Fiber Optic Particle Plasmon Resonance-Based Immunoassay Using a Novel Multi-Microchannel Biochip
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
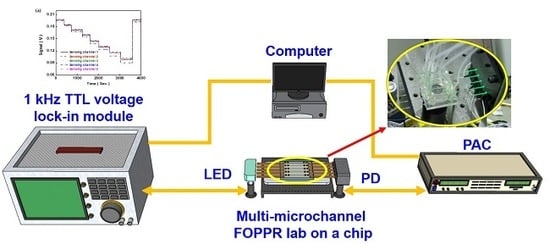
2.2. Multi-Microchannel Biochip Biosensing System
2.3. The Refractive Index Sensitivity Test by a Multi-Microchannel Biosensing System
2.4. Functionalization of Fiber-Optic Probe
2.5. Preparation of Standard Biological Samples and Statistical Analysis
3. Results and Discussion
3.1. Sensing Principle of the FOPPR
3.2. Stability and Reproducibility of FOPPR by Multi-Microchannel Biochip
3.3. Bio Selectivity—Nonspecific Adsorption Test
3.4. Detection of Receptor-Analyte Pairs by Multi-Microchannel FOPPR Lab on a Sensing Chip
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, X.; Modur, V.; Carayannopoulos, L.N.; Laterza, O.F. Biomarkers in Pharmaceutical Research. Clin. Chem. 2015, 61, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
- Tokonami, S.; Shiigi, H.; Nagaoka, T. Review: Micro- and nanosized molecularly imprinted polymers for high-throughput analytical applications. Anal. Chim. Acta 2009, 641, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Kingsmore, S.F. Multiplexed protein measurement: Technologies and applications of protein and antibody arrays. Nat. Rev. Drug Discov. 2006, 5, 310–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raman, B.; Meier, D.C.; Evju, J.K.; Semancik, S. Designing and optimizing microsensor arrays for recognizing chemical hazards in complex environments. Sens. Actuators B Chem. 2009, 137, 617–629. [Google Scholar] [CrossRef]
- Deyati, A.; Younesi, E.; Hofmann-Apitius, M.; Novac, N. Challenges and opportunities for oncology biomarker discovery. Drug Discov. Today 2013, 18, 614–624. [Google Scholar] [CrossRef]
- Kim, S.J.; Gobi, K.V.; Iwasaka, H.; Tanaka, H.; Miura, N. Novel miniature SPR immunosensor equipped with all-in-one multi-microchannel sensor chip for detecting low-molecular-weight analytes. Biosens. Bioelectron. 2007, 23, 701–707. [Google Scholar] [CrossRef]
- Qu, J.-H.; Dillen, A.; Saeys, W.; Lammertyn, J.; Spasic, D. Advancements in SPR biosensing technology: An overview of recent trends in smart layers design, multiplexing concepts, continuous monitoring and in vivo sensing. Anal. Chim. Acta 2020, 1104, 10–27. [Google Scholar] [CrossRef]
- Xie, L.; Yan, X.; Du, Y. An aptamer based wall-less LSPR array chip for label-free and high throughput detection of biomolecules. Biosens. Bioelectron. 2014, 53, 58–64. [Google Scholar] [CrossRef]
- Wu, C.-W.; Chiang, C.-Y.; Chen, C.-H.; Chiang, C.-S.; Wang, C.-T.; Chau, L.-K. Self-referencing fiber optic particle plasmon resonance sensing system for real-time biological monitoring. Talanta 2016, 146, 291–298. [Google Scholar] [CrossRef]
- Jagannathan, L.; Subramanian, V. DNA detection using organic thin film transistors: Optimization of DNA immobilization and sensor sensitivity. Biosens. Bioelectron. 2009, 25, 288–293. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, X.; Sun, C.; Li, J.; Zhu, R.; Gu, Z. Encoded Silica Colloidal Crystal Beads as Supports for Potential Multiplex Immunoassay. Anal. Chem. 2008, 80, 1598–1605. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Liu, T.; Zhang, X.-B.; Yang, Y.; Chen, T.; Wu, C.; Liu, Y.; Zhu, G.; Huan, S.; Fu, T.; et al. DLISA: A DNAzyme-Based ELISA for Protein Enzyme-Free Immunoassay of Multiple Analytes. Anal. Chem. 2015, 87, 7746–7753. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Z.; Miao, J.; Cui, L.; Guan, W. High-throughput and label-free parasitemia quantification and stage differentiation for malaria-infected red blood cells. Biosens. Bioelectron. 2017, 98, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Yuan, R.; Chai, Y. Magnetic Control of an Electrochemical Microfluidic Device with an Arrayed Immunosensor for Simultaneous Multiple Immunoassays. Clin. Chem. 2007, 53, 1323–1329. [Google Scholar] [CrossRef]
- Wu, J.; Yan, F.; Tang, J.; Zhai, C.; Ju, H. A Disposable Multianalyte Electrochemical Immunosensor Array for Automated Simultaneous Determination of Tumor Markers. Clin. Chem. 2007, 53, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- Morais, S.; Tortajada-Genaro, L.A.; Arnandis-Chover, T.; Puchades, R.; Maquieira, A. Multiplexed Microimmunoassays on a Digital Versatile Disk. Anal. Chem. 2009, 81, 5646–5654. [Google Scholar] [CrossRef]
- Tighe, P.J.; Ryder, R.R.; Todd, I.; Fairclough, L.C. ELISA in the multiplex era: Potentials and pitfalls. Proteom. Clin. Appl. 2015, 9, 406–422. [Google Scholar] [CrossRef]
- Lei, K.F.; Liu, T.-K.; Tsang, N.-M. Towards a high throughput impedimetric screening of chemosensitivity of cancer cells suspended in hydrogel and cultured in a paper substrate. Biosens. Bioelectron. 2018, 100, 355–360. [Google Scholar] [CrossRef]
- Li, G.; Xu, L.; Wu, W.; Wang, D.; Jiang, J.; Chen, X.; Zhang, W.; Poapolathep, S.; Poapolathep, A.; Zhang, Z.; et al. On-Site Ultrasensitive Detection Paper for Multiclass Chemical Contaminants via Universal Bridge-Antibody Labeling: Mycotoxin and Illegal Additives in Milk as an Example. Anal. Chem. 2019, 91, 1968–1973. [Google Scholar] [CrossRef]
- Qureshi, A.; Niazi, J.H.; Kallempudi, S.; Gurbuz, Y. Label-free capacitive biosensor for sensitive detection of multiple biomarkers using gold interdigitated capacitor arrays. Biosens. Bioelectron. 2010, 25, 2318–2323. [Google Scholar] [CrossRef]
- Cheng, S.-F.; Chau, L.-K. Colloidal Gold-Modified Optical Fiber for Chemical and Biochemical Sensing. Anal. Chem. 2003, 75, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Chau, L.-K.; Lin, Y.-F.; Cheng, S.-F.; Lin, T.-J. Fiber-optic chemical and biochemical probes based on localized surface plasmon resonance. Sens. Actuators B Chem. 2006, 113, 100–105. [Google Scholar] [CrossRef]
- Chen, C.-D.; Cheng, S.-F.; Chau, L.-K.; Wang, C.R.C. Sensing capability of the localized surface plasmon resonance of gold nanorods. Biosens. Bioelectron. 2007, 22, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Lai, N.-S.; Wang, C.-C.; Chiang, H.-L.; Chau, L.-K. Detection of antinuclear antibodies by a colloidal gold modified optical fiber: Comparison with ELISA. Anal. Bioanal. Chem. 2007, 388, 901–907. [Google Scholar] [CrossRef]
- Chiang, C.-Y.; Hsieh, M.-L.; Huang, K.-W.; Chau, L.-K.; Chang, C.-M.; Lyu, S.-R. Fiber-optic particle plasmon resonance sensor for detection of interleukin-1β in synovial fluids. Biosens. Bioelectron. 2010, 26, 1036–1042. [Google Scholar] [CrossRef]
- Hsu, W.-T.; Hsieh, W.-H.; Cheng, S.-F.; Jen, C.-P.; Wu, C.-C.; Li, C.-H.; Lee, C.-Y.; Li, W.-Y.; Chau, L.-K.; Chiang, C.-Y.; et al. Integration of fiber optic-particle plasmon resonance biosensor with microfluidic chip. Anal. Chim. Acta 2011, 697, 75–82. [Google Scholar] [CrossRef]
- Huang, K.-W.; Hsieh, C.-W.; Kan, H.-C.; Hsieh, M.-L.; Hsieh, S.; Chau, L.-K.; Cheng, T.-E.; Lin, W.-T. Improved performance of aminopropylsilatrane over aminopropyltriethoxysilane as a linker for nanoparticle-based plasmon resonance sensors. Sens. Actuators B Chem. 2012, 163, 207–215. [Google Scholar] [CrossRef]
- Chang, T.-C.; Wu, C.-C.; Wang, S.-C.; Chau, L.-K.; Hsieh, W.-H. Using A Fiber Optic Particle Plasmon Resonance Biosensor To Determine Kinetic Constants of Antigen–Antibody Binding Reaction. Anal. Chem. 2013, 85, 245–250. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Chiang, C.-Y.; Li, C.-H.; Chang, T.-C.; Chiang, C.-S.; Chau, L.-K.; Huang, K.-W.; Wu, C.-W.; Wang, S.-C.; Lyu, S.-R. Quantification of tumor necrosis factor-α and matrix metalloproteinases-3 in synovial fluid by a fiber-optic particle plasmon resonance sensor. Analyst 2013, 138, 4599–4606. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Huang, C.-H.; Lu, S.-H.; Kuo, I.T.; Chau, L.-K. Direct detection of orchid viruses using nanorod-based fiber optic particle plasmon resonance immunosensor. Biosens. Bioelectron. 2014, 51, 371–378. [Google Scholar] [CrossRef]
- Wu, W.-T.; Chen, C.-H.; Chiang, C.-Y.; Chau, L.-K. Effect of Surface Coverage of Gold Nanoparticles on the Refractive Index Sensitivity in Fiber-Optic Nanoplasmonic Sensing. Sensors 2018, 18, 1759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.-H.; Tsao, T.-C.; Li, W.-Y.; Shen, W.-C.; Cheng, C.-W.; Tang, J.-L.; Jen, C.-P.; Chau, L.-K.; Wu, W.-T. Novel U-shape gold nanoparticles-modified optical fiber for localized plasmon resonance chemical sensing. Microsyst. Technol. 2010, 16, 1207–1214. [Google Scholar] [CrossRef]
- Chen, C.-H.; Yeh, B.-K.; Tang, J.-L.; Wu, W.-T. Fabrication Quality Analysis of a Fiber Optic Refractive Index Sensor Created by CO2 Laser Machining. Sensors 2013, 13, 4067–4087. [Google Scholar]
- Chen, C.-H.; Tsao, T.-C.; Tang, J.-L.; Wu, W.-T. A Multi-D-Shaped Optical Fiber for Refractive Index Sensing. Sensors 2010, 10, 4794–4804. [Google Scholar] [CrossRef] [Green Version]
- Nath, N.; Chilkoti, A. A Colorimetric Gold Nanoparticle Sensor To Interrogate Biomolecular Interactions in Real Time on a Surface. Anal. Chem. 2002, 74, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Martinsson, E.; Sepulveda, B.; Chen, P.; Elfwing, A.; Liedberg, B.; Aili, D. Optimizing the Refractive Index Sensitivity of Plasmonically Coupled Gold Nanoparticles. Plasmonics 2014, 9, 773–780. [Google Scholar] [CrossRef]
- Fry, D.R.; Bobbitt, D.R. Hapten immobilization for antibody sensing using a dynamic modification protocol. Talanta 2001, 55, 1195–1203. [Google Scholar] [CrossRef]










| Slope | Correlation Coefficient (R) | Sensor Resolution (RIU) | |
|---|---|---|---|
| Sensing channel 1 | −7.14 | 0.9993 | 6.30 × 10−6 |
| Sensing channel 2 | −7.14 | 0.9984 | 6.30 × 10−6 |
| Sensing channel 3 | −7.23 | 0.9996 | 6.22 × 10−6 |
| Sensing channel 4 | −7.41 | 0.9978 | 6.07 × 10−6 |
| Sensing channel 5 | −7.19 | 0.9990 | 6.26 × 10−6 |
| Average | 6.23 ± 0.10 × 10−6 | ||
| Slope | Correlation Coefficient (R) | LOD (g/mL) | |
|---|---|---|---|
| Sensing channel 1 | −0.0149 | 0.9929 | 2.5 × 10−8 |
| Sensing channel 2 | −0.0130 | 0.9950 | 3.2 × 10−8 |
| Sensing channel 3 | −0.0144 | 0.9913 | 3.1 × 10−8 |
| Sensing channel 4 | −0.0139 | 0.9955 | 2.8 × 10−8 |
| Sensing channel 5 | −0.0143 | 0.9949 | 3.0 × 10−8 |
| Average | 2.92 ± 0.28 × 10−8 | ||
| ΔI1/I0 | ΔI2/I0 | ΔI3/I0 | ΔI4/I0 | ΔI5/I0 | ΔI6/I0 | |
|---|---|---|---|---|---|---|
| Sensing channel 1 | 0.0085 | 0.0153 | 0.0219 | 0.0324 | 0.0395 | 0.0462 |
| Sensing channel 2 | 0.0085 | 0.0146 | 0.0197 | 0.0297 | 0.0349 | 0.0418 |
| Sensing channel 3 | 0.0094 | 0.0147 | 0.0211 | 0.0314 | 0.0383 | 0.0457 |
| Sensing channel 4 | 0.0092 | 0.0159 | 0.0205 | 0.0309 | 0.0370 | 0.0451 |
| Sensing channel 5 | 0.0097 | 0.0157 | 0.0212 | 0.0318 | 0.0374 | 0.0467 |
| CV% | 5.95 | 4.36 | 4.02 | 3.25 | 4.53 | 4.31 |
| Slope | Correlation Coefficient (R) | LOD (g/mL) | |
|---|---|---|---|
| Sensing channel 1 | −0.0250 | 0.9977 | 7.5 × 10−8 |
| Sensing channel 2 | −0.0223 | 0.9970 | 7.3 × 10−8 |
| Sensing channel 3 | −0.0206 | 0.9929 | 7.8 × 10−8 |
| Sensing channel 4 | −0.0224 | 0.9988 | 6.9 × 10−8 |
| Sensing channel 5 | −0.0226 | 0.9984 | 7.9 × 10−8 |
| Average | 7.48 ± 0.40 × 10−8 | ||
| ΔI1/I0 | ΔI2/I0 | ΔI3/I0 | ΔI4/I0 | ΔI5/I0 | |
|---|---|---|---|---|---|
| Sensing channel 1 | 0.0059 | 0.016 | 0.0320 | 0.0433 | 0.0548 |
| Sensing channel 2 | 0.0070 | 0.016 | 0.03058 | 0.0408 | 0.0503 |
| Sensing channel 3 | 0.0067 | 0.0154 | 0.0304 | 0.0379 | 0.0468 |
| Sensing channel 4 | 0.0061 | 0.0155 | 0.0291 | 0.0388 | 0.0504 |
| Sensing channel 5 | 0.0064 | 0.0156 | 0.0305 | 0.0402 | 0.0503 |
| CV% | 7.12 | 2.65 | 3.59 | 5.14 | 5.64 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, C.-Y.; Chen, C.-H.; Wang, C.-T. Fiber Optic Particle Plasmon Resonance-Based Immunoassay Using a Novel Multi-Microchannel Biochip. Sensors 2020, 20, 3086. https://doi.org/10.3390/s20113086
Chiang C-Y, Chen C-H, Wang C-T. Fiber Optic Particle Plasmon Resonance-Based Immunoassay Using a Novel Multi-Microchannel Biochip. Sensors. 2020; 20(11):3086. https://doi.org/10.3390/s20113086
Chicago/Turabian StyleChiang, Chang-Yue, Chien-Hsing Chen, and Chien-Tsung Wang. 2020. "Fiber Optic Particle Plasmon Resonance-Based Immunoassay Using a Novel Multi-Microchannel Biochip" Sensors 20, no. 11: 3086. https://doi.org/10.3390/s20113086
APA StyleChiang, C.-Y., Chen, C.-H., & Wang, C.-T. (2020). Fiber Optic Particle Plasmon Resonance-Based Immunoassay Using a Novel Multi-Microchannel Biochip. Sensors, 20(11), 3086. https://doi.org/10.3390/s20113086