Polyelectrolytes Assembly: A Powerful Tool for Electrochemical Sensing Application
Abstract
:1. Introduction
2. Polyelectrolytes in Layer-by-Layer (LbL) Sensor Assembling
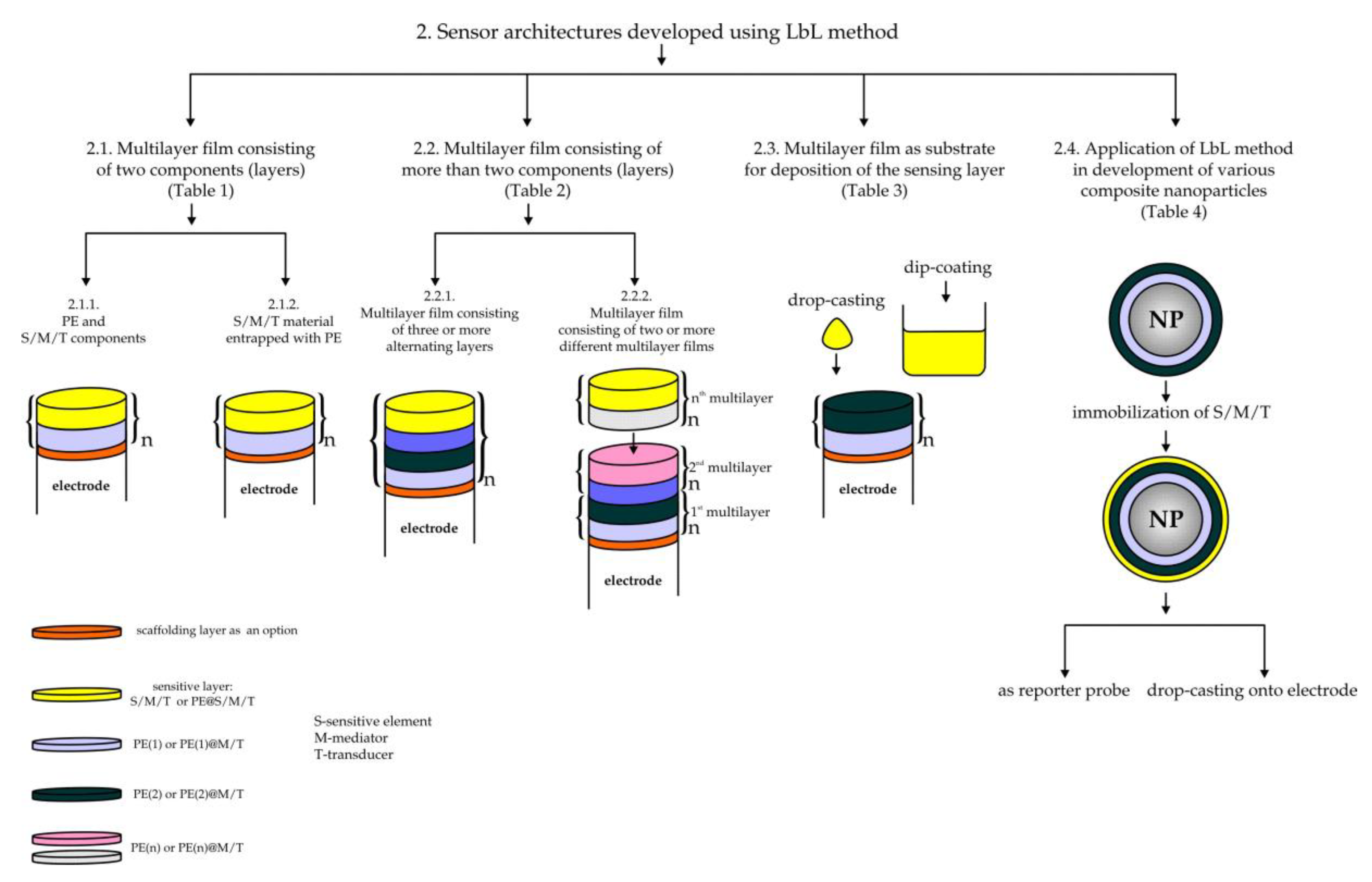
2.1. Multilayer Film Consisting of Two Components (Layer)
2.1.1. Sensing Film Consisting of Two Alternate Deposited Layers: PE and a Sensing/Mediating/Transducing Component
Redox Mediators (Complex Compound) as a Layer
Me or MeO NPs as a Layer
Carbon-Based Nanoparticles as a Layer
DNA as a Layer
Enzyme as a Layer
2.1.2. Sensing/Mediating/Transducing Material Is Entrapped with PE and Applied as a Self-Containing Layer in a Multilayer Film Consisting of Two Alternately Deposited Layers (Repeated Bilayers)
Me or MeO NPs–PE as a Layer
Carbon-Based Nanoparticle–PE as a Layer
2.2. Multilayer Film Consisting of More Than Two Components (Layers)
2.2.1. Multilayer Film Consisting of Three or More Alternately Deposited Layers
Redox Mediators (Complex Compounds) as a Layer
DNA as a Layer
Enzyme as a Layer
2.2.2. Multilayer Film Consisting of Two or More Multilayer Films
Me and MeO NPs as a Layer
Carbon-Based Nanomaterials as a Layer
Enzyme as a Layer
2.3. Multilayer Film as a Substrate (“Precursor” Film) for Deposition of a Sensing Layer
2.3.1. Redox Mediators (Complex Compounds) in a “Precursor” Multilayer Film
2.3.2. Me or MeO NPs in a “Precursor” Multilayer Film
2.3.3. DNA in a “Precursor” Multilayer Film
2.3.4. Unmodified PEs as a “Precursor” Multilayer Film
2.4. Application of the LbL Method in Development of Various Composite Nanoparticles (Nanospheres, Hollow-Shell Particles, Reporter Probes. etc.) and Other Sensor Architectures
2.4.1. Composite Nanoparticles (Nanospheres, Hollow-Shell Particles, Reported Probes)
2.4.2. LbL Procedure in Other Sensor Architectures
3. Polyelectrolytes in Casting/Coating Methods
3.1. Sensing/Mediating/Transducing Component Is Entrapped with PE
3.1.1. Redox Mediators (Complex Compounds)–PE Composite
3.1.2. Me or MeO NPs–PE Composite
3.1.3. Carbon-Based NPs–PE Composite
3.1.4. Other–PE Composite
3.2. Sensing Films Consisting of Three or More Components
3.2.1. Sensing Film Comprising Me or MeO NPs
3.2.2. Sensing Film Comprising DNA
3.2.3. Sensing Film Comprising an Enzyme
3.3. PE in Preparation of Scaffolding Layer by Drop-Casting
3.3.1. Redox Mediators (Complex Compounds)–PE Composite
3.3.2. Me or MeO NPs–PE Composite
3.3.3. Carbon-Based Nanomaterials–PE Composite
3.3.4. Me or MeO NPs–Carbon-Based Nanomaterials–PE Composite
3.3.5. PE or PEC as a Scaffolding Layer
3.3.6. Other Uses of PE in Casting/Coating Mode
3.4. PE or PEC as Sensing Layer
3.5. Other Methods Involved in Application of PEs in Sensor Architectures
3.5.1. Electrospinning and Spin-Coating of PE onto a Working Electrode
3.5.2. PEs in Construction of Reporter Probes, Nanoprobes, etc.
4. Conclusions and Future Perspectives
Funding
Conflicts of Interest
Abbreviations
| PEs: | |
| MP | carboxymethylpullulan; |
| CNCC | carboxylated nanocrystalline cellulose; |
| CS | chitosan; |
| HA | humic acid; |
| PAA(+) | poly(acrylamide); |
| PAA(−) | poly(acrylic acid); |
| PADA | poly[acrylamide-co-(diallyldimethylammonium chloride)]; |
| PAH | poly(allylamine hydrochloride); |
| PAM | polyacrylamide; |
| PAMAM | poly(amidoamine) dendrimer; |
| PAS | sodium poly(anethol sulphonate); |
| PC | pectin; |
| PDADMAC | poly(diallyldimethylammonium chloride); |
| PDDA | poly(diallyldimethylammonium chloride); |
| PEI PHD | poly(ethylenimine); poly(hydroxyethyl methacrylate-poly[2-(dimethylamino)ethyl methacrylate; |
| PHEMA-b PDMAEMA PSS | poly(hydroxyethyl methacrylate; poly[2-(dimethylamino)ethyl methacrylate;poly(styrene sulphonate); |
| PQ11 | poly [(2-ethyldimethylammonioethyl methacrylate ethyl sulphate)-co-(1-vinylpyrrolidone); |
| PVP | poly(vinyl pyrrolidone); |
| PVS | poly(vinylsulphate); |
| SCS | sulphonated chitosan; |
| SiPyCl | 3-n-propylpyridinium chloride silsesquioxane. |
| Carbon Nanomaterials: | |
| CNS | carbon nanosphere; |
| CNO | carbon nano-onions; |
| ErGO | electrochemically reduced graphene oxide; |
| GO | graphene oxide; |
| MWCNTs | multi-wall carbon nanotubes; |
| rGO | reduced graphene oxide; |
| SWCNTs | single-wall carbon nanotubes. |
| DNA: | |
| cDNA | complementary DNA; |
| ssDNA | single stranded DNA. |
| Enzymes: | |
| AChE | acetylcholinesterase; |
| ChOx | choline oxidase; |
| HRP | horse radish peroxidase; |
| GOx | glucose oxidase; |
| LOx | lactate oxidase; |
| SOD | sarcosine oxidase. |
| Electrode: | |
| FET | field-effect transistor; |
| FTO | fluorine doped tin oxide; |
| GCE | glassy carbon electrode; |
| IDE | planar interdigitated electrode; |
| ITO | indium tin oxide; |
| SPAu | screen-printed gold electrode; |
| SPCE | screen-printed carbon electrode; |
| SPGE | screen-printed graphite electrode. |
| Other: | |
| APTES | 3-aminopropyl-triethoxysilane; |
| BSA | bovine serum albumin; |
| CD | cyclodextrin; |
| GTH | glutaraldehyde; |
| MB | methylene blue; |
| MG | methylene green; |
| MPA | mercaptopropionic acid; |
| PANI | polyaniline; |
| PB | Prussian blue; |
| PEDOT | poly(3,4-ethylenedioxythiophene); |
| PSA | polystyrene-co-acrylic acid; |
| SDS | sodium dodecyl sulphate; |
| S-PEDOT | sulphonated poly(3,4-ethylenedioxythiophene); |
| TEOS | tetraethyl orthosilicate. |
| Electroanalytical Methods: | |
| AMP | amperometry; |
| CV | cyclic voltammetry; |
| DPASV | differential pulse anodic stripping voltammetry; |
| DPV | differential pulse voltammetry; |
| EIS | use of the electrochemical impedance spectroscopy; |
| IMP | impedimetry; |
| LSV | linear sweep voltammetry; |
| POT | potentiometry; |
| SWV | square-wave voltammetry |
References
- Scheuing, D. Size exclusion chromatography of polyelectrolytes in dimethylformamide. J. Appl. Polym. Sci. 1984, 29, 2819–2828. [Google Scholar] [CrossRef]
- Tuo, X.; Chen, D.; Wang, X. Preparation of azo polyelectrolyte self-assembled multilayers by using N,N-dimethylformamide/H2O mixtures as solvents. Front. Chem. China 2006, 1, 329–333. [Google Scholar] [CrossRef]
- Cohen Stuart, M.; de Vries, R.; Lykema, H. Polyelectrolytes. In Fundamentals of Interface and Colloid Science, 1st ed.; Lyklema, J., Ed.; Academic Press: Cambridge, MA, USA, 2005; Volume 5, pp. 2.1–2.84. [Google Scholar]
- Van der Gucht, J.; Spruijt, E.; Lemmers, M.; Cohen Stuart, M.A. Polyelectrolyte Complexes: Bulk Phases and Colloidal Systems. J. Colloid Interface Sci. 2011, 361, 407–422. [Google Scholar] [CrossRef]
- Izumrudov, V.; Mussabayeva, B.; Murzagulova, K. Polyelectrolyte multilayers: Preparation and applications. Russ. Chem. Rev. 2018, 87, 192–200. [Google Scholar] [CrossRef]
- Das, B.; Tsianou, M. From polyelectrolyte complexes to polyelectrolyte multilayers: Electrostatic assembly, nanostructure, dynamics, and functional properties. Adv. Colloid Interface Sci. 2017, 244, 71–89. [Google Scholar] [CrossRef] [PubMed]
- Fares, H.; Wang, Q.; Yang, M.; Schlenoff, J. Swelling and Inflation in Polyelectrolyte Complexes. Macromolecules 2018, 52, 610–619. [Google Scholar] [CrossRef]
- Insua, I.; Wilkinson, A.; Fernandez-Trillo, F. Polyion complex (PIC) particles: Preparation and biomedical applications. Eur. Poly. J. 2016, 81, 198–215. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.; Belbekhouche, S.; Dubot, P.; Carbonnier, B.; Grande, D. From the functionalization of polyelectrolytes to the development of a versatile approach to the synthesis of polyelectrolyte multilayer films with enhanced stability. J. Mater.Chem. A 2017, 5, 24472–24483. [Google Scholar] [CrossRef]
- Delcea, M.; Möhwald, H.; Skirtach, A. Stimuli-responsive LbL capsules and nanoshells for drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 730–747. [Google Scholar] [CrossRef]
- Pillay, V.; Tsai, T.; Choonara, Y.; du Toit, L.; Kumar, P.; Modi, G.; Naidoo, D.; Tomar, L.K.; Tyagi, C.; Ndesendo, V.M.K. A review of integrating electroactive polymers as responsive systems for specialized drug delivery applications. J. Biomed. Mat. Res. Part A 2013, 102, 2039–2054. [Google Scholar] [CrossRef]
- Al-Maadeed, P. Self-Repairing Composites for Corrosion Protection: A Review on Recent Strategies and Evaluation Methods. Materials 2019, 12, 2754. [Google Scholar] [CrossRef] [Green Version]
- Palencia, M.; Córdoba, A.; Melendrez, M. Nanocomposites based on cationic polyelectrolytes and silver nanoparticles: Synthesis, characterization, molybdate retention and antimicrobial activity. Arab. J. Chem. 2019, 12, 825–834. [Google Scholar] [CrossRef]
- Escorihuela, J.; Narducci, R.; Compañ, V.; Costantino, F. Proton Conductivity of Composite Polyelectrolyte Membranes with Metal-Organic Frameworks for Fuel Cell Applications. Adv. Mater. Interfaces 2018, 6, 1801146. [Google Scholar] [CrossRef]
- Kreuer, K.; Portale, G. A Critical Revision of the Nano-Morphology of Proton Conducting Ionomers and Polyelectrolytes for Fuel Cell Applications. Adv. Funct. Mater. 2013, 23, 5390–5397. [Google Scholar] [CrossRef]
- Li, L.; Pascal, T.; Connell, J.; Fan, F.; Meckler, S.; Ma, L.; Chiang, Y.M.; Prendergast, D.; Helms, B.A. Molecular understanding of polyelectrolyte binders that actively regulate ion transport in sulfur cathodes. Nat. Commun. 2017, 8, 2277. [Google Scholar] [CrossRef]
- Hou, W.; Xiao, Y.; Han, G.; Lin, J. The Applications of Polymers in Solar Cells: A Review. Polymers 2019, 11, 143. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Liu, B. Water-soluble conjugated polymers as the platform for protein sensors. Polym. Chem. 2010, 1, 252–259. [Google Scholar] [CrossRef]
- Jeong, J.; Woo, S.; Le, V.; Choi, H.; Woo, H. Combination of conjugated polyelectrolytes and biomolecules: A new optical platform for highly sensitive and selective chemo- and biosensors. Macromol. Res. 2014, 22, 461–473. [Google Scholar] [CrossRef]
- Ding, L.; Fang, Y. Chemically assembled monolayers of fluorophores as chemical sensing materials. Chem. Soc. Rev. 2010, 39, 4258–4273. [Google Scholar] [CrossRef]
- Rivero, P.; Goicoechea, J.; Arregui, F. Layer-by-Layer Nano-Assembly: A Powerful Tool for Optical Fiber Sensing Applications. Sensors 2019, 19, 683. [Google Scholar] [CrossRef] [Green Version]
- Ambade, A.; Sandanaraj, B.; Klaikherd, A.; Thayumanavan, S. Fluorescent polyelectrolytes as protein sensors. Polym. Int. 2007, 56, 474–481. [Google Scholar] [CrossRef]
- Feng, F.; He, F.; An, L.; Wang, S.; Li, Y.; Zhu, D. Fluorescent Conjugated Polyelectrolytes for Biomacromolecule Detection. Adv. Mat. 2008, 20, 2959–2964. [Google Scholar] [CrossRef]
- Feng, X.; Liu, L.; Wang, S.; Zhu, D. Water-soluble fluorescent conjugated polymers and their interactions with biomacromolecules for sensitive biosensors. Chem. Soc. Rev. 2010, 39, 2411–2419. [Google Scholar] [CrossRef]
- Liu, Y.; Ogawa, K.; Schanze, K. Conjugated polyelectrolytes as fluorescent sensors. J. Photochem. Photobiol. C 2009, 10, 173–190. [Google Scholar] [CrossRef]
- Barsan, M.M.; Brett, C.M.A. Graphene and carbon nanotube nanomaterials in layer-by-layer structured electrochemical enzymatic biosensors: A review. Studia Univ. Babes-Bolyia Chem. 2015, 60, 31–52. [Google Scholar]
- Prifitis, D. Polyelectrolyte-graphene nanocomposites for biosensing application. Curr. Org. Chem. 2015, 19, 1819–1827. [Google Scholar] [CrossRef]
- Lutkenhaus, J.; Hammond, P. Electrochemically enabled polyelectrolyte multilayer devices: From fuel cells to sensors. Soft. Matter. 2007, 3, 804–816. [Google Scholar] [CrossRef]
- Ariga, K.; Ji, Q.; Hill, J. Enzyme-Encapsulated Layer-by-Layer Assemblies: Current status and challenges toward ultimate nanodevices. Adv. Polym. Sci. 2010, 51–87. [Google Scholar] [CrossRef]
- Evtugyn, G.; Hianik, T. Electrochemical DNA sensors and aptasensors based on electropolymerized materials and polyelectrolyte complexes. TrAC Trends in Anal. Chem. 2016, 79, 168–178. [Google Scholar] [CrossRef]
- Del Mercato, L.L.; Ferraro, M.M.; Baldassarre, F.; Mancarella, S.; Greco, V.; Rinaldi, R.; Leporatti, S. Biological applications of LbL multilayer capsules: From drug delivery to sensing. Adv. Colloid Interface Sci. 2014, 207, 139–154. [Google Scholar] [CrossRef]
- Santos, C.; Ferreira, R.; Calixto, C.; Rufino, J.; Garcia, J.; Fujiwara, S.; Wohnrath, K.; Pessoa, C.A. The influence of organization of LbL films containing a silsesquioxane polymer on the electrochemical response of dopamine. J. Appl. Electrochem. 2014, 44, 1047–1058. [Google Scholar] [CrossRef]
- Shaik, M.; Rao, V.; Gupta, M.; Pandey, P. Layer-by-layer self-assembling copper tetrasulfonated phthalocyanine on carbon nanotube modified glassy carbon electrode for electro-oxidation of 2-mercaptoethanol. Thin Solid Films 2012, 526, 256–260. [Google Scholar] [CrossRef]
- Xu, J.; Xu, S.; Feng, S.; Hao, Y.; Wang, J. Electrochemical sensor for detecting both oxidizing and reducing compounds based on poly(ethyleneimine)/phosphotungstic acid multilayer film modified electrode. Electrochim. Acta. 2015, 174, 706–711. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, H.; Jang, A. Amperometric bromate-sensitive sensor via layer-by-layer assembling of metalloporphyrin and polyelectrolytes on carbon nanotubes modified surfaces. Sens. Actuat. B 2017, 244, 157–166. [Google Scholar] [CrossRef]
- Ammam, M.; Keita, B.; Nadjo, L.; Fransaer, J. Nitrite sensor based on multilayer film of Dawson-type tungstophosphate α-K7[H4PW18O62]·18H2O immobilized on glassy carbon. Talanta 2010, 80, 2132–2140. [Google Scholar] [CrossRef] [PubMed]
- Jović, M.; Hidalgo-Acosta, J.; Lesch, A.; Costa Bassetto, V.; Smirnov, E.; Cortés-Salazar, F.; Girault, H.H. Large-scale layer-by-layer inkjet printing of flexible iridium-oxide based pH sensors. J. Electroanal. Chem. 2018, 819, 384–390. [Google Scholar] [CrossRef]
- Zanardi, C.; Terzi, F.; Zanfrognini, B.; Pigani, L.; Seeber, R.; Lukkari, J.; Ääritalo, T. Effective catalytic electrode system based on polyviologen and Au nanoparticles multilayer. Sens. Actuat. B 2010, 144, 92–98. [Google Scholar] [CrossRef]
- Lu, W.; Luo, Y.; Chang, G.; Liao, F.; Sun, X. Layer-by-layer self-assembly of multilayer films of polyelectrolyte/Ag nanoparticles for enzymeless hydrogen peroxide detection. Thin Solid Films 2011, 520, 554–557. [Google Scholar] [CrossRef]
- Zhang, Y. Electrochemical Determination of Caffeine in Oolong Tea Based on Polyelectrolyte Functionalized Multi-Walled Carbon Nanotube. Int. J. Electrochem. Sci. 2017, 2552–2562. [Google Scholar] [CrossRef]
- Liu, H.; Li, S.; Sun, D.; Chen, Y.; Zhou, Y.; Lu, T. Layered graphene nanostructures functionalized with NH2-rich polyelectrolytes through self-assembly: Construction and their application in trace Cu(II) detection. J. Mat. Chem. B 2014, 2, 2212–2219. [Google Scholar] [CrossRef]
- Pang, Y.; Huang, Y.; Li, W.; Feng, L.; Shen, X. Conjugated Polyelectrolyte/Graphene Multilayer Films for Simultaneous Electrochemical Sensing of Three Monohydroxylated Polycyclic Aromatic Hydrocarbons. ACS. Appl. Nano Mater. 2019, 2, 7785–7794. [Google Scholar] [CrossRef]
- Wu, C.; Bronder, T.; Poghossian, A.; Werner, C.; Schöning, M. Label-free detection of DNA using a light-addressable potentiometric sensor modified with a positively charged polyelectrolyte layer. Nanoscale 2015, 7, 6143–6150. [Google Scholar] [CrossRef]
- Bronder, T.; Poghossian, A.; Scheja, S.; Wu, C.; Keusgen, M.; Mewes, D.; Schöning, M.J. DNA Immobilization and Hybridization Detection by the Intrinsic Molecular Charge Using Capacitive Field-Effect Sensors Modified with a Charged Weak Polyelectrolyte Layer. ACS Appl. Mater. Interfaces 2015, 7, 20068–20075. [Google Scholar] [CrossRef]
- Bronder, T.; Jessing, M.; Poghossian, A.; Keusgen, M.; Schöning, M. Detection of PCR-Amplified Tuberculosis DNA Fragments with Polyelectrolyte-Modified Field-Effect Sensors. Anal. Chem. 2018, 90, 7747–7753. [Google Scholar] [CrossRef]
- Bronder, T.; Poghossian, A.; Jessing, M.; Keusgen, M.; Schöning, M. Surface regeneration and reusability of label-free DNA biosensors based on weak polyelectrolyte-modified capacitive field-effect structures. Biosen. Bioelectron. 2019, 126, 510–517. [Google Scholar] [CrossRef]
- Evtugyn, G.; Stepanova, V.; Porfireva, A.; Zamaleeva, A.; Fakhrullin, R. Electrochemical DNA Sensors Based on Nanostructured Organic Dyes/DN.A/Polyelectrolyte Complexes. J. Nanosci. Nanotechnol. 2014, 14, 6738–6747. [Google Scholar] [CrossRef]
- Dontsova, E.; Zeifman, Y.; Budashov, I.; Eremenko, A.; Kalnov, S.; Kurochkin, I. Screen-printed carbon electrode for choline based on MnO2 nanoparticles and choline oxidase/polyelectrolyte layers. Sens. Actuat. B 2011, 159, 261–270. [Google Scholar] [CrossRef]
- Piccinini, E.; Bliem, C.; Reiner-Rozman, C.; Battaglini, F.; Azzaroni, O.; Knoll, W. Enzyme-polyelectrolyte multilayer assemblies on reduced graphene oxide field-effect transistors for biosensing applications. Biosen. Bioelectron. 2017, 92, 661–667. [Google Scholar] [CrossRef] [Green Version]
- Riedel, M.; Göbel, G.; Abdelmonem, A.; Parak, W.; Lisdat, F. Photoelectrochemical Sensor Based on Quantum Dots and Sarcosine Oxidase. ChemPhysChem. 2013, 14, 2338–2342. [Google Scholar] [CrossRef]
- Tanne, J.; Schäfer, D.; Khalid, W.; Parak, W.; Lisdat, F. Light-Controlled Bioelectrochemical Sensor Based on CdSe/ZnS Quantum Dots. Anal. Chem. 2011, 83, 7778–7785. [Google Scholar] [CrossRef]
- Abouzar, M.; Poghossian, A.; Siqueira, J.; Oliveira, O.; Moritz, W.; Schöning, M. Capacitive electrolyte-insulator-semiconductor structures functionalised with a polyelectrolyte/enzyme multilayer: New strategy for enhanced field-effect biosensing. Phys. Status Solidi A 2010, 207, 884–890. [Google Scholar] [CrossRef]
- Dos Santos, M.; Wrobel, E.; dos Santos, V.; Quináia, S.; Fujiwara, S.; Garcia, J.; Pessôa, C.A.; Scheffer, E.W.; Wohnrath, K. Development of an Electrochemical Sensor Based on LbL Films of Pt Nanoparticles and Humic Acid. J. Electrochem. Soc. 2016, 163, B499–B506. [Google Scholar] [CrossRef]
- Xiong, F.; Chen, C.; Liu, S. Preparation of Chitosan/Polystyrene Sulfonate Multilayered Composite Metal Nanoparticles and Its Application. J. Nanosci. Nanotechnol. 2016, 16, 6027–6031. [Google Scholar] [CrossRef]
- Kim, S.; Kim, Y.; Ko, Y.; Cho, J. Electrochemical sensors based on porous nanocomposite films with weak polyelectrolyte-stabilized gold nanoparticles. J. Mater. Chem. 2011, 21, 8008–8013. [Google Scholar] [CrossRef]
- Morais, P.; Silva, A.; Dantas, N.; Schöning, M.; Siqueira, J. Hybrid Layer-by-Layer Film of Polyelectrolytes-Embedded Catalytic CoFe2O4 Nanocrystals as Sensing Units in Capacitive Electrolyte-Insulator-Semiconductor Devices. Phys. Status Solidi A 2019, 216, 1900044. [Google Scholar] [CrossRef]
- Li, X.; Umar, A.; Chen, Z.; Tian, T.; Wang, S.; Wang, Y. Supramolecular fabrication of polyelectrolyte-modified reduced graphene oxide for NO2 sensing applications. Ceram. Int. 2015, 41, 12130–12136. [Google Scholar] [CrossRef]
- Firdoz, S.; Ma, F.; Yue, X.; Dai, Z.; Kumar, A.; Jiang, B. A novel amperometric biosensor based on single walled carbon nanotubes with acetylcholine esterase for the detection of carbaryl pesticide in water. Talanta 2010, 83, 269–273. [Google Scholar] [CrossRef]
- de Lucena, N.; Miyazaki, C.; Shimizu, F.; Constantino, C.; Ferreira, M. Layer-by-layer composite film of nickel phthalocyanine and montmorillonite clay for synergistic effect on electrochemical detection of dopamine. Appl. Surf. Sci. 2018, 436, 957–966. [Google Scholar] [CrossRef] [Green Version]
- Pajor-Świerzy, A.; Kruk, T.; Warszyński, P. Enhancement of the Electrocatalytic Properties of Prussian Blue Containing Multilayer Films Formed by Reduced Graphene Oxide. Colloid Interface Sci. Commun. 2014, 1, 6–9. [Google Scholar] [CrossRef] [Green Version]
- Pajor-Świerzy, A.; Kolasińska-Sojka, M.; Warszyński, P. The electroactive multilayer films of polyelectrolytes and Prussian blue nanoparticles and their application for H2O2 sensors. Colloid. Polym. Sci. 2013, 292, 455–465. [Google Scholar] [CrossRef]
- Pajor-Świerzy, A.; Kolasińska-Sojka, M.; Warszyński, P. Polyelectrolyte films with Prussian blue nanoparticles and conductive polymers. Surf. Innovations 2014, 2, 184–193. [Google Scholar] [CrossRef]
- Shen, J.; Pei, Y.; Dong, P.; Ji, J.; Cui, Z.; Yuan, J.; Baines, R.; Ajayan, P.M.; Ye, M. Layer-by-layer self-assembly of polyelectrolyte functionalized MoS2 nanosheets. Nanoscale 2016, 8, 9641–9647. [Google Scholar] [CrossRef]
- Paz Zanini, V.; Linarez Pérez, O.; Teijelo, M.; Labbé, P.; Lopez de Mishima, B.; Borsarelli, C. Development of a bioelectrode fabricated with a multilayer thin film of poly(diallyldimethylammonium)/gold-nanoparticle/lactate oxidase for analysis of L-lactate in food samples. Sens. Actuat. B 2017, 247, 830–839. [Google Scholar] [CrossRef]
- Kumlangdudsana, P.; Tuantranont, A.; Dubas, S.; Dubas, L. Fabrication of microelectrodes using flow layer-by-layer self assembly of gold nanoparticles. Superlattices Microstruct. 2012, 52, 1043–1051. [Google Scholar] [CrossRef]
- Rodrigues, G.; Miyazaki, C.; Rubira, R.; Constantino, C.; Ferreira, M. Layer-by-Layer Films of Graphene Nanoplatelets and Gold Nanoparticles for Methyl Parathion Sensing. ACS Appl. Nano Mater. 2019, 2, 1082–1091. [Google Scholar] [CrossRef]
- Lee, D.; Cui, T. A role of silica nanoparticles in layer-by-layer self-assembled carbon nanotube and In2O3 nanoparticle thin-film pH sensors: Tunable sensitivity and linearity. Sens. Actuat. A 2012, 188, 203–211. [Google Scholar] [CrossRef]
- Lee, D.; Cui, T. Layer-by-Layer Self-Assembly of Single-Walled Carbon Nanotubes with Amine-Functionalized Weak Polyelectrolytes for Electrochemically Tunable pH Sensitivity. Langmuir 2011, 27, 3348–3354. [Google Scholar] [CrossRef]
- Chen, H.; Xi, F.; Gao, X.; Chen, Z.; Lin, X. Bienzyme bionanomultilayer electrode for glucose biosensing based on functional carbon nanotubes and sugar–lectin biospecific interaction. Anal. Biochem. 2010, 403, 36–42. [Google Scholar] [CrossRef]
- Lee, D.; Cui, T. Carbon nanotube thin film pH electrode for potentiometric enzymatic acetylcholine biosensing. Microelectron. Eng. 2012, 93, 39–42. [Google Scholar] [CrossRef]
- Du, Y.; Chen, C.; Li, B.; Zhou, M.; Wang, E.; Dong, S. Layer-by-layer electrochemical biosensor with aptamer-appended active polyelectrolyte multilayer for sensitive protein determination. Biosens. Bioelectron. 2010, 25, 1902–1907. [Google Scholar] [CrossRef]
- Qin, H.; Liu, J.; Chen, C.; Wang, J.; Wang, E. An electrochemical aptasensor for chiral peptide detection using layer-by-layer assembly of polyelectrolyte-methylene blue/polyelectrolyte-graphene multilayer. Anal. Chim. Acta 2012, 712, 127–131. [Google Scholar] [CrossRef]
- de Jesus, C.; Lima, D.; dos Santos, V.; Wohnrath, K.; Pessôa, C. Glucose biosensor based on the highly efficient immobilization of glucose oxidase on layer-by-layer films of silsesquioxane polyelectrolyte. Sens. Actuat. B 2013, 186, 44–51. [Google Scholar] [CrossRef]
- Detsri, E.; Rujipornsakul, S.; Treetasayoot, T.; Siriwattanamethanon, P. Nanostructured multilayer thin films of multiwalled carbon nanotubes/gold nanoparticles/glutathione for the electrochemical detection of dopamine. Int. J. Miner. Metall. Mater. 2016, 23, 1204–1214. [Google Scholar] [CrossRef]
- Zhang, L.; Ning, L.; Zhang, Z.; Li, S.; Yan, H.; Pang, H.; Ma, H. Fabrication and electrochemical determination of L-cysteine of a composite film based on V-substituted polyoxometalates and Au@2Ag core–shell nanoparticles. Sens. Actuat. B 2015, 221, 28–36. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, L.; Wang, S.; Yang, W.; Wen, Y.; Zhang, X. An ultrasensitive electrochemical immunosensor for apolipoprotein E4 based on fractal nanostructures and enzyme amplification. Biosens. Bioelectron. 2015, 71, 396–400. [Google Scholar] [CrossRef]
- Liu, S.; Liu, J.; Wang, L.; Zhao, F. Development of electrochemical DNA biosensor based on gold nanoparticle modified electrode by electroless deposition. Bioelectrochemistry 2010, 79, 37–42. [Google Scholar] [CrossRef]
- Davletshina, R.; Ivanov, A.; Evtugyn, G. Acetylcholinesterase Sensor Based on Polyelectrolyte Complexes with DNA Inclusion for the Determination of Reversible Inhibitors. Electroanalysis 2019, 32, 308–316. [Google Scholar] [CrossRef]
- Ivanov, A.; Davletshina, R.; Sharafieva, I.; Evtugyn, G. Electrochemical biosensor based on polyelectrolyte complexes for the determination of reversible inhibitors of acetylcholinesterase. Talanta 2019, 194, 723–730. [Google Scholar] [CrossRef]
- Lakard, B.; Magnin, D.; Deschaume, O.; Vanlancker, G.; Glinel, K.; Demoustier-Champagne, S.; Nysten, B.; Jonas, A.M.; Bertrand, P.; Yunus, S. Urea potentiometric enzymatic biosensor based on charged biopolymers and electrodeposited polyaniline. Biosens. Bioelectron. 2011, 26, 4139–4145. [Google Scholar] [CrossRef] [PubMed]
- Lakard, B.; Magnin, D.; Deschaume, O.; Vanlancker, G.; Glinel, K.; Demoustier-Champagne, S.; Nysten, B.; Bertrand, P.; Yunus, B.; Jonas, A.M. Optimization of the structural parameters of new potentiometric pH and urea sensors based on polyaniline and a polysaccharide coupling layer. Sens. Actuat. B 2012, 166–167, 794–801. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhai, S.; Chen, G.; Zhang, K.; Yue, Q.; Wang, L.; Liu, J.; Jia, J. Effects of morphology of nanostructured ZnO on direct electrochemistry and biosensing properties of glucose oxidase. J. Electroanal. Chem. 2011, 656, 198–205. [Google Scholar] [CrossRef]
- Zhu, Z.; Gao, F.; Lei, J.; Dong, H.; Ju, H. A Competitive Strategy Coupled with Endonuclease-Assisted Target Recycling for DNA Detection Using Silver-Nanoparticle-Tagged Carbon Nanospheres as Labels. Chem. Eur. J. 2012, 18, 13871–13876. [Google Scholar] [CrossRef] [PubMed]
- Khunrattanaporn, N.; Rijiravanich, P.; Somasundrum, M.; Surareungchai, W. Highly sensitive electrochemical detection of genomic DNA based on stem loop probes structured for magnetic collection and measurement via metalised hollow polyelectrolyte shells. Biosens. Bioelectron. 2015, 73, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Snyder, A.; Bo, Z.; Sun, Q.; Martinez, C.; Stanciu, L. Electrochemical Biosensors Fabricated with Polyelectrolyte Microspheres. J. Electrochem. Soc. 2012, 159, B783–B788. [Google Scholar] [CrossRef]
- Dong, H.; Yan, F.; Ji, H.; Wong, D.; Ju, H. Quantum-Dot-Functionalized Poly(styrene-co-acrylic acid) Microbeads: Step-Wise Self-Assembly, Characterization, and Applications for Sub-femtomolar Electrochemical Detection of DNA Hybridization. Adv. Funct. Mat. 2010, 20, 1173–1179. [Google Scholar] [CrossRef]
- Kuan, G.; Sheng, L.; Rijiravanich, P.; Marimuthu, K.; Ravichandran, M.; Yin, L.; Lertanantawong, B.; Surareungchai, W. Gold-nanoparticle based electrochemical DNA sensor for the detection of fish pathogen Aphanomyces invadans. Talanta 2013, 117, 312–317. [Google Scholar] [CrossRef]
- Braham, Y.; Barhoumi, H.; Maaref, A.; Bakhrouf, A.; Jaffrezic-Renault, N. Modified insulator semiconductor electrode with functionalized nanoparticles for Proteus mirabilis bacteria biosensor development. Mater. Sci. Eng. C 2013, 33, 4504–4511. [Google Scholar] [CrossRef]
- Lee, S.; Kang, T.; Lee, S.; Lee, K.; Yi, H. Hydrodynamic Layer-by-Layer Assembly of Transferable Enzymatic Conductive Nanonetworks for Enzyme-Sticker-Based Contact Printing of Electrochemical Biosensors. ACS Appl. Mater. Interfaces 2018, 10, 36267–36274. [Google Scholar] [CrossRef]
- Buron, C.; Quinart, M.; Vrlinic, T.; Yunus, S.; Glinel, K.; Jonas, A.; Lakard, B. Application of original assemblies of polyelectrolytes, urease and electrodeposited polyaniline as sensitive films of potentiometric urea biosensors. Electrochim. Acta 2014, 148, 53–61. [Google Scholar] [CrossRef]
- Xu, G.; Liang, S.; Fan, J.; Sheng, G.; Luo, X. Amperometric sensing of nitrite using a glassy carbon electrode modified with a multilayer consisting of carboxylated nanocrystalline cellulose and poly(diallyldimethyl ammonium) ions in a PEDOT host. Microchim. Acta 2016, 183, 2031–2037. [Google Scholar] [CrossRef]
- Liang, J.; Chen, B.; Long, Y. A microgap impedance sensor for the determination of trace water in organic solvents. Analyst 2011, 136, 4053–4058. [Google Scholar] [CrossRef]
- Li, F.; Tang, C.; Liu, S.; Ma, G. Development of an electrochemical ascorbic acid sensor based on the incorporation of a ferricyanide mediator with a polyelectrolyte–calcium carbonate microsphere. Electrochim. Acta 2010, 55, 838–843. [Google Scholar] [CrossRef]
- Mathi, S.; Gupta, P.; Kumar, R.; Nagarale, R.; Sharma, A. Ferrocenium Ion Confinement in Polyelectrolyte for Electrochemical Nitric Oxide Sensor. ChemistrySelect. 2019, 4, 3833–3840. [Google Scholar] [CrossRef]
- Viswanathan, P.; Ramaraj, R. Polyelectrolyte assisted synthesis and enhanced catalysis of silver nanoparticles: Electrocatalytic reduction of hydrogen peroxide and catalytic reduction of 4-nitroaniline. J. Mol. Catal. A Chem. 2016, 424, 128–134. [Google Scholar] [CrossRef]
- Pedre, I.; Méndez DeLeo, L.; Sánchez-Loredo, M.; Battaglini, F.; González, G. Electrochemical sensor for thiourea focused on metallurgical applications of copper. Sens. Actuat. B 2016, 232, 383–389. [Google Scholar] [CrossRef]
- Viswanathan, P.; Manivannan, S.; Ramaraj, R. Polyelectrolyte stabilized bi-metallic Au/Ag nanoclusters modified electrode for nitric oxide detection. RSC Adv. 2015, 5, 54735–54741. [Google Scholar] [CrossRef]
- Kim, H.; Kim, J.; Park, S.; Kang, J.; Kim, S.; Choi, Y.; Shin, U.S. Carbon nanotubes immobilized on gold electrode as an electrochemical humidity sensor. Sens. Actuat. B 2019, 300, 127049. [Google Scholar] [CrossRef]
- Sobkowiak, M.; Rebis, T.; Milczarek, G. Electrocatalytic sensing of poly-nitroaromatic compounds on multiwalled carbon nanotubes modified with alkoxysulfonated derivative of PEDOT. Mat. Chem. Phys. 2017, 186, 108–114. [Google Scholar] [CrossRef]
- Yi, J.; Tang, S.; Wang, Z.; Yin, Y.; Yang, S.; Zhang, B.; Shu, S.; Liu, T.; Xu, L. Electrochemical determination of bisphenol A based on PHD/MWCNTs modified glassy carbon electrode. Int. J. Environ. Anal. Chem. 2015, 95, 158–174. [Google Scholar] [CrossRef]
- Breczko, J.; Plonska-Brzezinska, M.; Echegoyen, L. Electrochemical oxidation and determination of dopamine in the presence of uric and ascorbic acids using a carbon nano-onion and poly(diallyldimethylammonium chloride) composite. Electrochim. Acta 2012, 72, 61–67. [Google Scholar] [CrossRef]
- Xu, H.; Zheng, Q.; Yang, P.; Liu, J.; Jin, L. Sensitive voltammetricd of trace heavy metals in real water using multi-wall carbon nanotubes/Nafion composite film electrode. Chin. J. Chem. 2011, 29, 805–812. [Google Scholar] [CrossRef]
- Peng, D.; Zhang, J.; Qin, D.; Chen, J.; Shan, D.; Lu, X. An electrochemical sensor based on polyelectrolyte-functionalized graphene for detection of 4-nitrophenol. J. Electroanal. Chem. 2014, 734, 1–6. [Google Scholar] [CrossRef]
- Li, Y.; Deng, C.; Yang, M. Facilely prepared composites of polyelectrolytes and graphene as the sensing materials for the detection of very low humidity. Sens. Actuat. B 2014, 194, 51–58. [Google Scholar] [CrossRef]
- Schenkmayerová, A.; Bučko, M.; Gemeiner, P.; Katrlík, J. Microbial monooxygenase amperometric biosensor for monitoring of Baeyer–Villiger biotransformation. Biosens. Bioelectron. 2013, 50, 235–238. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, L.; Zhang, Y.; Zou, L.; Li, G.; Ye, B. Highly sensitive determination of gallic acid based on a Pt nanoparticle decorated polyelectrolyte-functionalized graphene modified electrode. Anal. Methods 2016, 8, 8474–8482. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, L.; Zhang, Y.; Zou, L.; Li, G.; Ye, B. Electrochemical behavior of amaranth and its sensitive determination based on Pd-doped polyelectrolyte functionalized graphene modified electrode. Talanta 2017, 168, 146–151. [Google Scholar] [CrossRef]
- Jin, L.; Gao, X.; Wang, L.; Wu, Q.; Chen, Z.; Lin, X. Electrochemical activation of polyethyleneimine-wrapped carbon nanotubes/in situ formed gold nanoparticles functionalised nanocomposite sensor for high sensitive and selective determination of dopamine. J. Electroanal. Chem. 2013, 692, 1–8. [Google Scholar] [CrossRef]
- Li, X.; Zhong, A.; Wei, S.; Luo, X.; Liang, Y.; Zhu, Q. Polyelectrolyte functionalized gold nanoparticles-reduced graphene oxide nanohybrid for electrochemical determination of aminophenol isomers. Electrochim. Acta 2015, 164, 203–210. [Google Scholar] [CrossRef]
- Rao, C. Polyelectrolyte-aided synthesis of gold and platinum nanoparticles: Implications in electrocatalysis and sensing. J. Appl. Polym. Sci. 2012, 124, 4765–4771. [Google Scholar] [CrossRef]
- Porfir’eva, A.; Shibaeva, K.; Evtyugin, V.; Yakimova, L.; Stoikov, I.; Evtyugin, G. An Electrochemical DNA Sensor for Doxorubicin Based on a Polyelectrolyte Complex and Aminated Thiacalix[4]Arene. J. Anal. Chem. 2019, 74, 707–714. [Google Scholar] [CrossRef]
- Jiang, Z.; Shangguan, Y.; Zheng, Q. Ferrocene-Modified Polyelectrolyte Film-Coated Electrode and Its Application in Glucose Detection. Polymers 2019, 11, 551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rassas, I.; Braiek, M.; Bonhomme, A.; Bessueille, F.; Raffin, G.; Majdoub, H.; Jaffrezic-Renault, N. Highly Sensitive Voltammetric Glucose Biosensor Based on Glucose Oxidase Encapsulated in a Chitosan/Kappa-Carrageenan/Gold Nanoparticle Bionanocomposite. Sensors 2019, 19, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Liu, J.; Ren, J.; Wang, J.; Wang, E. Mimetic biomembrane–AuNPs–graphene hybrid as matrix for enzyme immobilization and bioelectrocatalysis study. Talanta 2015, 143, 438–441. [Google Scholar] [CrossRef] [PubMed]
- Nouira, W.; Maaref, A.; Elaissari, H.; Vocanson, F.; Siadat, M.; Jaffrezic-Renault, N. Comparative study of conductometric glucose biosensor based on gold and on magnetic nanoparticles. Mater. Sci. Eng. C 2013, 33, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Hua, M.; Liu, Y.; Yang, H.; Tsai, R. Preparation of water-dispersible poly[aniline-co-sodium N-(1-one-butyric acid) aniline]–zinc oxide nanocomposite for utilization in an electrochemical sensor. J. Mater. Chem. 2012, 22, 13252–13259. [Google Scholar] [CrossRef]
- Pang, X.; Imin, P.; Zhitomirsky, I.; Adronov, A. Conjugated polyelectrolyte complexes with single-walled carbon nanotubes for amperometric detection of glucose with inherent anti-interference properties. J. Mater. Chem. 2012, 22, 9147–9154. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Du, D.; Shao, Y.; Li, Z.; Wang, J.; Engelhard, M.H.; Li, J.; Lin, Y. Self-assembly of acetylcholinesterase on a gold nanoparticles–graphene nanosheet hybrid for organophosphate pesticide detection using polyelectrolyte as a linker. J. Mater. Chem. 2011, 21, 5319–5325. [Google Scholar] [CrossRef]
- Kang, H.; Zhu, Y.; Yang, X.; Shen, J.; Chen, C.; Li, C. Gold/mesoporous silica-fiber core-shell hybrid nanostructure: A potential electron transfer mediator in a bio-electrochemical system. New J. Chem. 2010, 34, 2166–2175. [Google Scholar] [CrossRef]
- Wang, Z.; Yi, J.; Yang, S. Direct electrochemistry and electrocatalysis of hemoglobin incorporated in composite film based on diblock weak polyelectrolyte PHAEMA-b-PDMAEMA and multi-walled carbon nanotubes. Sens. Actuat. B 2013, 176, 211–216. [Google Scholar] [CrossRef]
- Gaviglio, C.; Battaglini, F. Hydrogen peroxide detection under physiological conditions by Prussian blue stabilized using a polyelectrolyte–surfactant complex matrix. Sens. Actuat. B 2013, 182, 53–57. [Google Scholar] [CrossRef]
- Cortez, M.; González, G.; Battaglini, F. An Electroactive Versatile Matrix for the Construction of Sensors. Electroanalysis 2010, 23, 156–160. [Google Scholar] [CrossRef]
- Mossanha, R.; Erdmann, C.; Santos, C.; Wohnrath, K.; Fujiwara, S.; Pessoa, C. Construction of a biosensor based on SAM of thiolactic acid on gold nanoparticles stabilized by silsesquioxane polyelectrolyte for cathecol determination. Sens. Actuat. B 2017, 252, 747–756. [Google Scholar] [CrossRef]
- Calfumán, K.; Quezada, D.; Isaacs, M.; Bollo, S. Enhanced Hydrogen Peroxide Sensing Based on Tetraruthenated Porphyrins/Nafion/Glassy Carbon-modified Electrodes via Incorporating of Carbon Nanotubes. Electroanalysis 2015, 27, 2778–2784. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Yang, Y.; Gu, H.; Zhou, T.; Shi, G. Size-tunable Pt nanoparticles assembled on functionalized ordered mesoporous carbon for the simultaneous and on-line detection of glucose and L-lactate in brain microdialysate. Biosens. Bioelectron. 2013, 41, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lai, G.; Zhang, H.; Yu, A. Amperometric aptasensing of chloramphenicol at a glassy carbon electrode modified with a nanocomposite consisting of graphene and silver nanoparticles. Microchim. Acta 2017, 184, 1445–1451. [Google Scholar] [CrossRef]
- Wang, Z.; Xia, J.; Guo, X.; Xia, Y.; Yao, S.; Zhang, F.; Li, Y.; Xia, L. Platinum/graphene functionalized by PDDA as a novel enzyme carrier for hydrogen peroxide biosensor. Anal. Methods 2013, 5, 483–488. [Google Scholar] [CrossRef]
- Hossain, M.; Barman, S.; Park, J. Seed-mediated growth of platinum nanoparticles anchored on chemically modified graphene and cationic polyelectrolyte composites for electrochemical multi-sensing applications. Sens. Actuat. B 2019, 282, 780–789. [Google Scholar] [CrossRef]
- Sun, H.; Jiang, Z.; Wang, H.; Zhao, H. Highly sensitive detection of peptide hormone prolactin using gold nanoparticles-graphene nanocomposite modified electrode. Int. J. Electrochem. Sci. 2015, 10, 9714–9724. [Google Scholar]
- Chen, Z.; Zhang, C.; Li, X.; Ma, H.; Wan, C.; Li, K.; Lin, Y. Aptasensor for electrochemical sensing of angiogenin based on electrode modified by cationic polyelectrolyte-functionalized graphene/gold nanoparticles composites. Biosens. Bioelectron. 2015, 65, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Ensafi, A.; Nasr-Esfahani, P.; Heydari-Bafrooei, E.; Rezaei, B. Redox targeting of DNA anchored to MWCNTs and TiO2 nanoparticles dispersed in poly dialyldimethylammonium chloride and chitosan. Colloids Surf. B 2014, 121, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.; Wang, S.; Chen, S. Electrodeposited indigotetrasulfonate film onto glutaraldehyde-cross-linked poly-L-lysine modified glassy carbon electrode for detection of dissolved oxygen. J. Electroanal. Chem. 2011, 659, 69–75. [Google Scholar] [CrossRef]
- Hao, X.; Xu, Z.; Li, N.; Li, N.; Luo, H. A cation exchange based electrochemical sensor for cetyltrimethylammonium bromide detection using an acridine orange/polystyrene sulfonate system. Anal. Methods 2015, 7, 3849–3854. [Google Scholar] [CrossRef]
- Sun, Y.; Ren, Q.; Liu, X.; Zhao, S.; Qin, Y. A simple route to fabricate controllable and stable multilayered all-MWNTs films and their applications for the detection of NADH at low potentials. Biosens. Bioelectron. 2013, 39, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Kogikoski, S.; Sousa, C.; Liberato, M.; Andrade-Filho, T.; Prieto, T.; Ferreira, F.; Rocha, A.R.; Guha, S.; Alves, W.A. Multifunctional biosensors based on peptide–polyelectrolyte conjugates. Phys. Chem. Chem. Phys. 2016, 18, 3223–3233. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, P.; Mutharani, B.; Chen, S.; Sireesha, P. Biocompatible chitosan-pectin polyelectrolyte complex for simultaneous electrochemical determination of metronidazole and metribuzin. Carbohydr. Polym. 2019, 214, 317–327. [Google Scholar] [CrossRef]
- Zilberg, R.; Maistrenko, V.; Kabirova, L.; Dubrovsky, D. Selective voltammetric sensors based on composites of chitosan polyelectrolyte complexes with cyclodextrins for the recognition and determination of atenolol enantiomers. Anal. Methods 2018, 10, 1886–1894. [Google Scholar] [CrossRef]
- Ning, J.; Luo, X.; Wang, M.; Li, J.; Liu, D.; Rong, H.; Chen, D.; Wang, J. Ultrasensitive Electrochemical Sensor Based on Polyelectrolyte Composite Film Decorated Glassy Carbon Electrode for Detection of Nitrite in Curing Food at Sub-Micromolar Level. Molecules 2018, 23, 2580. [Google Scholar] [CrossRef] [Green Version]
- Pedre, I.; Battaglini, F.; González, G. Disposable Electrochemical Sensor for Rapid Determination of Ethyl Xanthate in the Mining Industry. Electroanalysis 2018, 30, 2589–2596. [Google Scholar] [CrossRef]
- Rotariu, L.; Istrate, O.; Bala, C. Poly(allylamine hydrochloride) modified screen-printed carbon electrode for sensitive and selective detection of NADH. Sens. Actuat. B 2014, 191, 491–497. [Google Scholar] [CrossRef]
- Prakash, S.; Chakrabarty, T.; Michael Rajesh, A.; Shahi, V. Investigation of polyelectrolyte for electrochemical detection of uric acid in presence of ascorbic acid. Measurement 2012, 45, 500–506. [Google Scholar] [CrossRef]
- Menart, E.; Jovanovski, V.; Hočevar, S. Novel hydrazinium polyacrylate-based electrochemical gas sensor for formaldehyde. Sens. Actuat. B 2017, 238, 71–75. [Google Scholar] [CrossRef]
- Hua, M.; Chen, H.; Tsai, R.; Leu, Y.; Liu, Y.; Lai, J. Synthesis and characterization of carboxylated polybenzimidazole and its use as a highly sensitive and selective enzyme-free H2O2 sensor. J. Mater. Chem. 2011, 21, 7254–7262. [Google Scholar] [CrossRef]
- Bronder, T.; Poghossian, A.; Keusgen, M.; Schöning, M. Label-free detection of double-stranded DNA molecules with polyelectrolyte-modified capacitive field-effect sensors. Tech. Mess. 2017, 84, 628–634. [Google Scholar] [CrossRef]
- Li, Y.; Jiao, M.; Zhao, H.; Yang, M. Humidity sensing properties of the composite of electrospun crosslinked polyelectrolyte nanofibers decorated with Ag nanoparticles. Sens. Actuat. B 2018, 273, 133–142. [Google Scholar] [CrossRef]
- Mercante, L.; Pavinatto, A.; Iwaki, L.; Scagion, V.; Zucolotto, V.; Oliveira, O.; Mattoso, L.H.; Correa, D.S. Electrospun Polyamide 6/Poly(allylamine hydrochloride) Nanofibers Functionalized with Carbon Nanotubes for Electrochemical Detection of Dopamine. ACS Appl. Mater. Interfaces 2015, 7, 4784–4790. [Google Scholar] [CrossRef]
- Cortez, M.; Pallarola, D.; Ceolín, M.; Azzaroni, O.; Battaglini, F. Electron Transfer Properties of Dual Self-Assembled Architectures Based on Specific Recognition and Electrostatic Driving Forces: Its Application To Control Substrate Inhibition in Horseradish Peroxidase-Based Sensors. Anal.Chem. 2013, 85, 2414–2422. [Google Scholar] [CrossRef]
- Rozhanchuk, T.; Mariia, V.; Titov, M.; Tananaiko, O. Voltammetric Determination of Purine Bases Using a Carbon Electrode Modified With Hybrid Silica Film. Electroanalysis 2013, 25, 2045–2053. [Google Scholar] [CrossRef]
- Dong, X.; Mi, X.; Zhang, L.; Liang, T.; Xu, J.; Chen, H. DNAzyme-functionalized Pt nanoparticles/carbon nanotubes for amplified sandwich electrochemical DNA analysis. Biosens. Bioelectron. 2012, 38, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Lai, G.; Zhang, H.; Yu, A.; Ju, H. In situ deposition of Prussian blue on mesoporous carbon nanosphere for sensitive electrochemical immunoassay. Biosens. Bioelectron. 2015, 74, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yang, M. Electrochemical sensor utilizing ferrocene loaded porous polyelectrolyte nanoparticles as label for the detection of protein biomarker IL-6. Sens. Actuat. B 2011, 158, 361–365. [Google Scholar] [CrossRef]
- Kerr-Phillips, T.; Aydemir, N.; Chan, E.; Barker, D.; Malmström, J.; Plesse, C.; Travas-Sejdic, J. Conducting electrospun fibres with polyanionic grafts as highly selective, label-free, electrochemical biosensor with a low detection limit for non-Hodgkin lymphoma gene. Biosens. Bioelectron. 2018, 100, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.-X.; Pagliaro, M.; Xu, Y.-J.; Liu, B. Layer-by-Layer Assembly of versatile nanoarchitectures with diverse dimensionality: A new perspective for rational construction of multilayer assemblies. Chem. Soc. Rev. 2016, 45, 3088–3121. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.Q.; Zhang, Y.P.; Wang, M.; Liang, Y.H.; Ren, L.; Ren, L.Q. Recent progress in 4D printing of stimuli-responsive polymeric materials. Sci. China Tech. Sci. 2019, 63, 532–544. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, Q.; Li, X.; Serpe, M.J. Stimuli-responsive polymers for sensing and actuation. Mater. Horiz. 2019, 6, 1774–1793. [Google Scholar] [CrossRef]
- Zhang, D.; Ren, B.; Zhang, Y.; Xu, L.; Huang, Q.; He, Y.; Li, X.; Wu, J.; Yang, J.; Chen, Q.; et al. From design to applications of stimuli-responsive hydrogel strain sensors. J. Mat. Chem. B 2020, 8, 3171–3191. [Google Scholar] [CrossRef]















| 2.1. Multilayer Film Consisting of Two Components (Layers) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 2.1.1. PE and Sensing/Mediating/Transducing Component | ||||||||
| Redox Mediators (Complex Compounds) as a Layer | ||||||||
| PE(s) Used | Sensing Element | Modification Methods | Electrode/Substrate | Path Of Modification | Sensor Construction | Analyte | Analysis Method | Reference |
| SiPy(+)Cl(−) | Tetra sulpho phthalocyanine NiTsPc | LbL | Indium tin oxide (ITO) | LbL: SiPy(+)-NiTsPc(−) | ITO/LbL: SiPy-NiTsPc | Dopamine | Square-wave voltamm-etry (SWV) | [32] |
| Poly(diallyldimethylammonium chloride) (PDDA(+)) | Copper tetra-sulphonated phthalocyanine CuPcTS | Drop-casting/LbL | Glassy carbon electrode (GCE) | Drop-casting of the CNT(−); then LbL: PDDA(+)-CuPcTS(−) | GCE/carbon nanotube (CNT)/LbL: PDDA-CuPcTS | 2-mercaptoethanol | Ampero-metry (AMP) | [33] |
| Poly(ethylenimine) (PEI(+)) | Phosphotungstic acid-PTA H3PW12 O40 | LbL | ITO | LbL: PEI(+)-PTA(−) | ITO/LbL: PEI-PTA | Ascorbic acid and H2O2 | Cyclic voltamm-etry (CV) | [34] |
| Poly(styrene sulphonate) (PSS(−)); Nafion®(−) | Fe(III)porphyrine Fe(III)Ph | Drop-casting/LbL | Screen-printed carbon electrode (SPCE) | Suspension of multi-walled carbon nanotube (MWCNT)(−)@Nafion®(−) onto SPCE; then LbL: PSS(−)-Fe(III)P(+) | SPCE/MWCNT@Nafion®/LbL: PSS-Fe(III)P | Bromate | CV | [35] |
| Poly(allylamine hydrochloride) (PAH(+)); PSS(−); surfactant Triton-X® | Tungstophosphate (POM) | Silanization of GCE/immersion/LbL | GCE | Silanizated GCE by treatment with Aminopropyltriethoxysilane—ATS(+); then immersion in PSS(−); than LbL: PAH(+)@Trinton-X®-POM(−) | GCE@ATS/PSS/LbL: PAH@Trinton-X®-POM | Nitrite | AMP | [36] |
| Me or MeO NPs as a Layer | ||||||||
| PDDA(+) | IrOxNPs (nanoparticles) | LbL | ITO | LbL by printing: PDDA(+)-IrOxNPs(−) | ITO/LbL: PDDA-IrOxNPs | pH | Potentio-metry (POT) | [37] |
| Polyviologen (PV(+)) | AuNPs | LbL | Au | Au modified with 2-mercaptoethansulphonate (MESA)(−); then LbL: PV(+)-AuNPs(−) | Au@MESA/LbL: PV-AuNPs | H2O2 | AMP | [38] |
| Poly ((2-ethyldimethylammonioethyl methacrylate ethyl sulphate)-co-(1-vinylpyrrolidone) (PQ11(+)) | PQ11-AgNPs | LbL | ITO | LbL: PQ11(+)-AgNPs | GCE/LbL: PQ11-AgNPs | H2O2 | AMP | [39] |
| Carbon-Based Nanoparticles as a Layer | ||||||||
| PDDA(+) | MWCNTs | LbL | GCE | LbL: PDDA(+)-MWCNT(−) | GCE/LbL: PDDA-MWCNT | Caffeine | Different-ial pulse voltamm-etry (DPV) | [40] |
| PAH(+) | Graphene oxide (GO) | LbL | GCE | LbL: PAH(+)-GO; then reduction of GO with NaBH4 to obtain rGO. | GCE/LbL: PAH-rGO | Cu(II) | Different-ial pulse anodic stripping voltamm-etry (DPASV) | [41] |
| conjugated PE: PBCSO3(−) | Reduced graphene oxide (rGO) | Electrochem. deposition/LbL | GCE | Electrochemical deposition and simultaneous reduction of graphene oxide (GO) on GCE; then LbL: PBCSO3(−)-rGO | GCE/ErGO/LbL: PBCSO3-rGO | Polycyclic AromaticHydrocarbons | DPV | [42] |
| DNA as a Layer | ||||||||
| PAH(+) | Single stranded DNA (ssDNA) | LbL | SiO2 | SiO2(−) at physiological pH; then LbL: poly(acrylamide) (PAH(+))-ssDNA(−) | SiO2/LbL: PAH-ssDNA | cDNA | POT | [43] |
| PAH(+) | ssDNA | LbL | SiO2 | SiO2(−) at physiological pH; then LbL: PAH(+)-ssDNA(−) | SiO2/LbL: PAH-ssDNA | cDNA | Impedimetry (IMP) | [44] |
| PAH(+) | ssDNA | LbL | FE chip SiO2 | LbL: PAH(+)-ssDNA(−) | FE chip/LbL: PAH-ssDNA | cDNA | Electrochemic-al imped-ance spectro-scopy (EIS) | [45] |
| PAH(+) | ssDNA | LbL | FE chip SiO2 | LbL: PAH(+)-ssDNA(−) | FE chip/LbL: PAH-ssDNA | cDNA | IMP | [46] |
| PAH(+); PSS(−) | DNA | Electropolymerization/LbL | GCE | Electropolymerization of Methylene green (MG) or Methylene blue (MB) onto GCE; then different architectures were applied using the LbL method:: 1. PSS-PAH; 2. DNA-PAH; 3. PSS-PAH-PSS; 4. PSS-PAH-DNA; 5. DNA-PAH-PSS. | GCE/MG or MB/LbL: DNA-PAH | DNA | IMP | [47] |
| Enzyme as a Layer | ||||||||
| PDDA(+); PAS(−) | Choline oxidase (ChOx) | Drop-casting/LbL | SPCE | 1. Drop-casting of the sol MnO2; then drop-casting of PDDA(+); then drop-casting of ChOx 2. Drop-casting of the sol solution of MnO2; then LbL: PDDA(+)-PAS(−); then drop-casting of ChOx 3. Drop-casting of the sol solution of MnO2; then LbL: PDDA(+)-ChOx | 1. SPCE/MnO2/PDDA/ChOx 2. SPCE/MnO2/LbL:PDDA-PAS/ChOx 3. SPCE/MnO2/LbL: PDDA-ChOx | Choline | AMP | [48] |
| PEI(+) | Urease | LbL | Field-effect transistor (FET) (Si-SiO2) | glass substrate functionalized with APTES; then drop-casting of GO followed by its chemical reduction; then functionalization by SPS(−); then LbL: PEI(+)-urease(−) | FET@APTES/rGO/SPS/LbL: PEI-urease | Urea | AMP | [49] |
| PAH(+) | Sarcosine oxidase (SOD) | LbL | Au | Benzenedithiol (BDT) was immobilized onto Au; then immersion of the Au electrode into Cd/Se quantum dots (Cd/SeQD) to obtain Cd/SeQD@BDT; then LbL: SOD-PAH(+) | Au@BDT/Cd/SeQD@BDT/LbL: SOD-PAH | Sarcosine | AMP-photo current | [50] |
| PAH(+) | GOx | LbL | Au | immobilization of the benzenethiol functionalized CdSe/ZnQDs; then LbL: GOx-PAH(+); then GTH (cross-linking) | Au/QDs/LbL: GOx-PAH | Glucose | AMP-photo current | [51] |
| Poly(amidoamine) dendrimer (PAMAM(+)); PAH(+) | Penicilase | LbL | FET (Si/SiO2) | LbL: PAMAM(+) or PAH(+)-penicilase | FET/LbL: PAMAM or PAH-penicilase | Penicillin | EIS capacitive | [52] |
| 2.1.2. Sensing/Mediating/Transducing Material Entrapped within PE and Used as a Self-Containing Layer | ||||||||
| Me or MeO NPs–PE as a layer | ||||||||
| SiPy(+)Cl(−); HA(−) | PtNPs@SiPy | LbL | Fluorine doped tin oxide (FTO) | Reduction of the PtCl6− in the presence of SiPy(+); then LbL: HA(−)-PtNPs@SiPy(+) and vice versa; | FTO/LbL: HA-PtNPs@SiP | 17α-ethynylestradiol, EE2 | DPV | [53] |
| Chitosan (CS(+)); PSS(−) | Men+@CSMen+ = Cu2+, Ni2+, Ag+ | LbL | Quartz, silicon wafer; ITO | Substrate/LbL: Men+@CS(+)-PSS(−); then chemical reduction of Men+ in film | substrate/LbL: Me@CS-PSS | Glucose | CV | [54] |
| PAH(+); poly(acrylic acid) (PAA(−)); | AuNPs@PAH or AuNPs@PAA | LbL | ITO | Reduction of AuCl4− with NaBH4 in the presence of the PE; then LbL onto ITO: Au NPs@PAH(+)-AuNPs@PAA(−) | ITO/LbL: AuNPs@PAH-AuNPs@PAA | Nitrite | CV | [55] |
| poly(vinyl pyrrolidone) (PVP(0)); PAMAM(+) | CoFe2O4 | LbL | Chip: Al/p-Si/SiO2 | chips:Al/p-Si/SiO2; then LbL: PAMAM(+)-CoFe2O4@PVP(0) | Al/p-Si/SiO2/LbL:PAMAM-CoFe2O4 @PVP | H2O2 | IMP | [56] |
| Carbon-Based NPs–PE as a Layer | ||||||||
| PSS(−); PAH(+) | rGO@PSS/rGO@PAH | LbL | carbon film | LbL: rGO@PSS(−)-rGO@PAH(+) | carbon film/LbL: rGO@PSS-rGO@PAH | NO2 | Resistance | [57] |
| PDDA(+) | Enzyme-AChE | LbL | GCE | LbL: Single-wall carbon nanotubes (SWCNTs)@PDDA(+)-AChE(−) | GCE/LbL: SWCNTs@PDDA-AChE | Carbaryl pesticide | AMP | [58] |
| 2.2. Multilayer Film Consisting of More Than Two Components (Layers) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 2.2.1. Multilayer Film Consisting of Three or More Alternately Deposited Layers | ||||||||
| Redox Mediators (Complex Compounds) as a Layer | ||||||||
| PE(s) Used | Sensing Element | Modification Methods | Electrode/Substrate | Path of Modification | Sensor Construction | Analyte | Analysis Method | Reference |
| PEI(+) | NaMMT and NiTsPc | LbL | ITO | LbL: PEI(+)-MMT(−)-PEI(+)-NiTsPc | ITO/LbL: PEI-MMT-PEI-NiTsPc | Dopamine | DPV | [59] |
| PAH(+); PEI(+) | PB | Drop-casting/LbL | Au | Drop-casting of PEI(+) onto Au; then LbL: rGO-PAH(+)-PB(−) | Au/PEI/LbL: rGO-PAH-PB | H2O2 | CV | [60] |
| PEI(+); PAH(+) | PB | LbL | Au | LbL: PEI(+)-PB(−)-poly(3,4-ethylenedioxythiophene) (PEDOT):PSS-PAH(+) | Au/LbL: PEI-PB-PEDOT:PSS-PAH | H2O2 | CV | [61] |
| PEI(+); PAH(+) | PB | LbL | Au | LbL: PEI(+)-PB(−)-PPy or polyaniline (PANI)-PAH(+) | Au/LbL: PEI-PB-PPy or PANI-PAH | H2O2 | CV | [62] |
| PAA(−); PAM(+); PDDA(+) | MoS2@PAA or PAM | LbL | Quartz, silicon, ITO | LbL:PDDA(+)-MoS2@PAA(−)-MoS2@PAM(+)-PEDOT:PSS(−) | substrate/LbL: PDDA-MoS2@PAA-MoS2@PAM-PEDOT:PSS | H2O2 | AMP | [63] |
| DNA as a Layer | ||||||||
| PAH(+); PSS(−) | Methylene blue (MB) and Methylene green (MG); DNA | electropolymerization/LbL | GCE | Electropolymerization of MB or MG onto GCE; then different LbL architectures were obtained: 1. PSS-PAH; 2. DNA-PAH; 3. PSS-PAH-PSS; 4. PSS-PAH-DNA; 5. DNA-PAH-PSS. | GCE/LbL: DNA-PAH-PSS | DNA | IMP | [47] |
| Enzyme as a Layer | ||||||||
| PDDA(+) | LOx | LbL | Polycryst. Au (PGA) | LbL on the thiolated (MPS) PGA surface(−): PDDA(+)-AuNPs-LOx | Au@MPS/LbL: PDDA-AuNPs-LOx | L-lactate | AMP | [64] |
| 2.2.2. Multilayer Film Consisting of Two or More Multilayer Films | ||||||||
| Me and Meo Nps as a Layer | ||||||||
| Poly(diallyldimethylammonium chloride) (PDADMAC(+)); PSS(−) | AuNPs | LbL | PDMS pattern channel | LbL: PDADMAC(+)-PSS(−); then LbL: PDADMAC(+)-AuNPs | PDMS pattern channel/LbL: PDADMAC-PSS/LbL: PDADMAC-AuNPs | - | Conductivity | [65] |
| PDDA(+); PSS(−) | Au | LbL | ITO | Graphene nanopellets stabilized in PDDA(+) (GPDDA(+)) and PSS(−) (GPSS(−)); LbL: GPDDA(+)-GPSS(−); then LbL: AuNP(+)-GPSS(−) | ITO/LbL: GPDDA-GPSS/LbL: AuNP-GSS | Methyl parathion | DPV | [66] |
| PDDA(+); PSS(−) | SiO2NPs | LbL | ISFET | LbL: PDDA(+)-PSS(−); then LbL: PDDA(+)-SWCNT(−) or In2O3NPs(−); then LbL: PDDA(+)-SiO2NPs(−) | ISFET/LbL: PDDA-PSS/LbL: PDDA-SWCNT or In2O3NPs/LbL: PDDA-SiO2NPs | pH | chemoresistance | [67] |
| Carbon-Based Nanomaterials as a Layer | ||||||||
| CS(+); PAH(+); PDDA(+); PSS(−) | CS@SWCNTs | LbL | SiO2 plate covered with Cr-Au | SiO2@Cr-Au; then LbL: PDDA(+)-PSS(−); then LbL: PE(+)-SWCNTs(−) | SiO2@Cr-Au/LbL: PDDA-PSS/LbL: PE-SWCNTs | pH | chemo resistance | [68] |
| Enzyme as a Layer | ||||||||
| PAH(+); PSS(−) | GOx | Immersion/LbL | Au | Au modified with 3-mercapto-1-propanesulphonic acid (MPS) (−); then precursor bilayer film: PAH(+)@PSS(−); then LbL: MWCNTs(−)@PAH(+)-HRP (horse radish peroxidase); then LbL: ConA (bridge between HRP and GOx)-GOx | Au@MPS/PAH@PSS/LbL: MWCNTs@PAH-HRP/LbL: ConA-GOx | Glucose | AMP | [69] |
| PDDA(+); PSS(−) | SWCNTs; AChE. | LbL | SiO2/Cr/Au | LbL: PDDA(+)-PSS(−); then LbL: PDDA(+)-SWCNTs(−); then layer of the PDDA(+); then LbL: PDDA(+)-PSS-AChE | SiO2/Cr/Au/LbL: PDDA-PSS/LbL: PDDA-SWCNTs/PDDA/LbL: PSS-AChE | pH, acetylcholine | POT | [70] |
| 2.3. Multilayer Film as a Substrate (“Precursor” Film) for Deposition of a Sensing Layer | ||||||||
|---|---|---|---|---|---|---|---|---|
| 2.3.1. Redox Mediators (Complex Compounds) in a “Precursor” Multilayer Film | ||||||||
| PE(s) Used | Sensing Element | Modification Methods | Electrode/Substrate | Path of Modification | Sensor Construction | Analyte | Analysis Method | Reference |
| PEI(+) | Aptamers (TBA; LBA) | LbL/drop-casting | ITO | LbL: Fc@PEI(+)-CNTs(−); then drop-casting of the TBA or LBA; then bovine serum albumin (BSA) (prevent non-specific adsorption) | ITO/LbL: Fc@PEI-CNTs/TBA or LBA | Thrombin lysosome | DPV | [71] |
| PDDA(+); PSS(−) | Aptamer | LbL/drop-casting | ITO | LbL: MB(−)@PDDA(+)-rGO@PSS(−); then drop-casting of the aptamer; then BSA | ITO/LbL: MB@PDDA-rGO@PSS/aptamer | Chiral peptide detection | DPV | [72] |
| SiPy(+)Cl(−); Nafion®(−) | GOx | LbL/drop-casting | FTO | LbL: SiPy(+)-CuTsPc(−); then adsorption of the SiPy(+); then drop-casting of the GOx; then drop-casting of the Nafion®(−); | FTO/LbL: SiPy-CuTsPc/SiPy/GOx/Nafion® | Glucose | AMP | [73] |
| 2.3.2. Me or MeO NPs in a “Precursor” Multilayer Film | ||||||||
| PDADMAC(+); PSS(−) | GTH | LbL/drop-casting | ITO | ITO modified with PSS(−); then LbL: MWCNTs@PDADMAC(+)-AuNP; then drop-casting of the GTH | ITO/PSS/LbL: MWCNTs@PDADMAC-AuNP/GTH | Dopamine | CV | [74] |
| PEI(+); PSS(−) | P2Mo17V | LbL/dip-coating | ITO | LbL: PSS(−)-PEI(+); then immersion into AuCl4− followed by reduction with NaBH4; then immersion into AgNO3 followed by reduction with asorbic acid; then dip-coating into solution of P2Mo17V@PEI(+) | ITO/ LbL: PSS-PEI/ Au@2Ag/ P2Mo17V@PEI | L-cysteine | AMP | [75] |
| PSS(−); PDDA(+) | Anti-APOE-4 | LbL/electro deposition | ITO | LbL: PDDA(+)-PSS(−); then potentiostatic electrodeposition from AuCl4− to produce fractal Au (FracAu); then immobilization of anti-APOE-4 | ITO/LbL: PDDA-PSS/ FracAu/anti-APOE-4 | Protein APOE-4 | AMP | [76] |
| PAH(+); PSS(−) | Au@target DNA | LbL/dip-coating | Au | immersion of the Au electrode into mercaptopropionic acid (MPA(−)); then LbL: PAH(+)-PSS(−); then chemical reduction of the AuCl4− with NaBH4 onto Au; then immobilization of DNA probe | Au@MPA/ LbL: PAH-PSS/ AuNPs/DNA | Target DNA | DPV | [77] |
| 2.3.3. DNA in a “Precursor” Multilayer Film | ||||||||
| PAA(+); PSS(−) | Enzyme AChE | LbL/drop-casting | GCE | Drop casting of the suspension of Co-phtalocyanine (CoPc) and Carbon Black (CB); then LbL: PAA(+)-PSS(−) or PAA(+)-DNA(−); then drop-casting of AChE | GCE/CoPc@CB/LbL: PAA-PSS or PAA-DNA/AChE | Huperzine A and galantamine | AMP | [78] |
| 2.3.4. Unmodified PEs as a “Precursor” Multilayer Film | ||||||||
| PAA(+); PSS(−) | AChE | Drop casting/LbL/drop-casting | GCE | Drop-casting of suspension of the Co-phtalocyanine (CoPc) and Carbon Black (CB) onto GCE; then LbL: PAA(+)-PSS(−); then drop-casting of AChE | GCE/CoPh@CB/LbL: PAA-PSS/AChE | Donepezil and berberine | Measurement of anodic current | [79] |
| PDDA(+); PAS(−) | Choline oxidase (ChOx) | Drop-casting/LbL/drop-casting | SPCE | 1. Drop-casting of the sol solution of MnO2; then drop-casting of PDDA(+); then drop-casting of ChOx 2. Drop-casting of the sol solution of MnO2; then LbL: PDDA(+)-PAS(−); then drop-casting of ChOx 3. Drop-casting of the sol solution of MnO2; then LbL: PDDA(+)-ChOx | 1. SPCE/MnO2/PDDA(+)/ChOx 2. SPCE/MnO2/LbL: PDDA-PAS(−)/ChOx 3. SPCE/MnO2/LbL: PDDA-ChOx | Choline | AMP | [48] |
| Polysaccharide: CS(+); carboxymethylpullulan CMP(−) | Urease | LbL/ adsorption or covalent immobilization of enzyme | Carbon | PANI(−) electropolymerized over graphite; then LbL: CS(+)-CMP(−); then drop-cast or covalent immobilization of urease (via carbodiimide reaction) | C/PANI/LbL: CS-CMP/enzyme | Urea | POT | [80] |
| CMP(−); CS(+) | Urease and PEC | LbL/covalent immob. of enzyme | SPCE | Electropolymerized PANI over SPCE; then LbL: CMP(−)-CS(+); then covalent grafting via carbodiimide reaction of urease onto CMP | SPCE/LbL: CMP-CS/urease | pH and urea | POT | [81] |
| PDDA(+); PSS(−); Nafion® | GOx | LbL/dip-coating | ITO | ZnONRs or NPs deposited onto ITO; then LbL: PSS(−)-PDDA(+); then dip-coating of GOx; then Nafion® | ITO/ZnO NRs or NPs/ LbL: PSS-PDDA/ GOx/Nafion® | Glucose | AMP | [82] |
| 2.4. LbL Method in Development of Various Composite Nanoparticles and Other Sensor Architectures | ||||||||
|---|---|---|---|---|---|---|---|---|
| 2.4.1. Composite Nanoparticles (Nanospheres, Hollow-Shell Particles, Reporter Probes) | ||||||||
| PE(s) Used | Sensing Element | Modification Methods | Electrode/Substrate | Path Of Modification | Sensor Construction | Analyte | Analysis Method | Reference |
| PAH(+); PSS(−) | Streptavidin/ AgNP-tagged carbon nanosphere (CNSs) | LbL | Au; CNS | Reporter probe: LbL onto CNS: PAH(+)-PSS(−); then in solution of Ag+ and citrate; then into streptavidin. | CNS/LbL: PAH-PSS/ AgNPs/streptavidin | DNA | Linear sweep voltammetry (LSV) | [83] |
| PAA(+); PSS(−) | SPE | LbL | SPE | LbL: Hollow shall PAA(+)-PSS(−) capsules loaded with Au(III) followed by reduction to obtain AuNPs@PAA(+)-PSS(−); magnetic Fe3O4 covered with APTES and polystyrene-co-acrylic acid (PSA(−)) latex particles (Fe3O4@APTES(+)@PSA(−)); then attachment of alkaline phosphatase (AP) labeled anti-DIG onto Fe3O4@APTES(+)@PSA(−); bonding of AuNPs@PAA-PSS and AP labeled anti-DIG @Fe3O4@APTES@PSA via Au-S bond | AP labeled anti-DIG@ Fe3O4@APTES@PSA@ AuNPs@PAA-PSS; magnetic collection; dissolution and determination | DIG (DNA) | DPSAV | [84] |
| PAH(+): PEI(+); PAA(−); PSS(−) | AChE and HRP | LbL/drop-casting | SPCE | LbL on the PSS(−) (as template): PAH(+)-PAA(−) or PEI(+)-PAA(−); then immobilization of enzyme onto polyelectrolyte sphere by mixing it into enzyme solution; then drop-casting onto SPCE | SPCE/LbL onto PSS: PAA-PAA or PEI-PAA/HRP or AChE | Pesticide and H2O2 | AMP | [85] |
| PAH(+); PSS(−) | CdTeQD | LbL | GPTMS | Reporter probe: LbL onto polystyrene-co-acrylic acid (PSA) particles incubated with PAH(+)-PSS(−); then adding of CdTeQD onto PSA@PAH(+)-PSS(−); then conjugation of streptavidin onto PSA@PAH(+)-PSS(−)@CdTeQD; then electrostatic adsorption; then covered with BSA | PSA/LbL: PAH-PSS/CdTeQD/streptavidin | Target DNA | SWV | [86] |
| PAA(+); PSS(−) | DNA probe | LbL/adsorption | SPCE | Reporter probe: LbL onto polystyrene-co-acrylic acid (PSA) particles with PAA(+)-PSS(−); then adding of colloid AuNPs; then conjugation of DNA probe onto AuNPs; then electrostatic adsorption | PSA/LbL: PSS-PAA/AuNP/DNA | Target DNA | DPASV | [87] |
| 2.4.2. LbL Procedure in Other Sensor Architectures | ||||||||
| PAH(+); PSS(−) | Proteus mirabilis bacteria | LbL/drop-casting | Si/SiO2 | LbL on AuNPs: PAH(+)-PSS(−); then drop-casting onto Si/SiO2; then drop-casting of bacteria; then cross-linking with GSH | Si/SiO2/AuNPs@LbL: PAH-PSS/bacteria | Urea | POT | [88] |
| M13 phage(−); PEI(+) | GOx | LbL | SPAu | LbL on the cellulose acetate polymeric membrane by dialysis: SWCNTs@M13(−)-GOx@PEI(+); then wet contact-transfer-print onto SPAu | SPAu/LbL: SWCNTs@M13-GOx@PEI | Glucose | AMP | [89] |
| CMP(−); CS(+) | Urease | LbL/electropolymerization | SPCE | electropolymerization of PANI(+) on GCE; then immobilization of urease; then LbL: CMP(−)-CS(+); | GCE/PANI/urease/LbL: CMP-CS | Urea | POT | [90] |
| PDDA(+); CNNC(−) | PEDOT | LbL/electropolymerization | GCE | LbL: electrodeposition of PDDA(+)-then immersion into CNNC(−); then electrochemical polymerization of EDOT | GCE/LbL: PDDA-CNNC/PEDOT | Nitrite | AMP | [91] |
| 3.1. Sensing/Mediating/Transducing Component is Entrapped with PE | ||||||||
|---|---|---|---|---|---|---|---|---|
| 3.1.1. Redox Mediators (Complex Compounds)–PE Composite | ||||||||
| PE(s) Used | Sensing Element | Modification Methods | Electrode/Substrate | Path of Modification | Sensor Construction | Analyte | Analysis Method | Reference |
| PDMDAAC (+) | Fe(CN)63−/4− | Dip-coating | ITO | Dip-coating of the solution of PDMDAAC(+)@Fe(CN)63−/4− | ITO/PDMDAAC@Fe(CN)63−/4− | Water in organic solvents | IMP | [92] |
| PAH(+); PSS(−); CS(+) | Fe(CN)63−/4− | Drop-casting | GCE | Drop-casting of the suspension of prepared porous material: CaCO3@PSS(−)@PAH(+)@Fe(CN)63−/4−@CS(+) | GCE/CaCO3@PSS@PAH@Fe(CN)63−/4−@CS | Ascorbic acid | AMP | [93] |
| PSS(−); Nafion®(−) | Ferrocenium (Fe(C10H10+) | Drop-casting | GCE | ferrocenium ion entangled and extracted with Nafion® or PSS(−); then drop-casting of the Fe(C10H10+)(+)@Nafion®(−) or Fe(C10H10+)(+)@PSS(−) | GCE/Fe(C10H10+)@Nafion® or Fe(C10H10+)@PSS | Nitrite | CV | [94] |
| 3.1.2. Me or MeO NPs–PE Composite | ||||||||
| PADA(+) | AgNP | Drop-casting | GCE | Mixing solutions of PADA(+), TPDT (as reducing agent) and Ag+ to obtain PADA(+)@AgNP@TPDT; then drop-casting onto GCE | GCE/PADA@Ag@TPDT | H2O2 and 4-nitroaniline | SWV and LSV | [95] |
| PAA(+) sodium dodecyl sulphate (SDS(−)) surfactant | AgNP | Drop-casting | SPGE | solution of the AgNP and SDS (surfactant) (e.g., AgNP@SDS(−)) and PAA(+); then drop-casting onto SPGE | SPGE/AgNP@SDS@PAA | Thiourea | SWV | [96] |
| PADA(+) | PADA@Au50 Ag50NCs | Drop-casting | GCE | Drop-casting of solution of the bi-metallic Au/Ag nanoclusters embedded in PDDA(+): PADA(+)@Au50Ag50NCs | GCE/PADA@Au50Ag50NCs | NO | AMP | [97] |
| 3.1.3. Carbon-Based NPs–PE Composite | ||||||||
| CS(+) | MWCNTs | Drop-casting | Au | Drop-casting suspension of MWCNTs@CS(+) onto Au | Au/MWCNTs@CS | Humidity | resistivity | [98] |
| PEDOT-S(−) (sulphonated PEDOT) | PEDOT-S@ MWCNTs | Drop-casting | GCE | Drop-casting of the suspension of the PEDOT-S(−)@MWCNTs onto GCE | GCE/PEDOT-S@MWCNTs | Nitrobenzenes | DPV;SWV | [99] |
| PHEMA-b-PDMAEMA(PHD(+)) | MWCNTs@ PHD | Drop-casting | GCE | Drop-casting of the suspension of MWCNTs@PHD(+) onto GCE | GCE/MWCNTs@PHD | Bisphenol-A | AMP | [100] |
| PDDA(+) | Carbon nano-onions (CNO) | Drop-casting | GCE | Drop-casting of the suspension of CNO@PDDA(+) onto GCE | GCE/CNO@PDDA | Dopamine | DPV;SWV | [101] |
| Nafion® (−) | MWCNTs@ Nafion® | Drop-casting | GCE | drop-casting of MWCNTs(−)@ Nafion®(−) onto GCE | GCE/MWCNTs@Nafion® | Pb, Cd | DPASV | [102] |
| PDDA(+) | rGO@PDDA | Drop-casting | GCE | Drop-casting of the suspension of rGO@PDDA(+) onto GCE | GCE/MWCNTs@PDDA | 4-Nitrophenol | LSV | [103] |
| PDDA(+); PSS(−) | GO@PDDA or rGO@PSS | Dip-coating | Au | Dip-coating of the rGO@PDDA(+) or rGO@PSS(−) onto Au | Au/rGO@PDDA or rGO@PSS | Humidity | IMP | [104] |
| 3.1.4 Other–PE Composite | ||||||||
| Cellulose sulphate (−); sodium alginate (−); poly(methylene-co-guanidine (+) | Cell entrapped in PEC membrane | Attach | Oxygen electrode | Suspension of biomass into solution of PEC; obtained gel placed onto oxygen electrode | Oxygen electrode/cell@PEC | Biotransformation based on Baeyer–Villiger oxidation-CBCH | AMP | [105] |
| 3.2 Sensing Film Consisting of Three or More Components | ||||||||
|---|---|---|---|---|---|---|---|---|
| 3.2.1. Sensing Film Comprising Me or MeO NPs | ||||||||
| PE(s) Used | Sensing Element | Modification Methods | Electrode/Substrate | Path of Modification | Sensor Construction | Analyte | Analysis Method | Reference |
| PDDA(+) | rGO@PDDA@ PtNPs | Drop-casting | GCE | Drop-casting of the rGO *@PDDA(+)@PtNPs onto GCE * rGO—graphene sheets | GCE/Gr@PDDA@Pt | Gallic acid | DPV | [106] |
| PSS(−) | rGO@PSS@ PdNP | Drop-casting | GCE | Drop-casting of the suspension of graphene rGO *@PSS(−)@PdNP * rGO—graphene | GCE/Gr@PSS@PdNP | Amaranth | DPV | [107] |
| PEI(+) | MWCNTs@ PEI@AuNP | Drop-casting | Au | Drop-casting of MWCNTs(−)@PEI(+)@AuNP | Au/MWCNTs@PEI@AuNP | Dopamine | DPV | [108] |
| PDDA(+) | rGO@PDDA@ | Drop-casting | GCE | Reduction of the GO with PDDA(+); then addition of Au(III) in the rGO solution and reduction of Au(III) with PDDA(+); then drop-casting of the suspension of rGO@PDDA(+)@AuNP onto GCE | GCE/rGO@PDDA@AuNP | Aminophenol isomers | DPV | [109] |
| PDDMAC (+); Nafion® | Carbon ink@ Nafion®@AuNPs | Drop-casting | GCE | Electrodeposition of AuNPs from AuCl4− and PDDMAC(+); drop-casting of the carbon ink@Nafion®(−)@AuNPs | GCE/carbon ink@ Nafion®@AuNPs | Dopamine | CV | [110] |
| 3.2.2. Sensing Film Comprising DNA | ||||||||
| PAA(+); PSS(−) | DNA | Electropolym./Drop-casting | GCE | Electropolymerization of Neutral Red (NR) or PANI onto GCE; then drop casting of TC@PAA(+) or PSS(−)@DNA(−) | GCE/NR or PANI/TC@PAA or PSS@DNA | Doxorubicin | IMP | [111] |
| 3.2.3 Sensing Film Comprising an Enzyme | ||||||||
| PAA(+); PEI(+); PAM(−) | GOx | Drop-casting | GCE | Entrapping of the ferrocene (Fc) with PE; then preparation of hybrid composite GO@MWCNTs@PE@Fc@GOx; then drop-casting of the GO@MWCNTs@PE@Fc@GOx | GCE/GO@MWCNTs@PE@Fc@GOx | Glucose | AMP | [112] |
| CS(+); kappa-carrageenan (−) | GOx | Drop-casting | Au | GOx encapsuled in PEC (CS(+)@kappa-carrageenan(−)); then mixed with AuNPs to obtain AuNPs@PEC@GOx; then drop-casting of the AuNPs@PEC@GOx onto Au | GCE/Au/AuNPs@PEC@GOx | Glucose | SWV | [113] |
| PDDA(+) | microperoxidase-11 (MP-11) | Drop-casting | GCE | Graphene@PDDA(+) mixed with AuNPs@DMPG 1,2-Dimyristoyl-sn-gly-cero-3-phosphatidil glycerol (DMPG) to obtain graphen@PDDA(+)@AuNPs@DMPG; then mixed with MP-11; then drop-casting onto GCE | GCE/graphen@PDDA/AuNPs@DMPG@MP-11 | H2O2 | CV | [114] |
| PAH(+) | GOx | Drop-casting | planar interdigitated electrode (IDE) | nanoparticles were coated with an initial layer of PAH; then dispersion of the PAH(+)@NPs into GOx; then Drop-casting of NPs@PAH(+)@GOx onto IDEs; then cross-linking in the vapor of GTH | IDE/NPs@PAH@GOx | Glucose | conductometry | [115] |
| SPAnNa(−) | HRP | Drop-casting | Au | poly[aniline-co-sodium N-(1-one-butyric acid) aniline]-zinc oxide (SPAnNa@ZnO) mixed with HRP; then drop-cast onto Au | Au/SPAnNa@ZnO@HRP | H2O2 | AMP | [116] |
| Conjugated PEs: P3TOPS(−); aPPE(−); | GOx | Drop-casting | GCE | Preparation of complex (by mixing) of the SWCNTs, GOx and P3TOPS (−) or PPE(−); then drop-casting onto GCE | GCE/SWCNTs@PE@GOx | Glucose | AMP | [117] |
| PDDA(+) | AChE | Drop-casting | SPCE | GO and AuCl4− chemically reduced with NaBH4 in the presence of PDDA(+); then AuNPs@PDDA@rGO mixed with AChE; then drop-cast onto SPCE | SPCE/AuNPs@PDDA@rGO@AChE | Paraoxone | AMP | [118] |
| PAH(+) | GOx | Drop-casting | ITO | SiO2NFs coated with PAH(+); then immersion into AuCl4− followed by reduction by NaBH4; then mixing with GOx, L-cysteine and GTH; then immobilization onto ITO | ITO/SiO2@PAH@AuNPs@L-cysteine@GOx | Glucose | CV | [119] |
| PHEMA-b-PDMAEMA (PHD(+)) | hemoglobin | Drop-casting | GCE | Drop-casting of the Hb@PHD(+)@MWCNTs GCE | GCE/Hb@PHD@MWCNTs | H2O2 | AMP | [120] |
| 3.3. PE in Preparation of Scaffolding Layer by Drop-Casting | ||||||||
|---|---|---|---|---|---|---|---|---|
| 3.3.1. Redox Mediators (Complex Compounds)–PE Composite | ||||||||
| PE(s) Used | Sensing Element | Modification Methods | Electrode/Substrate | Path of Modification | Sensor Construction | Analyte | Analysis Method | Reference |
| PAA(+); dodecyl sulphate (DS(−)) as surfactant | PB | Drop-casting/electrodeposition | Graphite; ITO | Drop-casting of the PAA(+)@DS(−) onto electrode; then electrodeposition of the PB | Graphite or ITO/PAA@DS/PB | H2O2 | AMP | [121] |
| PAA(+) derivatized with pyridine Os complex (PAO(+)); SDS (−) as surfactants | GOx | Drop-casting | Graphite | Precipitation of PAO(+) and SDS(−); then solution of PAO(+)@SDS(−) in dimethylformamide DMF onto G electrode; then drop-casting of GOx | Graphite/PAO@SDS/GOx | Glucose | CV | [122] |
| 3.3.2. Me or MeO NPs–PE Composite | ||||||||
| silsesquioxane polymer SiPy(+)Cl(−) | HRP | Drop-casting | GCE | Drop-casting of the suspension of AuNPs stabilized with SiPy(+) onto GCE; then TLA (thiolactic acid); then immobilization of HRP via carbodiimide reaction (EDC/NHS) | GCE/AuNPs@SiPy/TLA/HRP | Catechol | DPV | [123] |
| 3.3.3. Carbon-Based Nanomaterials–PE Composite | ||||||||
| Nafion®(−) | MWCNTs @ M(II)Ph; M = Zn, Co, Ni | Drop-coating/dip-coating | GCE | drop-coating of the solution of Nafion®(−) onto GCE; than in Nafion®(−)@MWCNTs; then in suspension of M(II)Ph(+)@Nafion®(−) | GCE/Nafion®@MWCNTs/M(II)Ph@Nafion® | H2O2 | AMP | [124] |
| 3.3.4. Me or MeO NPs–Carbon-Based Nanomaterials–PE Composite | ||||||||
| PDDA(+); PEI(+) | GOx and LOD | Drop-casting | Dual GCE | Drop-casting of the dispersion of the Pt@PDDA(+)@carbon mesoporous material (CMM) onto GCE; then drop-casting of the GOx and LOD@PEI(+); then cross-linking with GTH | GCE/Pt@PDDA@CMM/GOx or LOD@PEI | Glucose and L-lactate | AMP | [125] |
| PDDA (+) | Aptamer | Drop-casting | GCE | Drop-casting of the suspension of rGO@PDDA(+)@AgNP; then drop of solution of aptamer | GCE/rGO@PDDA@AgNP/aptamer | Chloramphenicol | LSV | [126] |
| PDDA(+) | HRP | Drop-casting | GCE | Graphene modified with PDDA(+); then electrostatic interaction with PtCl62-; then PtCl62- microwave reduction; then drop-casting of the composite rGO@PDDA@PtNPs onto GCE; then drop-casting of the HRP | GCE/rGO@PDDA@PtNPs/HRP | H2O2 | CV | [127] |
| PDDA(+) | rGO@PDDA(+)@PtNP | Drop-casting | SiO2/Ti or Au film | drop-casting of the rGO@PDDA(+) onto substrate; then immersion in the PtCl6− followed by reduction of adsorbed Pt(IV) into PtNP by ascorbic acid | SiO2/Ti or Au film/rGO@PDDA@PtNP | Ascorbic acid (AA) dopamine (DA) and uric acid (UA) | AMP | [128] |
| CS(+) | Ab1 | Drop-casting | GCE | drop-casting of the graphene@CS(+) onto GCE; then immersion of electrode in AuCl4− followed by electroreduction; then drop-casting of Ab1 (anti-body) | GCE/graphene@CS@AuNPs/Ab1 | Prolactin | DPV | [129] |
| PDDA(+) | Aptamer | Drop-casting | GCE | GO@PDDA(+) into AuCl4− followed by chemical reduction to obtain GO@PDDA(+)@AuNPs; then drop-casting onto GCE; then incubation in aptamer solution | GCE/GO@PDDA@AuNPs/ssDNA | cDNA | SWV | [130] |
| PDDA(+); CS(+) | dsDNA | Dip-coating | PGE (pencil graphite electrode) | Various modification paths. Example: The PGEs were immersed into a suspension containing MWCNTs, TiO2NPs dispersed in PDDA(+); the electrodes were dipped into the dsDNA | PGE/MWCNTs@TiO2NPs@PDDA/dsDNA | Guanine and adenine | DPV | [131] |
| 3.3.5. PE or PEC as a Scaffolding Layer | ||||||||
| PLL(+); Indigo tetra sulphonate (ITS)(−) | PLL(+)@GTH/ITS (−) | Drop-casting/electrodeposition | GCE | Drop-casting of the solution PLL(+)@GTH (as cross-linker) onto GCE; then electrodeposition of ITS(−) | GCE/PLL@GTH/ITS | Oxygen | RDE voltammetry | [132] |
| PSS(−); PEI(+) | Acridine orange (AO) | Dip-coating | GCE | Dip-coating of PEI(+); then dip-coating of PSS(−); then dip-coating AO(+) | GCE/PEI/PSS/AO | CTAB(−) | AMP | [133] |
| PAA(+) | CMWCNTs-AMWCNTs bonded via carbodiimide ester bond | Dip-coating/LbL | GCE | Dip-coating of GCE into PAA(+); then LbL: CMWCNTs-AMWCNTs/then EDC/NHS to form carbodiimide ester NOTE: AMWCNTs: MWNTs functionalized with amino terminated silanes (APS) CMWCNT: carboxylated MWCNT | GCE/PAA/LbL: CMWCNTs-AMWCNTs | NADH | AMP | [134] |
| 3.3.6 Other Uses of PE in Casting/Coating Mode | ||||||||
| Nafion®(−); PAH(+) | Bienzymesensor: FFMNSs@PAH@MP11/GOx | Drop-casting | GCE | FF-MNSs modified with PAH(+) and MP11 to obtain FFMNSs@PAH(+)@MP11; then drop-casting onto GCE; then drop-casting GOx/then Nafion®(−); | GCE/FFMNSs@PAH@MP11/GOx/Nafion® | Glucose | AMP | [135] |
| 3.4. PE or PEC as a Sensing Layer | ||||||||
|---|---|---|---|---|---|---|---|---|
| PE(s) Used | Sensing Element | Modification Methods | Electrode/Substrate | Path of Modification | Sensor Construction | Analyte | Analysis Method | Reference |
| CS(+); Pectin(−) (PC) | CS@PC | Drop-casting | GCE | Dispersion of biopolymer polyelectrolyte complex (CS(+)@PC(−)) | GCE/CS@PC | Metronidazole and metribuzin | DPV | [136] |
| CS(+); Sulphonated CS(−) (SCS) | Cyclodextrin (CD) | Drop-casting | GCE | Homogenous solution of the of CS(+)@SCS(−)@CD onto GCE | GCE/CS@SCS and GCE/CS@SCS@CD | Atenolol | DPV | [137] |
| PDADMAC (+); CS-Cellulose sulphate(−) | PDMDAAC@CS | Sequential drop-casting | GCE | Sequential drop-casting of the PDADMAC(+) solution and CS(−) solution onto GCE | GCE/PDADMAC@CS | Nitrite | DPV | [138] |
| PAA(+); surfactant SDS(−) | SDS@PAA | Drop-casting | SPCE | Drop-casting of the suspension of PAA(+) and SDS(−) | SPCE/SDS@PAA | Xanthate | IMP | [139] |
| PAH(+) | PAH | Drop-casting | SPCE | Drop-casting of PAH(+) on oxidized SPCE | SPCE/PAH | NADH | DPV | [140] |
| PDDA(+) | PDDA | from the solution | GCE | PDDA(+) in sample solution | GCE covered with PDDA during analysis | uric acid and ascorbic acid | DPV | [141] |
| PAA(+); HPA (+) | Hydrazinium poly acrylate (HPA) | Drop-casting | SPCE | Drop-casting of the PAA(+) or HPA(+) onto SPCE | SPCE/PAA or HPA | Form aldehyde | CV | [142] |
| PBI-BA(−) | poly[N-(1-one-butyric acid) benzimidazole] (PBI-BA) | Drop-casting | Au | Drop-casting of the solution of the PBI-BA(−) or graphene modified PBI-BA(−) (PBI-BA(−)@ graphene) onto Au | Au/PBI-BA or graphene@PBI-BA | H2O2 | AMP | [143] |
| PAH(+) | ssDNA | Drop-casting | Chip (pSi-SiO2) | Drop-coating of the PAH(+) onto chip/then immobilization of ssDNA(−) | Chip/PAH/ssDNA | cDNA | IMP | [144] |
| 3.5. Other Method Involved in Application of PEs in Sensor Architectures | ||||||||
|---|---|---|---|---|---|---|---|---|
| 3.5.1. Electrospinning and Spin-Coating of PE onto a Working Electrode | ||||||||
| PE(s) Used | Sensing Element | Modification Methods | Electrode/Substrate | Path of Modification | Sensor Construction | Analyte | Analysis Method | Reference |
| Crosslinked and quaternized QC-P4VP(+) | AgNPs | Electro spinning/dip-coating | Au | Electrospinning of P4PVP; then crosslinking and quaternization with 1,4-dibromobutane (DBB) to obtain QC-P4VP(+); then dip-coating into Ag+ followed by reduction by UV lamp | Au/QC-P4VP@AgNPs | Humidity | IMP | [145] |
| PAH(+) | PA6@PAH(+)@MWCNTs | Electrospinning | ITO | Electrospinning of polyamide (PA6) and PAH(+) onto ITO; then immersion into dispersion of the MWCNTs | ITO/PA6@PAH@MWCNTs | Dopamine | DPV | [146] |
| PAA(+) SDS(−) surfactant | HRP | Spin-coating | Si coated with Pd, Ti, Au | spin-coated redox-active glycoppolyelectrolyte containing Os(bpy)2 (GOsPA) mixed with SDS(−) onto electrode; then building of the protein building blocks such as Con A, Os-Con A; then immobilization of HRP | Au/GOsPA-SDS/Con A or Os-Con A/HRP | H2O2 | CV; AMP | [147] |
| PDDA(+); PVS(−) | Silica@PEC | Spin coating/drop-casting | GCE; glass | mixing solution of tetraethyl orthosilicate (TEOS) and PDDA(+) or PEC (PDDA(+)@PVS(−)) during preparation of silica sol (in various ratio depending on ion-exchange power); then addition of adenine; then spin-coating onto electrode; then removing adenine from film in ethanol. | GCE/silica@PEC | Adenine and guanine | DPV;SWV | [148] |
| 3.5.2. PEs in Construction of Reporter Probes, Nanoprobes, etc. | ||||||||
| PDDA(+) | DNAzyme/PtNPs/CNTs | Hybridization | Au | Capture probe (electrode): hybridization of target DNA onto Au electrode modified with capture DNA probe Reporter probe: mixture of CNTs(−) and PDDA(+) to obtain CNTs(−)@PDDA(+); then mixing with PtNPs; then immobilization of DNAzyme (DNA labeled with enzyme) by mixing; then immobilization onto modified Au electrode by hybridization. | Reporter probe (nanoprobe): CNTs@PDDA@PtNPs@DNAzyme | DNA | AMP | [149] |
| PDDA(+) | Anti-HigG | Drop-casting | SPCE | Capture probe (electrode): drop-casting of the Au(III) onto SPCE followed by electroreduction; then immobilization of the anti-HigG; then BSA (blocking surface); Reporter probe (nanoprobe): Prussian blue (PB) functionalized mesoporous carbon nanosphere (MCN) (obtained using silica template) (MCN@PB(−)); then coated with PDDA(+); then reduction of the AuCl4− with citrate to obtain MCN@PB(−)@AuNPs; then anti-HigG and GOx through the inherent interaction between Au NPs and protein | Reporter probe (nanoprobe): MCN@PB/PDDA@AuNPs@HigG and GOx | HigG | DPV | [150] |
| PDDA(+); PSS(−) | FC@PE@Ab2 | Drop-casting | GCE | Capture probe (electrode): immobilization of Ab1 by amidation onto oxidized GCE(−) Reporter probe (nanoprobe): CaCO3 (as templates) nanoparticles mixed with PDDA(+), PSS(−) and FC; then mixing of the obtained modified CaCO3@FC@PDDA(+)@PSS(−) with Ab2 | Reporter probe (nanoprobe): CaCO3@FC@PDDA@PSS@Ab2 | Interleukin-6 (IL-6) | SWV | [151] |
| PAA(+) | Gene sensing material | Rubber | PEDOT embedded electrospun rubber; then grafting PAA(+) as brushes; then potentiodynamically polymerized ssDNA(−) | Rubber/PEDOT/PAA/gene sensing material | Non-Hodgkin lymphoma gene (cDNA) | IMP | [152] | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Škugor Rončević, I.; Krivić, D.; Buljac, M.; Vladislavić, N.; Buzuk, M. Polyelectrolytes Assembly: A Powerful Tool for Electrochemical Sensing Application. Sensors 2020, 20, 3211. https://doi.org/10.3390/s20113211
Škugor Rončević I, Krivić D, Buljac M, Vladislavić N, Buzuk M. Polyelectrolytes Assembly: A Powerful Tool for Electrochemical Sensing Application. Sensors. 2020; 20(11):3211. https://doi.org/10.3390/s20113211
Chicago/Turabian StyleŠkugor Rončević, Ivana, Denis Krivić, Maša Buljac, Nives Vladislavić, and Marijo Buzuk. 2020. "Polyelectrolytes Assembly: A Powerful Tool for Electrochemical Sensing Application" Sensors 20, no. 11: 3211. https://doi.org/10.3390/s20113211





