef]
- Li, T.; Zhou, C.; Jiang, M. UV absorption spectra of polystyrene. Polym. Bull. 1991, 25, 211–216. [Google Scholar] [CrossRef]
- Koban, W.; Koch, J.D.; Hanson, R.K.; Schulz, C. Absorption and fluorescence of toluene vapor at elevated temperatures. Phys. Chem. Chem. Phys. 2004, 6, 2940–2945. [Google Scholar] [CrossRef]
- Benesi, H.A.; Hildebrand, J.H. A Spectrophotometric Investigation of the Interaction of Iodine with Aromatic Hydrocarbons. J. Am. Chem. Soc. 1949, 71, 2703–2707. [Google Scholar] [CrossRef]
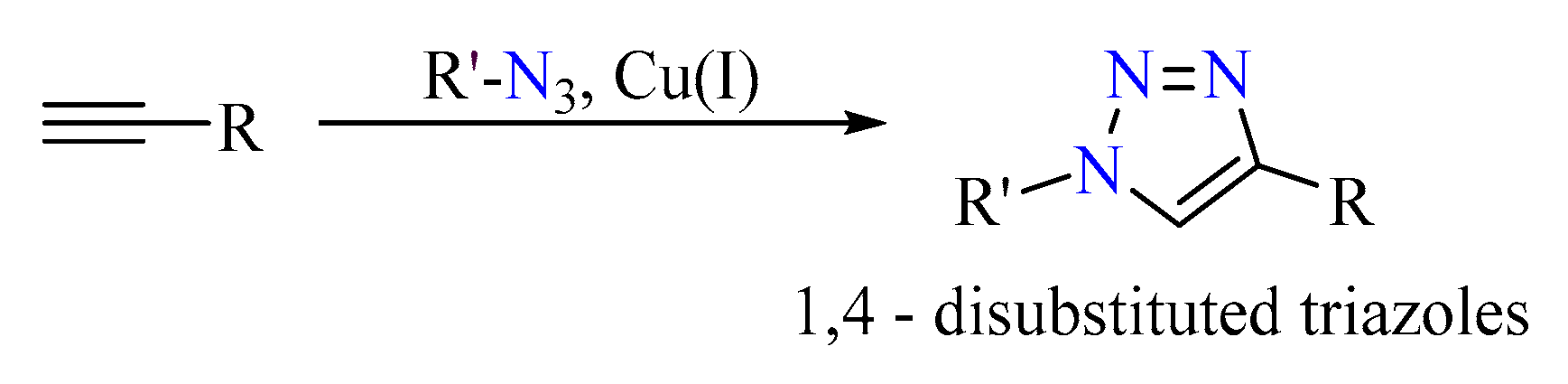







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ugboya, A.; Monroe, K.; Ofulue, U.; Yates, K.; Ghosh, D.; Landge, S.M.; Quirino, R.L.; Aiken, K.S. Exploring the Effects of Various Polymeric Backbones on the Performance of a Hydroxyaromatic 1,2,3-Triazole Anion Sensor. Sensors 2020, 20, 2973. https://doi.org/10.3390/s20102973
Ugboya A, Monroe K, Ofulue U, Yates K, Ghosh D, Landge SM, Quirino RL, Aiken KS. Exploring the Effects of Various Polymeric Backbones on the Performance of a Hydroxyaromatic 1,2,3-Triazole Anion Sensor. Sensors. 2020; 20(10):2973. https://doi.org/10.3390/s20102973
Chicago/Turabian StyleUgboya, Aikohi, Khristal Monroe, Unodinma Ofulue, Kayley Yates, Debanjana Ghosh, Shainaz M. Landge, Rafael Lopes Quirino, and Karelle S. Aiken. 2020. "Exploring the Effects of Various Polymeric Backbones on the Performance of a Hydroxyaromatic 1,2,3-Triazole Anion Sensor" Sensors 20, no. 10: 2973. https://doi.org/10.3390/s20102973
APA StyleUgboya, A., Monroe, K., Ofulue, U., Yates, K., Ghosh, D., Landge, S. M., Quirino, R. L., & Aiken, K. S. (2020). Exploring the Effects of Various Polymeric Backbones on the Performance of a Hydroxyaromatic 1,2,3-Triazole Anion Sensor. Sensors, 20(10), 2973. https://doi.org/10.3390/s20102973






