Fifteen Years of Wireless Sensors for Balance Assessment in Neurological Disorders
Abstract
1. Introduction
2. Physiology and Pathophysiology of Balance
3. Clinical Assessment of Balance
4. Static and Dynamic Posturography
5. Wearable Technologies
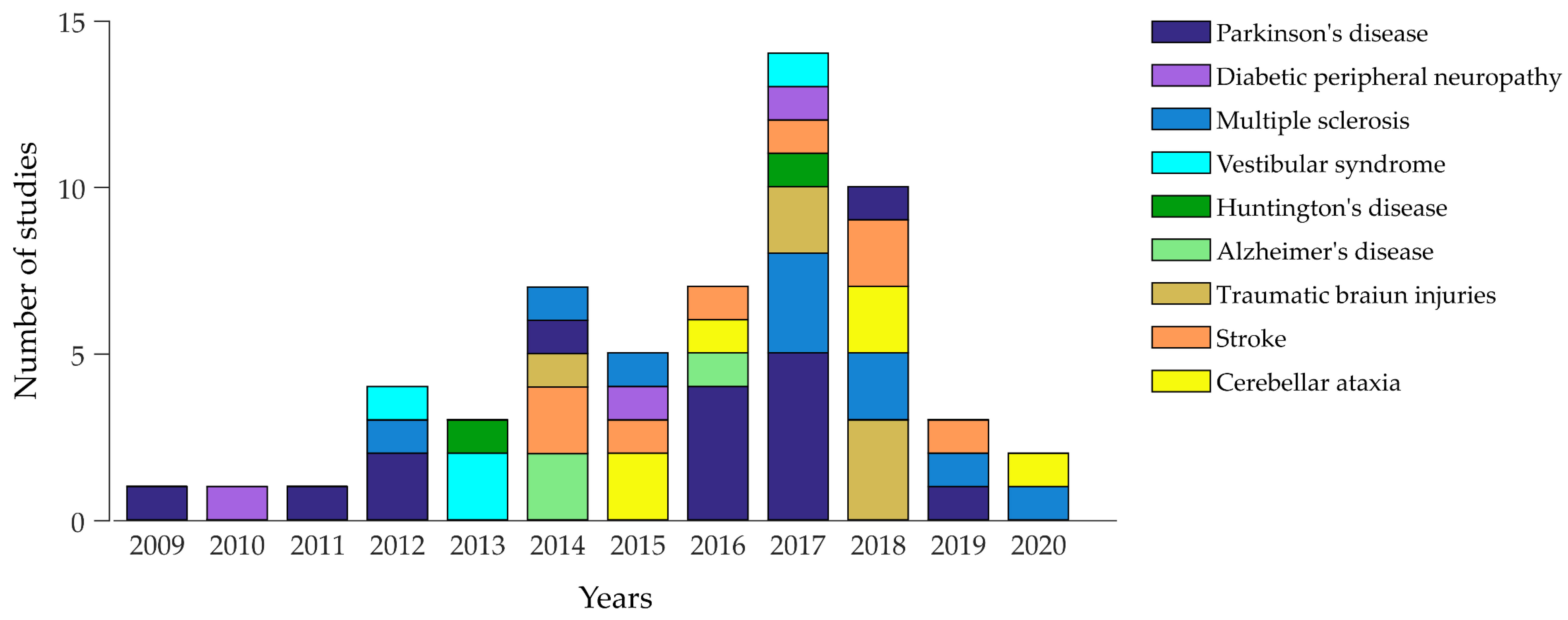
6. Literature Research Strategy and Criteria
7. Wearable Technologies in Neurological Disorders
8. Teleneurology and Telerehabilitation for Balance: Prospects and Challenges
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| APA | Anticipatory postural adjustment |
| AT | Adaptation Test |
| BOS | Base of support |
| IMU | Inertial measurements unit |
| COM | Centre of mass |
| COP | Centre of pressure |
| MCT | Motor Control Test |
| sEMG | Surface electromyography |
| SOT | Sensory Organisation Test |
References
- Ageing and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 16 March 2020).
- World Health Organization (Ed.) WHO Global Report on Falls Prevention in Older Age; World Health Organization: Geneva, Switzerland, 2008; ISBN 978-92-4-156353-6. [Google Scholar]
- Matheron, E.; Dubost, V.; Mourey, F.; Pfitzenmeyer, P.; Manckoundia, P. Analysis of postural control in elderly subjects suffering from Psychomotor Disadaptation Syndrome (PDS). Arch. Gerontol. Geriatr. 2010, 51, e19–e23. [Google Scholar] [CrossRef] [PubMed]
- Florence, C.S.; Bergen, G.; Atherly, A.; Burns, E.; Stevens, J.; Drake, C. Medical Costs of Fatal and Nonfatal Falls in Older Adults. J. Am. Geriatr. Soc. 2018, 66, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Trisan, R. Balance Problems and Fall Risks in the Elderly. Clin. Geriatr. Med. 2019, 35, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Sturnieks, D.L.; St George, R.; Lord, S.R. Balance disorders in the elderly. Neurophysiol. Clin. 2008, 38, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Stolze, H.; Klebe, S.; Zechlin, C.; Baecker, C.; Friege, L.; Deuschl, G. Falls in frequent neurological diseases-prevalence, risk factors and aetiology. J. Neurol. 2004, 251, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Sai, A.J.; Gallagher, J.C.; Smith, L.M.; Logsdon, S. Fall predictors in the community dwelling elderly: A cross sectional and prospective cohort study. J. Musculoskelet. Neuronal Interact. 2010, 10, 142–150. [Google Scholar] [PubMed]
- Pickering, R.M.; Grimbergen, Y.A.M.; Rigney, U.; Ashburn, A.; Mazibrada, G.; Wood, B.; Gray, P.; Kerr, G.; Bloem, B.R. A meta-analysis of six prospective studies of falling in Parkinson’s disease. Mov. Disord. 2007, 22, 1892–1900. [Google Scholar] [CrossRef]
- Bloem, B.R.; Steijns, J.A.G.; Smits-Engelsman, B.C. An update on falls. Curr. Opin. Neurol. 2003, 16, 15–26. [Google Scholar] [CrossRef]
- Dorsey, E.R.; Glidden, A.M.; Holloway, M.R.; Birbeck, G.L.; Schwamm, L.H. Teleneurology and mobile technologies: The future of neurological care. Nat. Rev. Neurol. 2018, 14, 285–297. [Google Scholar] [CrossRef]
- Bernhard, F.P.; Sartor, J.; Bettecken, K.; Hobert, M.A.; Arnold, C.; Weber, Y.G.; Poli, S.; Margraf, N.G.; Schlenstedt, C.; Hansen, C.; et al. Wearables for gait and balance assessment in the neurological ward-study design and first results of a prospective cross-sectional feasibility study with 384 inpatients. BMC Neurol. 2018, 18, 114. [Google Scholar] [CrossRef]
- Pollock, A.S.; Durward, B.R.; Rowe, P.J.; Paul, J.P. What is balance? Clin. Rehabil. 2000, 14, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Le Huec, J.C.; Saddiki, R.; Franke, J.; Rigal, J.; Aunoble, S. Equilibrium of the human body and the gravity line: The basics. Eur. Spine J. 2011, 20, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Vassar, R.L.; Rose, J. Motor systems and postural instability. Handb. Clin. Neurol. 2014, 125, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Takakusaki, K. Functional Neuroanatomy for Posture and Gait Control. J. Mov. Disord. 2017, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- MacKinnon, C.D. Chapter 1—Sensorimotor anatomy of gait, balance, and falls. In Handbook of Clinical Neurology; Balance, G., Day, B.L., Lord, S.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 159, pp. 3–26. [Google Scholar]
- Varghese, J.P.; Merino, D.M.; Beyer, K.B.; McIlroy, W.E. Cortical control of anticipatory postural adjustments prior to stepping. Neuroscience 2016, 313, 99–109. [Google Scholar] [CrossRef]
- Jacobs, J.V.; Horak, F.B. Cortical control of postural responses. J. Neural. Transm. 2007, 114, 1339–1348. [Google Scholar] [CrossRef]
- Forbes, P.A.; Chen, A.; Blouin, J.-S. Sensorimotor control of standing balance. Handb. Clin. Neurol. 2018, 159, 61–83. [Google Scholar] [CrossRef]
- Proske, U.; Gandevia, S.C. The proprioceptive senses: Their roles in signaling body shape, body position and movement, and muscle force. Physiol. Rev. 2012, 92, 1651–1697. [Google Scholar] [CrossRef]
- Rogers, M.W.; Mille, M.-L. Balance perturbations. Handb. Clin. Neurol. 2018, 159, 85–105. [Google Scholar] [CrossRef]
- Bronte-Stewart, H.M.; Minn, A.Y.; Rodrigues, K.; Buckley, E.L.; Nashner, L.M. Postural instability in idiopathic Parkinson’s disease: The role of medication and unilateral pallidotomy. Brain 2002, 125, 2100–2114. [Google Scholar] [CrossRef]
- Woollacott, M.; Shumway-Cook, A. Attention and the control of posture and gait: A review of an emerging area of research. Gait Posture 2002, 16, 1–14. [Google Scholar] [CrossRef]
- Peterka, R.J. Sensory integration for human balance control. Handb. Clin. Neurol. 2018, 159, 27–42. [Google Scholar] [CrossRef]
- Mileti, I.; Taborri, J.; Rossi, S.; Del Prete, Z.; Paoloni, M.; Suppa, A.; Palermo, E. Reactive Postural Responses to Continuous Yaw Perturbations in Healthy Humans: The Effect of Aging. Sensors 2019, 20, 63. [Google Scholar] [CrossRef]
- Apostolova, L.G. Alzheimer Disease. Continuum 2016, 22, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.W.; Lee, H.; Chung, I.S.; Yi, H.A. Relationship between postural instability and subcortical volume loss in Alzheimer’s disease. Medicine 2017, 96, e7286. [Google Scholar] [CrossRef] [PubMed]
- Gordon, B.; Carson, K. The basis for choice reaction time slowing in Alzheimer’s disease. Brain Cognit. 1990, 13, 148–166. [Google Scholar] [CrossRef]
- Uhlmann, R.F.; Larson, E.B.; Koepsell, T.D.; Rees, T.S.; Duckert, L.G. Visual impairment and cognitive dysfunction in Alzheimer’s disease. J. Gen. Intern. Med. 1991, 6, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Moreland, J.D.; Richardson, J.A.; Goldsmith, C.H.; Clase, C.M. Muscle weakness and falls in older adults: A systematic review and meta-analysis. J. Am. Geriatr. Soc. 2004, 52, 1121–1129. [Google Scholar] [CrossRef]
- Gago, M.F.; Fernandes, V.; Ferreira, J.; Silva, H.; Rocha, L.; Bicho, E.; Sousa, N. Postural Stability Analysis with Inertial Measurement Units in Alzheimer’s Disease. Dement. Geriatr. Cognit. Dis. Extra 2014, 4, 22–30. [Google Scholar] [CrossRef]
- Kim, S.D.; Allen, N.E.; Canning, C.G.; Fung, V.S.C. Parkinson disease. Handb. Clin. Neurol. 2018, 159, 173–193. [Google Scholar] [CrossRef]
- Crouse, J.J.; Phillips, J.R.; Jahanshahi, M.; Moustafa, A.A. Postural instability and falls in Parkinson’s disease. Rev. Neurosci. 2016, 27, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Di Giulio, I.; St George, R.J.; Kalliolia, E.; Peters, A.L.; Limousin, P.; Day, B.L. Maintaining balance against force perturbations: Impaired mechanisms unresponsive to levodopa in Parkinson’s disease. J. Neurophysiol. 2016, 116, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.V.; Horak, F.B. Abnormal proprioceptive-motor integration contributes to hypometric postural responses of subjects with Parkinson’s disease. Neuroscience 2006, 141, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Maschke, M.; Gomez, C.M.; Tuite, P.J.; Konczak, J. Dysfunction of the basal ganglia, but not the cerebellum, impairs kinaesthesia. Brain 2003, 126, 2312–2322. [Google Scholar] [CrossRef]
- Wright, W.G.; Gurfinkel, V.S.; Nutt, J.; Horak, F.B.; Cordo, P.J. Axial hypertonicity in Parkinson’s disease: Direct measurements of trunk and hip torque. Exp. Neurol. 2007, 208, 38–46. [Google Scholar] [CrossRef]
- Allcock, L.M.; Rowan, E.N.; Steen, I.N.; Wesnes, K.; Kenny, R.A.; Burn, D.J. Impaired attention predicts falling in Parkinson’s disease. Parkinsonism Relat. Disord. 2009, 15, 110–115. [Google Scholar] [CrossRef]
- What Is MS? Available online: http://www.nationalmssociety.org/What-is-MS (accessed on 15 March 2020).
- Fjeldstad, C.; Pardo, G.; Bemben, D.; Bemben, M. Decreased postural balance in multiple sclerosis patients with low disability. Int. J. Rehabil. Res. 2011, 34, 53–58. [Google Scholar] [CrossRef]
- Gunn, H.J.; Newell, P.; Haas, B.; Marsden, J.F.; Freeman, J.A. Identification of risk factors for falls in multiple sclerosis: A systematic review and meta-analysis. Phys. Ther. 2013, 93, 504–513. [Google Scholar] [CrossRef]
- Vuong, K.; Canning, C.G.; Menant, J.C.; Loy, C.T. Gait, balance, and falls in Huntington disease. Handb. Clin. Neurol. 2018, 159, 251–260. [Google Scholar] [CrossRef]
- Ross, C.A.; Aylward, E.H.; Wild, E.J.; Langbehn, D.R.; Long, J.D.; Warner, J.H.; Scahill, R.I.; Leavitt, B.R.; Stout, J.C.; Paulsen, J.S.; et al. Huntington disease: Natural history, biomarkers and prospects for therapeutics. Nat. Rev. Neurol. 2014, 10, 204–216. [Google Scholar] [CrossRef]
- Medina, L.D.; Pirogovsky, E.; Salomonczyk, D.; Goldstein, J.; Panzera, R.; Gluhm, S.; Simmons, R.; Corey-Bloom, J.; Gilbert, P.E. Postural limits of stability in premanifest and manifest Huntington’s disease. J. Huntingt. Dis. 2013, 2, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Kegelmeyer, D.A.; Kostyk, S.K.; Fritz, N.E.; Fiumedora, M.M.; Chaudhari, A.; Palettas, M.; Young, G.; Kloos, A.D. Quantitative biomechanical assessment of trunk control in Huntington’s disease reveals more impairment in static than dynamic tasks. J. Neurol. Sci. 2017, 376, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Ashizawa, T.; Xia, G. Ataxia. Continuum 2016, 22, 1208–1226. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, H. Stroke. Handb. Clin. Neurol. 2013, 110, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Handelzalts, S.; Melzer, I.; Soroker, N. Analysis of Brain Lesion Impact on Balance and Gait Following Stroke. Front. Hum. Neurosci. 2019, 13, 149. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.E.; Hedman, L.D. Sensory dysfunction following stroke: Incidence, significance, examination, and intervention. Top. Stroke Rehabil. 2008, 15, 200–217. [Google Scholar] [CrossRef] [PubMed]
- Tasseel-Ponche, S.; Yelnik, A.P.; Bonan, I.V. Motor strategies of postural control after hemispheric stroke. Neurophysiol. Clin. 2015, 45, 327–333. [Google Scholar] [CrossRef]
- Rahimzadeh Khiabani, R.; Mochizuki, G.; Ismail, F.; Boulias, C.; Phadke, C.P.; Gage, W.H. Impact of Spasticity on Balance Control during Quiet Standing in Persons after Stroke. Stroke Res. Treat. 2017, 2017, 6153714. [Google Scholar] [CrossRef]
- Jehkonen, M.; Ahonen, J.P.; Dastidar, P.; Koivisto, A.M.; Laippala, P.; Vilkki, J. How to detect visual neglect in acute stroke. Lancet 1998, 351, 727–728. [Google Scholar] [CrossRef]
- Sand, K.M.; Midelfart, A.; Thomassen, L.; Melms, A.; Wilhelm, H.; Hoff, J.M. Visual impairment in stroke patients—A review. Acta Neurol. Scand. 2013, 127, 52–56. [Google Scholar] [CrossRef]
- Delavaran, H.; Jönsson, A.-C.; Lövkvist, H.; Iwarsson, S.; Elmståhl, S.; Norrving, B.; Lindgren, A. Cognitive function in stroke survivors: A 10-year follow-up study. Acta Neurol. Scand. 2017, 136, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Hyder, A.A.; Wunderlich, C.A.; Puvanachandra, P.; Gururaj, G.; Kobusingye, O.C. The impact of traumatic brain injuries: A global perspective. NeuroRehabilitation 2007, 22, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.H.; Yi, J.H.; Kwon, H.G. Injury of the inferior cerebellar peduncle in patients with mild traumatic brain injury: A diffusion tensor tractography study. Brain Inj. 2016, 30, 1271–1275. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.F.; Liou, T.H.; Hu, C.J.; Ma, H.P.; Ou, J.C.; Chiang, Y.H.; Chiu, W.T.; Tsai, S.H.; Chu, W.C. Balance function and sensory integration after mild traumatic brain injury. Brain Inj. 2015, 29, 41–46. [Google Scholar] [CrossRef]
- Ramdharry, G. Peripheral nerve disease. Handb. Clin. Neurol. 2018, 159, 403–415. [Google Scholar] [CrossRef]
- Nicholson, M.; King, J.; Smith, P.F.; Darlington, C.L. Vestibulo-ocular, optokinetic and postural function in diabetes mellitus. Neuroreport 2002, 13, 153–157. [Google Scholar] [CrossRef]
- Young, A.S.; Rosengren, S.M.; Welgampola, M.S. Disorders of the inner-ear balance organs and their pathways. Handb. Clin. Neurol. 2018, 159, 385–401. [Google Scholar] [CrossRef]
- Fernández, L.; Breinbauer, H.A.; Delano, P.H. Vertigo and Dizziness in the Elderly. Front. Neurol. 2015, 6, 144. [Google Scholar] [CrossRef]
- Mancini, M.; Horak, F.B. The relevance of clinical balance assessment tools to differentiate balance deficits. Eur. J. Phys. Rehabil. Med. 2010, 46, 239–248. [Google Scholar]
- Khasnis, A.; Gokula, R.M. Romberg’s test. J. Postgrad. Med. 2003, 49, 169. [Google Scholar]
- Hunt, A.L.; Sethi, K.D. The pull test: A history. Mov. Disord. 2006, 21, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Margolesky, J.; Singer, C. How tandem gait stumbled into the neurological exam: A review. Neurol. Sci. 2018, 39, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Fregly, A.R.; Graybiel, A. An ataxia test battery not requiring rails. Aerosp. Med. 1968, 39, 277–282. [Google Scholar] [PubMed]
- Mathias, S.; Nayak, U.S.; Isaacs, B. Balance in elderly patients: The “get-up and go” test. Arch. Phys. Med. Rehabil. 1986, 67, 387–389. [Google Scholar] [PubMed]
- Tinetti, M.E. Performance-oriented assessment of mobility problems in elderly patients. J. Am. Geriatr. Soc. 1986, 34, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Duncan, P.W.; Weiner, D.K.; Chandler, J.; Studenski, S. Functional reach: A new clinical measure of balance. J. Gerontol. 1990, 45, M192–M197. [Google Scholar] [CrossRef]
- Berg, K.O.; Wood-Dauphinee, S.L.; Williams, J.I.; Maki, B. Measuring balance in the elderly: Validation of an instrument. Can. J. Public Health 1992, 83, S7–S11. [Google Scholar]
- Powell, L.E.; Myers, A.M. The Activities-specific Balance Confidence (ABC) Scale. J. Gerontol. A Biol. Sci. Med. Sci. 1995, 50A, M28–M34. [Google Scholar] [CrossRef]
- Lord, S.R.; Menz, H.B.; Tiedemann, A. A physiological profile approach to falls risk assessment and prevention. Phys. Ther. 2003, 83, 237–252. [Google Scholar] [CrossRef]
- Horak, F.B.; Wrisley, D.M.; Frank, J. The Balance Evaluation Systems Test (BESTest) to differentiate balance deficits. Phys. Ther. 2009, 89, 484–498. [Google Scholar] [CrossRef]
- Blum, L.; Korner-Bitensky, N. Usefulness of the Berg Balance Scale in stroke rehabilitation: A systematic review. Phys. Ther. 2008, 88, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Dye, D.C.; Eakman, A.M.; Bolton, K.M. Assessing the validity of the dynamic gait index in a balance disorders clinic: An application of Rasch analysis. Phys. Ther. 2013, 93, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Horak, F.B.; Shupert, C.L.; Mirka, A. Components of postural dyscontrol in the elderly: A review. Neurobiol. Aging 1989, 10, 727–738. [Google Scholar] [CrossRef]
- Piirtola, M.; Era, P. Force platform measurements as predictors of falls among older people-a review. Gerontology 2006, 52, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bronstein, A.M.; Pavlou, M. Chapter 16—Balance. In Handbook of Clinical Neurology; Neurological Rehabilitation; Barnes, M.P., Good, D.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 110, pp. 189–208. [Google Scholar]
- Visser, J.E.; Carpenter, M.G.; van der Kooij, H.; Bloem, B.R. The clinical utility of posturography. Clin. Neurophysiol. 2008, 119, 2424–2436. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, M.; Geroin, C.; Picelli, A.; Smania, N.; Bartolo, M. Assessment of Balance Disorders. In Advanced Technologies for the Rehabilitation of Gait and Balance Disorders; Sandrini, G., Homberg, V., Saltuari, L., Smania, N., Pedrocchi, A., Eds.; Springer International Publishing: Cham, Switzerland, 2018; Volume 19, pp. 47–67. [Google Scholar]
- Peterka, R.J. Sensorimotor integration in human postural control. J. Neurophysiol. 2002, 88, 1097–1118. [Google Scholar] [CrossRef] [PubMed]
- Cenciarini, M.; Loughlin, P.J.; Sparto, P.J.; Redfern, M.S. Stiffness and damping in postural control increase with age. IEEE Trans. Biomed. Eng. 2010, 57, 267–275. [Google Scholar] [CrossRef]
- Winter, D.A.; Patla, A.E.; Prince, F.; Ishac, M.; Gielo-Perczak, K. Stiffness control of balance in quiet standing. J. Neurophysiol. 1998, 80, 1211–1221. [Google Scholar] [CrossRef]
- Chaudhry, H.; Bukiet, B.; Ji, Z.; Findley, T. Measurement of balance in computer posturography: Comparison of methods—A brief review. J. Bodyw. Mov. Ther. 2011, 15, 82–91. [Google Scholar] [CrossRef]
- Parijat, P.; Lockhart, T.E. Effects of moveable platform training in preventing slip-induced falls in older adults. Ann. Biomed. Eng. 2012, 40, 1111–1121. [Google Scholar] [CrossRef]
- Lee, A.J.Y.; Lin, W.-H. Twelve-week biomechanical ankle platform system training on postural stability and ankle proprioception in subjects with unilateral functional ankle instability. Clin. Biomech. 2008, 23, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Newstead, A.H.; Hinman, M.R.; Tomberlin, J.A. Reliability of the Berg Balance Scale and balance master limits of stability tests for individuals with brain injury. J. Neurol. Phys. Ther. 2005, 29, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Kalron, A.; Fonkatz, I.; Frid, L.; Baransi, H.; Achiron, A. The effect of balance training on postural control in people with multiple sclerosis using the CAREN virtual reality system: A pilot randomized controlled trial. J. Neuroeng. Rehabil. 2016, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, P.B.; Knight, C.A.; Barela, J.A. Postural reactions following forward platform perturbation in young, middle-age, and old adults. J. Electromyogr. Kinesiol. 2010, 20, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Halická, Z.; Lobotková, J.; Bzdúšková, D.; Hlavačka, F. Age-related changes in postural responses to backward platform translation. Physiol. Res. 2012, 61, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Kharboutly, H.; Ma, J.; Benali, A.; Thoumie, P.; Pasqui, V.; Bouzit, M. Design of multiple axis robotic platform for postural stability analysis. IEEE Trans. Neural. Syst. Rehabil. Eng. 2015, 23, 93–103. [Google Scholar] [CrossRef]
- Taborri, J.; Mileti, I.; Del Prete, Z.; Rossi, S.; Palermo, E. Yaw Postural Perturbation Through Robotic Platform: Aging Effects on Muscle Synergies. In Proceedings of the 2018 7th IEEE International Conference on Biomedical Robotics and Biomechatronics (Biorob), Enschede, The Netherlands, 26–29 August 2018; pp. 916–921. [Google Scholar]
- Dimitrova, D.; Horak, F.B.; Nutt, J.G. Postural muscle responses to multidirectional translations in patients with Parkinson’s disease. J. Neurophysiol. 2004, 91, 489–501. [Google Scholar] [CrossRef]
- Kanekar, N.; Aruin, A.S. Aging and balance control in response to external perturbations: Role of anticipatory and compensatory postural mechanisms. Age 2014, 36, 9621. [Google Scholar] [CrossRef]
- Horak, F.B.; Nashner, L.M. Central programming of postural movements: Adaptation to altered support-surface configurations. J. Neurophysiol. 1986, 55, 1369–1381. [Google Scholar] [CrossRef]
- Grin, L.; Frank, J.; Allum, J.H.J. The effect of voluntary arm abduction on balance recovery following multidirectional stance perturbations. Exp. Brain Res. 2007, 178, 62–78. [Google Scholar] [CrossRef][Green Version]
- Chen, C.L.; Lee, J.Y.; Horng, R.F.; Lou, S.Z.; Su, F.C. Development of a three-degrees-of-freedom moveable platform for providing postural perturbations. Proc. Inst. Mech. Eng. H 2009, 223, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Cappa, P.; Patanè, F.; Rossi, S.; Petrarca, M.; Castelli, E.; Berthoz, A. Effect of changing visual condition and frequency of horizontal oscillations on postural balance of standing healthy subjects. Gait Posture 2008, 28, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Gazzellini, S.; Petrarca, M.; Patanè, F.; Salfa, I.; Castelli, E.; Cappa, P. Compensation to whole body active rotation perturbation. Gait Posture 2014, 39, 621–624. [Google Scholar] [CrossRef] [PubMed]
- Amori, V.; Petrarca, M.; Patané, F.; Castelli, E.; Cappa, P. Upper body balance control strategy during continuous 3D postural perturbation in young adults. Gait Posture 2015, 41, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Corna, S.; Tarantola, J.; Nardone, A.; Giordano, A.; Schieppati, M. Standing on a continuously moving platform: Is body inertia counteracted or exploited? Exp. Brain Res. 1999, 124, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.A.; Jensen, J.L.; Korff, T.; Woollacott, M.H. The translating platform paradigm: Perturbation displacement waveform alters the postural response. Gait Posture 2001, 14, 256–263. [Google Scholar] [CrossRef]
- De Nunzio, A.M.; Nardone, A.; Schieppati, M. Head stabilization on a continuously oscillating platform: The effect of a proprioceptive disturbance on the balancing strategy. Exp. Brain Res. 2005, 165, 261–272. [Google Scholar] [CrossRef]
- Santos, M.J.; Kanekar, N.; Aruin, A.S. The role of anticipatory postural adjustments in compensatory control of posture: 1. Electromyographic analysis. J. Electromyogr. Kinesiol. 2010, 20, 388–397. [Google Scholar] [CrossRef]
- Santos, M.J.; Kanekar, N.; Aruin, A.S. The role of anticipatory postural adjustments in compensatory control of posture: 2. Biomechanical analysis. J. Electromyogr. Kinesiol. 2010, 20, 398–405. [Google Scholar] [CrossRef]
- Schmid, M.; Bottaro, A.; Sozzi, S.; Schieppati, M. Adaptation to continuous perturbation of balance: Progressive reduction of postural muscle activity with invariant or increasing oscillations of the center of mass depending on perturbation frequency and vision conditions. Hum. Mov. Sci. 2011, 30, 262–278. [Google Scholar] [CrossRef]
- Mileti, I.; Taborri, J.; Rossi, S.; Prete, Z.D.; Paoloni, M.; Suppa, A.; Palermo, E. Measuring age-related differences in kinematic postural strategies under yaw perturbation. In Proceedings of the 2018 IEEE International Symposium on Medical Measurements and Applications, MeMeA, Rome, Italy, 11–13 June 2018; p. 8438804. [Google Scholar]
- Claudino, R.; dos Santos, E.C.C.; Santos, M.J. Compensatory but not anticipatory adjustments are altered in older adults during lateral postural perturbations. Clin. Neurophysiol. 2013, 124, 1628–1637. [Google Scholar] [CrossRef] [PubMed]
- Inglis, J.T.; Shupert, C.L.; Hlavacka, F.; Horak, F.B. Effect of galvanic vestibular stimulation on human postural responses during support surface translations. J. Neurophysiol. 1995, 73, 896–901. [Google Scholar] [CrossRef] [PubMed]
- Paquette, C.; Fung, J. Temporal facilitation of gaze in the presence of postural reactions triggered by sudden surface perturbations. Neuroscience 2007, 145, 505–519. [Google Scholar] [CrossRef] [PubMed]
- Ford-Smith, C.D.; Wyman, J.F.; Elswick, R.K.; Fernandez, T.; Newton, R.A. Test-retest reliability of the sensory organization test in noninstitutionalized older adults. Arch. Phys. Med. Rehabil. 1995, 76, 77–81. [Google Scholar] [CrossRef]
- Hale, L.; Miller, R.; Barach, A.; Skinner, M.; Gray, A. Motor Control Test responses to balance perturbations in adults with an intellectual disability. J. Intellect. Dev. Disabil. 2009, 34, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.; Salarian, A.; Carlson-Kuhta, P.; Zampieri, C.; King, L.; Chiari, L.; Horak, F.B. ISway: A sensitive, valid and reliable measure of postural control. J. Neuroeng. Rehabil. 2012, 9, 59. [Google Scholar] [CrossRef]
- Ghislieri, M.; Gastaldi, L.; Pastorelli, S.; Tadano, S.; Agostini, V. Wearable Inertial Sensors to Assess Standing Balance: A Systematic Review. Sensors 2019, 19, 4075. [Google Scholar] [CrossRef]
- Winter, D.A. Biomechanics and Motor Control of Human Movement, 4th ed.; Wiley: Hoboken, NJ, USA, 2009; ISBN 978-0-470-39818-0. [Google Scholar]
- Park, J.-H.; Mancini, M.; Carlson-Kuhta, P.; Nutt, J.G.; Horak, F.B. Quantifying effects of age on balance and gait with inertial sensors in community-dwelling healthy adults. Exp. Gerontol. 2016, 85, 48–58. [Google Scholar] [CrossRef]
- Craig, J.J.; Bruetsch, A.P.; Lynch, S.G.; Horak, F.B.; Huisinga, J.M. Instrumented balance and walking assessments in persons with multiple sclerosis show strong test-retest reliability. J. Neuroeng. Rehabil. 2017, 14, 43. [Google Scholar] [CrossRef]
- Chen, C.L.; Lou, S.Z.; Wu, H.W.; Wu, S.K.; Yeung, K.T.; Su, F.C. Postural responses to yaw rotation of support surface. Gait Posture 2013, 37, 296–299. [Google Scholar] [CrossRef]
- Nardone, A.; Grasso, M.; Tarantola, J.; Corna, S.; Schieppati, M. Postural coordination in elderly subjects standing on a periodically moving platform. Arch. Phys. Med. Rehabil. 2000, 81, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- De Nunzio, A.M.; Nardone, A.; Schieppati, M. The control of equilibrium in Parkinson’s disease patients: Delayed adaptation of balancing strategy to shifts in sensory set during a dynamic task. Brain Res. Bull. 2007, 74, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Curtze, C.; Nutt, J.G.; Carlson-Kuhta, P.; Mancini, M.; Horak, F.B. Objective Gait and Balance Impairments Relate to Balance Confidence and Perceived Mobility in People with Parkinson Disease. Phys. Ther. 2016, 96, 1734–1743. [Google Scholar] [CrossRef] [PubMed]
- King, L.A.; Mancini, M.; Fino, P.C.; Chesnutt, J.; Swanson, C.W.; Markwardt, S.; Chapman, J.C. Sensor-Based Balance Measures Outperform Modified Balance Error Scoring System in Identifying Acute Concussion. Ann. Biomed. Eng. 2018, 45, 2135–2145. [Google Scholar] [CrossRef]
- Baston, C.; Mancini, M.; Rocchi, L.; Horak, F. Effects of Levodopa on Postural Strategies in Parkinson’s disease. Gait Posture 2016, 46, 26–29. [Google Scholar] [CrossRef]
- Chen, T.; Fan, Y.; Zhuang, X.; Feng, D.; Chen, Y.; Chan, P.; Du, Y. Postural sway in patients with early Parkinson’s disease performing cognitive tasks while standing. Neurol. Res. 2018, 40, 491–498. [Google Scholar] [CrossRef]
- Baracks, J.; Casa, D.J.; Covassin, T.; Sacko, R.; Scarneo, S.E.; Schnyer, D.; Yeargin, S.W.; Neville, C. Acute Sport-Related Concussion Screening for Collegiate Athletes Using an Instrumented Balance Assessment. J. Athl. Train. 2018, 53, 597–605. [Google Scholar] [CrossRef]
- Greene, B.R.; McGrath, D.; Walsh, L.; Doheny, E.P.; McKeown, D.; Garattini, C.; Cunningham, C.; Crosby, L.; Caulfield, B.; Kenny, R.A. Quantitative falls risk estimation through multi-sensor assessment of standing balance. Physiol. Meas. 2012, 33, 2049–2063. [Google Scholar] [CrossRef]
- Whitney, S.L.; Roche, J.L.; Marchetti, G.F.; Steed, D.P.; Furman, G.R.; Redfern, M.S.; Consulting, C. A comparison of accelerometry and center of pressure measures during computerized dynamic posturography: A measure of balance. Gait Posture 2016, 33, 594–599. [Google Scholar] [CrossRef]
- Sozzi, S.; Nardone, A.; Schieppati, M. Vision does not necessarily stabilize the head in space during continuous postural perturbations. Front. Neurol. 2019, 10, 1–13. [Google Scholar] [CrossRef]
- Nguyen, N.; Phan, D.; Pathirana, P.N.; Horne, M.; Power, L.; Szmulewicz, D. Quantification of Axial Abnormality Due to Cerebellar Ataxia with Inertial Measurements. Sensors 2018, 18, 2791. [Google Scholar] [CrossRef] [PubMed]
- Szturm, T.; Fallang, B. Effects of Varying Acceleration of Platform Translation and Toes-Up Rotations on the Pattern and Magnitude of Balance Reactions in Humans. J. Vestib. Res. 1998, 8, 381–397. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Sienko, K.H. The Design of a Cell-Phone Based Balance-Training Device. J. Med. Devices 2009, 3. [Google Scholar] [CrossRef]
- Weiss, A.; Herman, T.; Plotnik, M.; Brozgol, M.; Maidan, I.; Giladi, N.; Gurevich, T.; Hausdorff, J.M. Can an accelerometer enhance the utility of the Timed Up & Go Test when evaluating patients with Parkinson’s disease? Med. Eng. Phys. 2010, 32, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Giggins, O.M.; Sweeney, K.T.; Caulfield, B. Rehabilitation exercise assessment using inertial sensors: A cross-sectional analytical study. J. Neuroeng. Rehabil. 2014, 11, 158. [Google Scholar] [CrossRef]
- Leardini, A.; Lullini, G.; Giannini, S.; Berti, L.; Ortolani, M.; Caravaggi, P. Validation of the angular measurements of a new inertial-measurement-unit based rehabilitation system: Comparison with state-of-the-art gait analysis. J. Neuroeng. Rehabil. 2014, 11, 136. [Google Scholar] [CrossRef]
- Grimm, B.; Bolink, S. Evaluating physical function and activity in the elderly patient using wearable motion sensors. EFORT Open Rev. 2016, 1, 112–120. [Google Scholar] [CrossRef]
- Horak, F.; King, L.; Mancini, M. Role of body-worn movement monitor technology for balance and gait rehabilitation. Phys. Ther. 2015, 95, 461–470. [Google Scholar] [CrossRef]
- Tokuçoğlu, F. Monitoring Physical Activity with Wearable Technologies. Noro Psikiyatr. Ars. 2018, 55, S63–S65. [Google Scholar] [CrossRef]
- Dobkin, B.H.; Martinez, C. Wearable Sensors to Monitor, Enable Feedback, and Measure Outcomes of Activity and Practice. Curr. Neurol. Neurosci. Rep. 2018, 18, 87. [Google Scholar] [CrossRef]
- Tien, I.; Glaser, S.D.; Aminoff, M.J. Characterization of gait abnormalities in Parkinson’s disease using a wireless inertial sensor system. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2010, 2010, 3353–3356. [Google Scholar] [CrossRef] [PubMed]
- Buganè, F.; Benedetti, M.G.; D’Angeli, V.; Leardini, A. Estimation of pelvis kinematics in level walking based on a single inertial sensor positioned close to the sacrum: Validation on healthy subjects with stereophotogrammetric system. Biomed. Eng. Online 2014, 13, 146. [Google Scholar] [CrossRef] [PubMed]
- Mason, B.S.; Rhodes, J.M.; Goosey-Tolfrey, V.L. Validity and reliability of an inertial sensor for wheelchair court sports performance. J. Appl. Biomech. 2014, 30, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, A.; Ko, S.; Lee, S.; Lee, J.A.; Kim, K. Quantitative Assessment of Balance Impairment for Fall-Risk Estimation Using Wearable Triaxial Accelerometer. IEEE Sens. J. 2017, 17, 6743–6751. [Google Scholar] [CrossRef]
- Gouwanda, D.; Gopalai, A.A.; Khoo, B.H. A Low Cost Alternative to Monitor Human Gait Temporal Parameters–Wearable Wireless Gyroscope. IEEE Sens. J. 2016, 16, 9029–9035. [Google Scholar] [CrossRef]
- Adkin, A.L.; Bloem, B.R.; Allum, J.H.J. Trunk sway measurements during stance and gait tasks in Parkinson’s disease. Gait Posture 2005, 22, 240–249. [Google Scholar] [CrossRef]
- Spain, R.I.; St George, R.J.; Salarian, A.; Mancini, M.; Wagner, J.M.; Horak, F.B.; Bourdette, D. Body-worn motion sensors detect balance and gait deficits in people with multiple sclerosis who have normal walking speed. Gait Posture 2012, 35, 573–578. [Google Scholar] [CrossRef]
- Hubble, R.P.; Naughton, G.A.; Silburn, P.A.; Cole, M.H. Wearable sensor use for assessing standing balance and walking stability in people with Parkinson’s disease: A systematic review. PLoS ONE 2015, 10, e0123705. [Google Scholar] [CrossRef]
- Mazzetta, I.; Gentile, P.; Pessione, M.; Suppa, A.; Zampogna, A.; Bianchini, E.; Irrera, F. Stand-Alone Wearable System for Ubiquitous Real-Time Monitoring of Muscle Activation Potentials. Sensors 2018, 18, 1748. [Google Scholar] [CrossRef]
- Mileti, I.; Zampogna, A.; Taborri, J.; Martelli, F.; Rossi, S.; Del Prete, Z.; Paoloni, M.; Suppa, A.; Palermo, E. Parkinson’s disease and Levodopa effects on muscle synergies in postural perturbation. In Proceedings of the 2019 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Istanbul, Turkey, 26–28 June 2019; pp. 1–6. [Google Scholar]
- Jiang, S.; Pang, Y.; Wang, D.; Yang, Y.; Yang, Z.; Yang, Y.; Ren, T.L. Gait Recognition Based on Graphene Porous Network Structure Pressure Sensors for Rehabilitation Therapy. In Proceedings of the 2018 IEEE International Conference on Electron Devices and Solid State Circuits (EDSSC), Shenzhen, China, 6–8 June 2018; pp. 1–2. [Google Scholar]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.-F.; Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef]
- Li, G.; Wen, D. Wearable biochemical sensors for human health monitoring: Sensing materials and manufacturing technologies. J. Mater. Chem. B 2020, 8, 3423–3436. [Google Scholar] [CrossRef] [PubMed]
- Goud, K.Y.; Moonla, C.; Mishra, R.K.; Yu, C.; Narayan, R.; Litvan, I.; Wang, J. Wearable Electrochemical Microneedle Sensor for Continuous Monitoring of Levodopa: Toward Parkinson Management. ACS Sens. 2019, 4, 2196–2204. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.; King, L.; Salarian, A.; Holmstrom, L.; McNames, J.; Horak, F.B. Mobility Lab to Assess Balance and Gait with Synchronized Body-worn Sensors. J. Bioeng. Biomed. Sci. 2011, 12, 007. [Google Scholar] [CrossRef]
- Mazzetta, I.; Zampogna, A.; Suppa, A.; Gumiero, A.; Pessione, M.; Irrera, F. Wearable Sensors System for an Improved Analysis of Freezing of Gait in Parkinson’s Disease Using Electromyography and Inertial Signals. Sensors 2019, 19, 948. [Google Scholar] [CrossRef]
- Mancini, M.; Zampieri, C.; Carlson-Kuhta, P.; Chiari, L.; Horak, F.B. Anticipatory postural adjustments prior to step initiation are hypometric in untreated Parkinson’s disease: An accelerometer-based approach. Eur. J. Neurol. 2009, 16, 1028–1034. [Google Scholar] [CrossRef]
- Mancini, M.; Horak, F.B.; Zampieri, C.; Carlson-Kuhta, P.; Nutt, J.G.; Chiari, L. Trunk accelerometry reveals postural instability in untreated Parkinson’s disease. Parkinsonism Relat. Disord. 2011, 17, 557–562. [Google Scholar] [CrossRef]
- Maetzler, W.; Mancini, M.; Liepelt-Scarfone, I.; Müller, K.; Becker, C.; van Lummel, R.C.; Ainsworth, E.; Hobert, M.; Streffer, J.; Berg, D.; et al. Impaired trunk stability in individuals at high risk for Parkinson’s disease. PLoS ONE 2012, 7, e32240. [Google Scholar] [CrossRef]
- Mancini, M.; Chiari, L.; Holmstrom, L.; Salarian, A.; Horak, F.B. Validity and reliability of an IMU-based method to detect APAs prior to gait initiation. Gait Posture 2016, 43, 125–131. [Google Scholar] [CrossRef]
- Baston, C.; Mancini, M.; Schoneburg, B.; Horak, F.; Rocchi, L. Postural strategies assessed with inertial sensors in healthy and parkinsonian subjects. Gait Posture 2014, 40, 70–75. [Google Scholar] [CrossRef]
- De Souza Fortaleza, A.C.; Mancini, M.; Carlson-Kuhta, P.; King, L.A.; Nutt, J.G.; Chagas, E.F.; Freitas, I.F.; Horak, F.B. Dual task interference on postural sway, postural transitions and gait in people with Parkinson’s disease and freezing of gait. Gait Posture 2017, 56, 76–81. [Google Scholar] [CrossRef]
- Ozinga, S.J.; Linder, S.M.; Alberts, J.L. Use of Mobile Device Accelerometry to Enhance Evaluation of Postural Instability in Parkinson Disease. Arch. Phys. Med. Rehabil. 2017, 98, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Ozinga, S.J.; Koop, M.M.; Linder, S.M.; Machado, A.G.; Dey, T.; Alberts, J.L. Three-dimensional evaluation of postural stability in Parkinson’s disease with mobile technology. NeuroRehabilitation 2017, 41, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Bonora, G.; Mancini, M.; Carpinella, I.; Chiari, L.; Horak, F.B.; Ferrarin, M. Gait initiation is impaired in subjects with Parkinson’s disease in the OFF state: Evidence from the analysis of the anticipatory postural adjustments through wearable inertial sensors. Gait Posture 2017, 51, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Bonora, G.; Mancini, M.; Carpinella, I.; Chiari, L.; Ferrarin, M.; Nutt, J.G.; Horak, F.B. Investigation of Anticipatory Postural Adjustments during One-Leg Stance Using Inertial Sensors: Evidence from Subjects with Parkinsonism. Front. Neurol. 2017, 8, 361. [Google Scholar] [CrossRef]
- Falaki, A.; Huang, X.; Lewis, M.M.; Latash, M.L. Impaired Synergic Control of Posture in Parkinson’s Patients without Postural Instability. Gait Posture 2016, 44, 209–215. [Google Scholar] [CrossRef]
- Falaki, A.; Huang, X.; Lewis, M.M.; Latash, M.L. Dopaminergic modulation of multi-muscle synergies in postural tasks performed by patients with Parkinson’s disease. J. Electromyogr. Kinesiol. 2017, 33, 20–26. [Google Scholar] [CrossRef]
- Lang, K.C.; Hackney, M.E.; Ting, L.H.; McKay, J.L. Antagonist muscle activity during reactive balance responses is elevated in Parkinson’s disease and in balance impairment. PLoS ONE 2019, 14, e0211137. [Google Scholar] [CrossRef]
- Spain, R.I.; Mancini, M.; Horak, F.B.; Bourdette, D. Body-worn sensors capture variability, but not decline, of gait and balance measures in multiple sclerosis over 18 months. Gait Posture 2014, 39, 958–964. [Google Scholar] [CrossRef]
- Solomon, A.J.; Jacobs, J.V.; Lomond, K.V.; Henry, S.M. Detection of postural sway abnormalities by wireless inertial sensors in minimally disabled patients with multiple sclerosis: A case-control study. J. Neuroeng. Rehabil. 2015, 12, 74. [Google Scholar] [CrossRef]
- El-Gohary, M.; Peterson, D.; Gera, G.; Horak, F.B.; Huisinga, J.M. Validity of the Instrumented Push and Release Test to Quantify Postural Responses in Persons With Multiple Sclerosis. Arch. Phys. Med. Rehabil. 2017, 98, 1325–1331. [Google Scholar] [CrossRef]
- Witchel, H.J.; Oberndorfer, C.; Needham, R.; Healy, A.; Westling, C.E.I.; Guppy, J.H.; Bush, J.; Barth, J.; Herberz, C.; Roggen, D.; et al. Thigh-Derived Inertial Sensor Metrics to Assess the Sit-to-Stand and Stand-to-Sit Transitions in the Timed Up and Go (TUG) Task for Quantifying Mobility Impairment in Multiple Sclerosis. Front. Neurol. 2018, 9, 684. [Google Scholar] [CrossRef] [PubMed]
- Huisinga, J.; Mancini, M.; Veys, C.; Spain, R.; Horak, F. Coherence analysis of trunk and leg acceleration reveals altered postural sway strategy during standing in persons with multiple sclerosis. Hum. Mov. Sci. 2018, 58, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Moon, Y.; McGinnis, R.S.; Seagers, K.; Motl, R.W.; Sheth, N.; Wright, J.A.; Ghaffari, R.; Patel, S.; Sosnoff, J.J. Assessment of Postural Sway in Individuals with Multiple Sclerosis Using a Novel Wearable Inertial Sensor. Digit. Biomark. 2018, 2, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Arpan, I.; Fino, P.C.; Fling, B.W.; Horak, F. Local dynamic stability during long-fatiguing walks in people with multiple sclerosis. Gait Posture 2020, 76, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Chitnis, T.; Glanz, B.I.; Gonzalez, C.; Healy, B.C.; Saraceno, T.J.; Sattarnezhad, N.; Diaz-Cruz, C.; Polgar-Turcsanyi, M.; Tummala, S.; Bakshi, R.; et al. Quantifying neurologic disease using biosensor measurements in-clinic and in free-living settings in multiple sclerosis. NPJ Digit. Med. 2019, 2, 123. [Google Scholar] [CrossRef] [PubMed]
- Gera, G.; Fling, B.W.; Horak, F.B. Cerebellar White Matter Damage Is Associated with Postural Sway Deficits in People with Multiple Sclerosis. Arch. Phys. Med. Rehabil. 2020, 101, 258–264. [Google Scholar] [CrossRef]
- Perez-Cruzado, D.; González-Sánchez, M.; Cuesta-Vargas, A.I. Parameterization and reliability of single-leg balance test assessed with inertial sensors in stroke survivors: A cross-sectional study. Biomed. Eng. Online 2014, 13, 127. [Google Scholar] [CrossRef]
- Merchán-Baeza, J.A.; González-Sánchez, M.; Cuesta-Vargas, A.I. Comparison of kinematic variables obtained by inertial sensors among stroke survivors and healthy older adults in the Functional Reach Test: Cross-sectional study. Biomed. Eng. Online 2015, 14, 49. [Google Scholar] [CrossRef]
- Merchán-Baeza, J.A.; González-Sánchez, M.; Cuesta-Vargas, A.I. Reliability in the parameterization of the functional reach test in elderly stroke patients: A pilot study. Biomed. Res. Int. 2014, 2014, 637671. [Google Scholar] [CrossRef]
- Iosa, M.; Bini, F.; Marinozzi, F.; Fusco, A.; Morone, G.; Koch, G.; Martino Cinnera, A.; Bonnì, S.; Paolucci, S. Stability and Harmony of Gait in Patients with Subacute Stroke. J. Med. Biol. Eng. 2016, 36, 635–643. [Google Scholar] [CrossRef]
- Belluscio, V.; Bergamini, E.; Iosa, M.; Tramontano, M.; Morone, G.; Vannozzi, G. The iFST: An instrumented version of the Fukuda Stepping Test for balance assessment. Gait Posture 2018, 60, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.R.; Chiu, Y.L.; Chiang, S.L.; Chen, H.Y.; Sung, W.H. Feasibility of a smartphone-based balance assessment system for subjects with chronic stroke. Comput. Methods Programs Biomed. 2018, 161, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.R.; Chiu, Y.L.; Chiang, S.-L.; Chen, H.Y.; Sung, W.H. Development of a Smartphone-Based Balance Assessment System for Subjects with Stroke. Sensors 2019, 20, 88. [Google Scholar] [CrossRef]
- Furman, G.R.; Lin, C.C.; Bellanca, J.L.; Marchetti, G.F.; Collins, M.W.; Whitney, S.L. Comparison of the balance accelerometer measure and balance error scoring system in adolescent concussions in sports. Am. J. Sports Med. 2013, 41, 1404–1410. [Google Scholar] [CrossRef] [PubMed]
- Doherty, C.; Zhao, L.; Ryan, J.; Komaba, Y.; Inomata, A.; Caulfield, B. Quantification of postural control deficits in patients with recent concussion: An inertial-sensor based approach. Clin. Biomech. 2017, 42, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Alkathiry, A.A.; Sparto, P.J.; Freund, B.; Whitney, S.L.; Mucha, A.; Furman, J.M.; Collins, M.W.; Kontos, A.P. Using Accelerometers to Record Postural Sway in Adolescents With Concussion: A Cross-Sectional Study. J. Athl. Train. 2018, 53, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Gera, G.; Chesnutt, J.; Mancini, M.; Horak, F.B.; King, L.A. Inertial Sensor-Based Assessment of Central Sensory Integration for Balance After Mild Traumatic Brain Injury. Mil. Med. 2018, 183, 327–332. [Google Scholar] [CrossRef]
- King, L.A.; Horak, F.B.; Mancini, M.; Pierce, D.; Priest, K.C.; Chesnutt, J.; Sullivan, P.; Chapman, J.C. Instrumenting the Balance Error Scoring System for use with patients reporting persistent balance problems after mild traumatic brain injury. Arch. Phys. Med. Rehabil. 2014, 95, 353–359. [Google Scholar] [CrossRef]
- Van de Warrenburg, B.P.C.; Bakker, M.; Kremer, B.P.H.; Bloem, B.R.; Allum, J.H.J. Trunk sway in patients with spinocerebellar ataxia. Mov. Disord. 2005, 20, 1006–1013. [Google Scholar] [CrossRef]
- Hejda, J.; Cakrt, O.; Socha, V.; Schlenker, J.; Kutilek, P. 3-D trajectory of body sway angles: A technique for quantifying postural stability. Biocybern. Biomed. Eng. 2015, 35, 185–191. [Google Scholar] [CrossRef]
- Kutílek, P.; Socha, V.; Čakrt, O.; Svoboda, Z. Assessment of postural stability in patients with cerebellar disease using gyroscope data. J. Bodyw. Mov. Ther. 2015, 19, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Melecky, R.; Socha, V.; Kutilek, P.; Hanakova, L.; Takac, P.; Schlenker, J.; Svoboda, Z. Quantification of Trunk Postural Stability Using Convex Polyhedron of the Time-Series Accelerometer Data. J. Healthc. Eng. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Adamová, B.; Kutilek, P.; Cakrt, O.; Svoboda, Z.; Viteckova, S.; Smrcka, P. Quantifying postural stability of patients with cerebellar disorder during quiet stance using three-axis accelerometer. Biomed. Signal Process. Control 2018, 40, 378–384. [Google Scholar] [CrossRef]
- Widener, G.L.; Conley, N.; Whiteford, S.; Gee, J.; Harrell, A.; Gibson-Horn, C.; Block, V.; Allen, D.D. Changes in standing stability with balance-based torso-weighting with cerebellar ataxia: A pilot study. Physiother. Res. Int. 2020, 25, e1814. [Google Scholar] [CrossRef]
- Cohen, H.S.; Mulavara, A.P.; Peters, B.T.; Sangi-Haghpeykar, H.; Bloomberg, J.J. Tests of walking balance for screening vestibular disorders. J. Vestib. Res. 2012, 22, 95–104. [Google Scholar] [CrossRef]
- Kapoula, Z.; Gaertner, C.; Yang, Q.; Denise, P.; Toupet, M. Vergence and Standing Balance in Subjects with Idiopathic Bilateral Loss of Vestibular Function. PLoS ONE 2013, 8, e66652. [Google Scholar] [CrossRef]
- Kim, S.C.; Kim, M.J.; Kim, N.; Hwang, J.H.; Han, G.C. Ambulatory balance monitoring using a wireless attachable three-axis accelerometer. J. Vestib. Res. 2013, 23, 217–225. [Google Scholar] [CrossRef]
- D’Silva, L.J.; Kluding, P.M.; Whitney, S.L.; Dai, H.; Santos, M. Postural sway in individuals with type 2 diabetes and concurrent benign paroxysmal positional vertigo. Int. J. Neurosci. 2017, 127, 1065–1073. [Google Scholar] [CrossRef]
- Najafi, B.; Horn, D.; Marclay, S.; Crews, R.T.; Wu, S.; Wrobel, J.S. Assessing postural control and postural control strategy in diabetes patients using innovative and wearable technology. J. Diabetes Sci. Technol. 2010, 4, 780–791. [Google Scholar] [CrossRef]
- Toosizadeh, N.; Mohler, J.; Armstrong, D.G.; Talal, T.K.; Najafi, B. The influence of diabetic peripheral neuropathy on local postural muscle and central sensory feedback balance control. PLoS ONE 2015, 10, e0135255. [Google Scholar] [CrossRef]
- Gago, M.F.; Yelshyna, D.; Bicho, E.; Silva, H.D.; Rocha, L.; Lurdes Rodrigues, M.; Sousa, N. Compensatory Postural Adjustments in an Oculus Virtual Reality Environment and the Risk of Falling in Alzheimer’s Disease. Dement. Geriatr. Cogniy. Dis. Extra 2016, 6, 252–267. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.L.; Chung, P.C.J.; Wang, W.H.; Pai, M.C.; Wang, C.Y.; Lin, C.W.; Wu, H.L.; Wang, J.S. Gait and balance analysis for patients with Alzheimer’s disease using an inertial-sensor-based wearable instrument. IEEE J. Biomed. Health Inform. 2014, 18, 1822–1830. [Google Scholar] [CrossRef] [PubMed]
- Dalton, A.; Khalil, H.; Busse, M.; Rosser, A.; van Deursen, R.; Ólaighin, G. Analysis of gait and balance through a single triaxial accelerometer in presymptomatic and symptomatic Huntington’s disease. Gait Posture 2013, 37, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Palermo, E.; Rossi, S.; Marini, F.; Patanè, F.; Cappa, P. Experimental evaluation of accuracy and repeatability of a novel body-to-sensor calibration procedure for inertial sensor-based gait analysis. Measurement 2014, 52, 145–155. [Google Scholar] [CrossRef]
- Ancillao, A.; Tedesco, S.; Barton, J.; O’flynn, B. Indirect measurement of ground reaction forces and moments by means of wearable inertial sensors: A systematic review. Sensors 2018, 18, 2564. [Google Scholar] [CrossRef] [PubMed]
- Carroll, W.M. The global burden of neurological disorders. Lancet Neurol. 2019, 18, 418–419. [Google Scholar] [CrossRef]
- Suppa, A.; Kita, A.; Leodori, G.; Zampogna, A.; Nicolini, E.; Lorenzi, P.; Rao, R.; Irrera, F. l-DOPA and Freezing of Gait in Parkinson’s Disease: Objective Assessment through a Wearable Wireless System. Front. Neurol. 2017, 8, 406. [Google Scholar] [CrossRef]
- Hatcher-Martin, J.M.; Adams, J.L.; Anderson, E.R.; Bove, R.; Burrus, T.M.; Chehrenama, M.; Dolan O’Brien, M.; Eliashiv, D.S.; Erten-Lyons, D.; Giesser, B.S.; et al. Telemedicine in neurology: Telemedicine Work Group of the American Academy of Neurology update. Neurology 2020, 94, 30–38. [Google Scholar] [CrossRef]
- Wu, G.; Xue, S. Portable preimpact fall detector with inertial sensors. IEEE Trans. Neural. Syst. Rehabil. Eng. 2008, 16, 178–183. [Google Scholar] [CrossRef]
- Ma, C.Z.H.; Wong, D.W.C.; Lam, W.K.; Wan, A.H.P.; Lee, W.C.C. Balance improvement effects of biofeedback systems with state-of-the-art wearable sensors: A systematic review. Sensors 2016, 16, 434. [Google Scholar] [CrossRef]
- Erra, C.; Mileti, I.; Germanotta, M.; Petracca, M.; Imbimbo, I.; De Biase, A.; Rossi, S.; Ricciardi, D.; Pacilli, A.; Di Sipio, E.; et al. Immediate effects of rhythmic auditory stimulation on gait kinematics in Parkinson’s disease ON/OFF medication. Clin. Neurophysiol. 2019, 130, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Altilio, R.; Liparulo, L.; Panella, M.; Proietti, A.; Paoloni, M. Multimedia and Gaming Technologies for Telerehabilitation of Motor Disabilities [Leading Edge]. IEEE Technol. Soc. Mag. 2015, 34, 23–30. [Google Scholar] [CrossRef]
- Porciuncula, F.; Roto, A.V.; Kumar, D.; Davis, I.; Roy, S.; Walsh, C.J.; Awad, L.N. Wearable Movement Sensors for Rehabilitation: A Focused Review of Technological and Clinical Advances. PM R 2018, 10, S220–S232. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.L.; McKay, J.L.; Sawers, A.; Hackney, M.E.; Ting, L.H. Increased neuromuscular consistency in gait and balance after partnered, dance-based rehabilitation in Parkinson’s disease. J. Neurophysiol. 2017, 118, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Gordt, K.; Gerhardy, T.; Najafi, B.; Schwenk, M. Effects of Wearable Sensor-Based Balance and Gait Training on Balance, Gait, and Functional Performance in Healthy and Patient Populations: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Gerontology 2017, 64, 74–89. [Google Scholar] [CrossRef] [PubMed]
- Grewal, G.S.; Schwenk, M.; Lee-Eng, J.; Parvaneh, S.; Bharara, M.; Menzies, R.A.; Talal, T.K.; Armstrong, D.G.; Najafi, B. Sensor-Based Interactive Balance Training with Visual Joint Movement Feedback for Improving Postural Stability in Diabetics with Peripheral Neuropathy: A Randomized Controlled Trial. Gerontology 2015, 61, 567–574. [Google Scholar] [CrossRef]
- Schwenk, M.; Grewal, G.S.; Holloway, D.; Moucha, A.; Garland, L.; Najafi, B. Interactive sensor-based balance training in older cancer patients with chemotherapy-induced peripheral neuropathy: A randomized controlled trial. Gerontology 2016, 62, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Schwenk, M.; Sabbagh, M.; Lin, I.; Morgan, P.; Grewal, G.S.; Mohler, J.; Coon, D.W.; Najafi, B. Sensor-based balance training with motion feedback in people with mild cognitive impairment. J. Rehabil. Res. Dev. 2016, 53, 945–958. [Google Scholar] [CrossRef]
- Carpinella, I.; Cattaneo, D.; Bonora, G.; Bowman, T.; Martina, L.; Montesano, A.; Ferrarin, M. Wearable Sensor-Based Biofeedback Training for Balance and Gait in Parkinson Disease: A Pilot Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2017, 98, 622–630.e3. [Google Scholar] [CrossRef]
- Esteva, A.; Robicquet, A.; Ramsundar, B.; Kuleshov, V.; DePristo, M.; Chou, K.; Cui, C.; Corrado, G.; Thrun, S.; Dean, J. A guide to deep learning in healthcare. Nat. Med. 2019, 25, 24–29. [Google Scholar] [CrossRef]
- Rajkomar, A.; Dean, J.; Kohane, I. Machine Learning in Medicine. N. Engl. J. Med. 2019, 380, 1347–1358. [Google Scholar] [CrossRef] [PubMed]
- Kubota, K.J.; Chen, J.A.; Little, M.A. Machine learning for large-scale wearable sensor data in Parkinson’s disease: Concepts, promises, pitfalls, and futures. Mov. Disord. 2016, 31, 1314–1326. [Google Scholar] [CrossRef] [PubMed]
- Handelman, G.S.; Kok, H.K.; Chandra, R.V.; Razavi, A.H.; Lee, M.J.; Asadi, H. eDoctor: Machine learning and the future of medicine. J. Intern. Med. 2018, 284, 603–619. [Google Scholar] [CrossRef]
- Cilliers, L. Wearable devices in healthcare: Privacy and information security issues. Health Inf. Manag. 2019, 1833358319851684. [Google Scholar] [CrossRef] [PubMed]
- Guideline on Data Integrity. Available online: https://www.who.int/medicines/areas/quality_safety/quality_assurance/QAS19_819_data_integrity.pdf?ua=1 (accessed on 23 May 2020).
- Mourcou, Q.; Fleury, A.; Diot, B.; Vuillerme, N. iProprio: A smartphone-based system to measure and improve proprioceptive function. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2016, 2016, 2622–2625. [Google Scholar] [CrossRef] [PubMed]

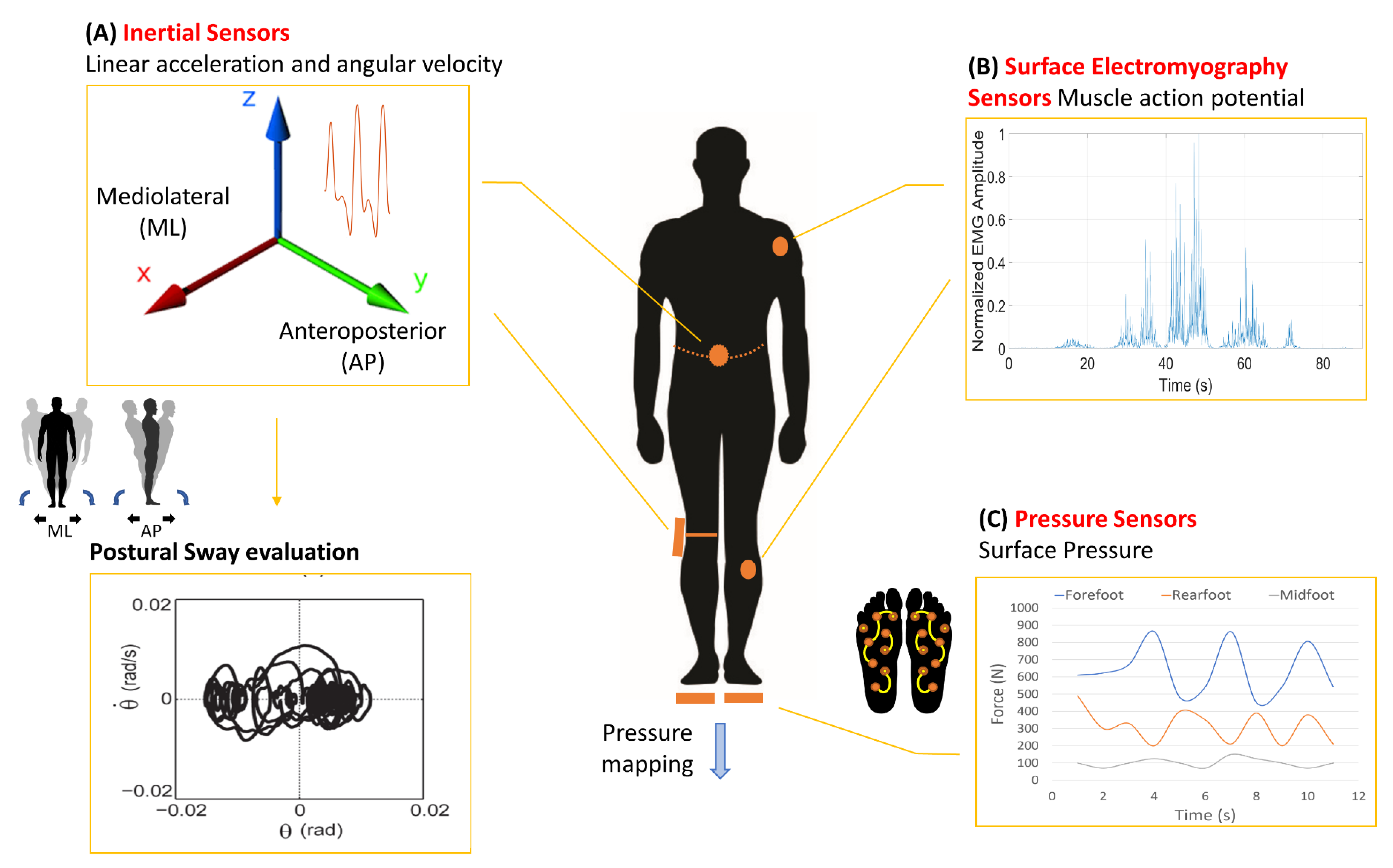
| Disease Definition | Nervous Structures Involved | Pathophysiological Mechanisms | Main Clinical Consequence | |
|---|---|---|---|---|
| Alzheimer’s disease | Neurodegenerative dementia associated with progressive cognitive and functional dysfunction [27] | Cerebral cortex and subcortical structures, prominently involving nucleus accumbens and putamen [28] | Cognitive impairment, abnormal sensorimotor function and vision, peripheral sensory loss, muscle weakness [29,30,31,32] | Hallucinations, inattention, abnormal sensory reweighting |
| Parkinson’s disease | Neurodegenerative movement disorder associated with progressive motor and cognitive dysfunction [33] | Basal ganglia, locus coeruleus and pedunculopontine nucleus [34] | Impaired scaling of postural responses [35], abnormal central proprioceptive-motor integration [36], reduced kinaesthesia [37], axial rigidity [38], cognitive dysfunction [39] | Postural instability, disrupted trunk-legs coordination, freezing of gait |
| Multiple sclerosis | Acquired demyelinating disease of the central nervous system [40] | Cortico-spinal tract, cerebellum, proprioceptive pathways, vestibular system, brainstem structures for eye movement control [41] | Abnormal sensorimotor, visual, cerebellar, vestibular and cognitive functions [41], muscle weakness and spasticity [42] | Abnormal coordination and sensory reweighting, reduced attentional resources, strength impairment |
| Huntington’s disease | Neurodegenerative disease with autosomal dominant pattern of inheritance [43], associated with cognitive and motor impairment, psychiatric disorders and involuntary movements (chorea) [44] | Basal ganglia, prominently interesting caudate and putamen [45] | Involuntary movements, trunk muscles weakness, hip flexor tightness, impairment in visual and vestibular integration, ocular pursuit movements and proprioception [46] | Chorea, abnormal sensory reweighting, increased stride variability |
| Cerebellar ataxia | Acquired or hereditary, as well as acute or progressive, disorder associated with dysfunction of cerebellum and/or its connections [47] | Cerebellum (primarily vermis and anterior lobe) and/or its connections, including spinocerebellar tracts [47] | Impaired coordination of movements | Axial motor impairment and asynergic movement |
| Stroke | Acute neurologic syndrome due to the interruption of blood supply to a part of the central nervous system by an ischemic or haemorrhagic vascular injury [48] | Cortico-spinal tract, cerebellum, proprioceptive pathways, vestibular system and brainstem structures [49] | Somatosensory and motor dysfunction [50,51], spasticity [52], visual and perceptual disorders [53,54], including impaired perception of upright body position, cognitive impairment [55] | Hemispatial neglect, strength impairment, abnormal coordination, sensory reweighting |
| Traumatic brain injury | Acute blunt head traumas or acceleration forces to the head [56] | Vestibular nuclei, cerebellar peduncles, medial lemniscus, dentato-rubro-thalamic and cortico-reticular pathways [57] | Impairment in cognitive and motor functionality [58] | Dizziness, visual-spatial deficits and inattention |
| Neuropathies | Acute or progressive disorders of the peripheral nervous system, associate with the disruption of nerve action potentials transmission [59] | Peripheral nervous system (nerves) | Sensory and/or motor impairment [59], retinopathy, vestibular and muscle impairment [60], sensory ataxia | Proprioception and strength impairment |
| Vestibular syndromes | Acute or chronic disorders of the inner-ear balance organs and/or their nervous structures [61] (e.g., Meniere’s disease, benign positional vertigo, bilateral vestibular loss, vestibular neuritis, posterior circulation strokes) | Vestibular system (i.e., inner-ear balance organs, vestibular nerve and central nuclei) | Abnormal spatial orientation and motion perception [62], ataxia, eye movement abnormalities [61] | Dizziness and vertigo |
| Clinical Test or Scale | Aim of the Test/Scale | Procedures | Outcome Measures |
|---|---|---|---|
| Romberg test [64] | Postural ability and pathophysiological mechanisms | The subject stands with feet close together, arms by the side, and with eyes open, and then closes eyes while maintaining the same position (removal of vision possibly compensatory proprioceptive deficits) | Unbalance and fall |
| Pull test [65] | Postural ability | The subject undergoes a sudden body displacement by a quick and forceful pull on the shoulders during upright stance | Number of backward steps or falling (qualitative) |
| Tandem gait test [66] | Postural ability | The subject walks a straight line while touching the heel of one foot to the toe of the other (narrowed base of support) | Unbalance, falls or need to enlarge the base of support |
| One-leg stance test [67] | Postural ability | The subject stands unassisted on one leg with opened eyes and arms on the hips as long as possible | Time of performance in seconds |
| Timed up and go test [68] | Gait and postural ability | The subject sits on a chair, stands up, walks 3 m, turns around, walks back and sits down | Time of performance in seconds |
| Tinetti balance and mobility scale - Performance-oriented mobility assessment [69] | Gait and postural ability | The subject performs postural and walking motor tasks reflecting common daily activities, such as rising from a chair, maintaining upright stance after a nudge, walking and turning (total 24 items consisting of 14 balance items and 10 gait items) | Total score (sum of gait and balance scores) by using a 2/3-point ordinal scale for each item |
| Functional reach test [70] | Postural ability | The subject reaches as far forward as he can with arms at 90° flexion, keeping feet on the floor | Maximum distance (cm) that the subject can reach forward beyond arm’s length |
| Berg balance scale [71] | Postural ability | The subject performs functional activities reflecting different components of postural control, such as reaching, bending, transferring and standing (total 14 items) | Total score by using a 5-point ordinal scale for each item |
| Activities of balance confidence scale [72] | Postural ability | The subject performs a self-report questionnaire on subjective impact of balance dysfunction on 16 daily activities, such as walking in different environmental and postural conditions (total 16 items) | Average score in percentage (each item rated from 0% to 100% of balance confidence) |
| Physiological profile assessment [73] | Pathophysiological mechanisms | The subject performs different sensorimotor tasks to assess vision (e.g., dual contrast visual acuity chart), lower limb sensation (e.g., tests of proprioception), legs strength, step reaction times, vestibular function (e.g., visual field dependence) and postural sway | Falls risk assessment based on the scores of sensorimotor tasks |
| Balance evaluation systems test [74] | Pathophysiological mechanisms | The subject performs several motor tasks reflecting different systems underlying balance control (e.g., stance on a firm or foam surface, stepping over obstacles, alternate stair touching); (total 36 items categorised into 6 underlying systems: "Biomechanical Constraints," "Stability Limits/Verticality," "Anticipatory Postural Adjustments," “Postural Responses,” “Sensory Orientation” and “Stability in Gait”) | Total score in percentage referring to the partial score of systems that involve a 4-point ordinal scale for each item |
| Name | Meaning | Static | Dynamic |
|---|---|---|---|
| RANGE | Range of acceleration/displacement in the AP, ML, and V direction. Impaired motor strategies report high values of Range Index | [114,117,118] | [111,119] |
| STD | Standard deviation of reference body landmarks. It is an index of average amplitude of body displacements. | [102,104,120,121] | |
| DIST | Mean distance from the centre of acceleration/displacement trajectory. It is an index of desertion. In static evaluation, high values indicate poor motor control. | [114,117,122,123] | |
| RMS | Root mean square of the acceleration/displacement in AP, ML, and V direction. High values represent larger dispersion and poor motor control. | [114,117,122,123,124,125,126,127] | [128] |
| MEAN | Average acceleration/velocity/displacement in the AP, ML, V direction. High values represent unstable postural adjustments and poor motor control. | [118,122,127] | |
| PATH | Total length of the acceleration/displacement in static condition larger values represent poor motor control. | [114,117] | [26,102,128] |
| MV | Mean velocity. It is the first derivative of the acceleration signal in the AP, ML and V direction. Impaired motor strategies report High values of Mean Velocity Index. | [114,117] | |
| AREA | Total area that encapsulates the total sway path in AP and ML directions. In a static condition, higher values represent poor motor control. | [114,117,118,123,127] | |
| EA95 | 95% ellipse sway area. It is the ellipse area that encapsulates the 95% of the sway path in the AP and ML direction. High values represent poor motor control. | [114,117,126,127] | |
| JERK | Time derivative of the acceleration signal. It represents the range of changes in the acceleration signal. High values represent accelerating and decelerating pattern attesting more unstable condition and poor motor control. | [114,117,118,122,125] | |
| Cross-correlation | Cross-correlation between displacements of two body points. It is an index of coupling between the motion behaviour of two body segments or between the movable platform and the human body | [102,104,120,129] | |
| PWR | Total power of the power spectrum of the acceleration signal. | [114,123] | [102] |
| F95 or F50 | Frequency below which is present the 95% or 50% of the total power. High values indicate a larger amount of postural adjustments and poor motor control. | [114,118] | |
| CF | Centroidal frequency of the signal in the AP, ML and V direction. It is the frequency at which the power is balanced, i.e., the total power above this frequency is equal to the one below. Poor motor control is identified by low values of CF. | [114,117,122] | |
| FD | Frequency dispersion. It is a measure of the variability of the frequencies of the power spectral density. Values close to zero indicate pure sinusoidal patterns of the signal and a more stable motor control. | [114,117,118,122] | |
| Entropy | It is the power spectrum entropy of the signal. It is an index of movement smoothness and the inability to regulate postural fluctuations. | [127,130] | |
| Magnitude | It the area below the EMG curve over a specific range of time, starting from the onset of the perturbation. Mostly this index of muscular intensity is computed during the early response (0–200 ms), the intermediate response (201–400 ms) and the late response (401–600 ms). Impaired postural strategies report lower values of muscle activation. | [111,119] | |
| Onset latency | Time delay between onset of perturbation and muscle activation. It represents how fast a muscle reacts after a perturbation. Impaired balancing strategies report high values of onset latency. | [86,90,111,119] | |
| Time to peak | Time between the onset of perturbation and the maximum activation of the muscle or the maximum peak of joint angle. It indicates how quickly a muscle/joint reaches its maximal value. In dynamic evaluation, lower values indicate high capability in counteracting perturbation. | [86,90,111,119,129,131] | |
| Coactivation | It is the ratio between the magnitude of the agonist and antagonist muscles activity. Impaired postural strategies present an increased coactivation of agonist-antagonist muscles. | [86,90] | |
| Peak angle | Peak of the angular displacement of two adjacent body segment. | [86,129,131] | |
| APAs–CPAs | Anticipatory and compensatory postural adjustments. EMG activity and principal component analysis are estimated over four-time windows in relation to perturbation onset, i.e., APA1 (from −250 ms to −100 ms); APA2 (from −100 ms to +50 ms); CPA1 (from +50 ms to +200 ms); CPA2 (from 200 ms to +350 ms). Impaired motor control reports smaller and delayed APAs during unexpected perturbation. | [95,105,106,109] |
| Wireless Sensor | Strengths | Limitations | Challenges |
|---|---|---|---|
| IMU | Low cost and high accuracy | Possible magnetic interferences, errors of misalignment, orthogonality and offset and energy consumption | New algorithms for position and orientation correction |
| sEMG | Noninvasive analysis and unobtrusiveness | Crosstalk due to adjacent muscles, skin-electrode interface noise and electrode positioning | New implantable EMG sensors and dry electrodes composed of conductive fabric |
| Pressure | Outdoor measurements and easy integrability | Low comfortability during gait, limited sensitive area and high cost | New capacitive sensors composed of fabric |
| Disease and Number of Studies | Studies with a Control Group | Type and Main Locations of Sensors | Other Measurements | Main Experimental Setups | Main Postural Measures | Main Findings | Clinical-Behavioural Correlations |
|---|---|---|---|---|---|---|---|
| Alzheimer’s disease N = 3 [32,202,203] | N = 3 [32,202,203] | 1 to 5 IMUs on trunk, waist, legs and thighs | Not performed | Upright stance with open or closed eyes, different BOS amplitudes and surfaces (e.g., firm and foam), as well as during virtual perturbations | Pitch and roll angles; COM displacement; sway velocity, area and path; RMS acceleration | Lower minimal roll angle, larger COM displacement, higher sway area and RMS acceleration in AD than HS | Not significant or not performed |
| Parkinson’s disease N = 17 [114,122,124,125,156,157,158,159,160,161,162,163,164,165,166,167,168] | N = 16 [114,122,124,125,156,157,158,159,160,161,162,163,164,165,166,168] | 1 to 8 IMUs on trunk, waist, wrists, thighs, shanks and feet; 10 to 22 sEMG on lower limb muscles, lumbar erector spinae, thoracic erector spinae and rectus abdominis | Force plate (COP measures) and infrared optical system | Gait initiation; upright stance with open or closed eyes, different BOS amplitudes and surfaces (e.g., firm and foam), under and not under cognitive load; SOT; ISAW; self-triggered and external postural perturbations; OLS | IMUs: APAs; mean velocity; RMS acceleration; jerkiness; peak-to-peak sway; 95% ellipse area; strategy index. sEMG: amount of variance accounted for; synergy index; ASAs; modulation index | Correlation between inertial, COP and optical measures; hypometric APAs, higher mean velocity, acceleration size and jerkiness, larger peak-to peak sway and 95% ellipse area, predominant ankle strategy; lower VAF and synergy index, reduced ASAs and muscle modulation in PD than HS | Acceleration changes correlated with PIGD and UPDRS-III scores, strategy index with ABC scores, muscle modulation with postural ability and disease severity in PD |
| Multiple sclerosis N = 11 [118,146,169,170,171,172,173,174,175,176,177] | N = 10 [118,146,169,170,171,172,173,174,175,177] | 1 to 6 IMUs on trunk, waist, wrists, thighs, shanks and feet | Force plate (COP measures) and infrared optical system | Upright stance with open or closed eyes and different surfaces (e.g., firm and foam); walking tasks (e.g., TUG, timed 25-foot walk, 6-minute walk test); external perturbations (e.g., push and release test, backward perturbation) | RMS acceleration; mean velocity; sway jerk, path length, area; F95%; time to reach stability; coherence of acceleration between trunk and legs | Correlation between inertial and COP measures; larger sway acceleration amplitude, angular trunk range of motion in roll and yaw axes, sway path length and area, reduced ML sway jerk, higher F95%, longer time to reach stability and lower acceleration coherence between trunk and legs in MS than HS | Sway acceleration correlated with ABC and MSWS12 scores; RMS acceleration, displacement, mean frequency and time to reach stability correlated with EDSS scores |
| Huntington’s disease N = 2 [46,204] | N = 2 [46,204] | 1 to 2 IMUs on trunk and waist | Not performed | Upright stance with open or closed eyes and different BOS amplitudes; sitting, standing and walking | RMS acceleration; total, peak and mean angular excursion | Higher RMS acceleration; larger peak and total excursions in HD than HS | Not significant or not performed |
| Cerebellar ataxia N = 7 [130,190,191,192,193,194,195] | N = 7 [130,190,191,192,193,194,195] | 1 to 6 IMUs on trunk, waist, wrists, ankles and feet | Force plate (COP measures) | Upright stance with open or closed eyes and different surfaces (e.g., firm and foam); walking tasks and external perturbations (e.g., retropulsion test) | Trunk angular displacement and velocity, sway path length, area of the convex hull, convex polyhedron volume, entropy, 95% of the ellipse sway area | Correlation between inertial and COP measures; larger trunk angular displacement and velocity, sway path length, area of the convex hull, convex polyhedron volume, entropy and 95% of the ellipse sway area in CA than HS | Inertial measures (e.g., trunk angular displacement and velocity) correlated with ICARS scores, Tinetti’s Mobility Index and SARA scores |
| Stroke N = 8 [52,178,179,180,181,182,183,184] | N = 5 [179,181,182,183,184] | 1 to 5 IMUs on head, trunk, waist and shins | Force plate (COP measures) | Upright stance with open or closed eyes and different BOS amplitudes; walking tasks; functional reach test; Fukuda stepping test; OLS | Body displacement (time, velocity, acceleration); RMS acceleration | Higher maximum and minimum acceleration, LL trunk acceleration, angular velocity in ST than HS | Gyroscope data negatively correlated with Berg balance scale scores |
| Traumatic brain injury N = 7 [123,126,185,186,187,188,189] | N = 6 [123,126,185,186,188,189] | 1 IMU on waist | Force plate (COP measures) | Upright stance with open or closed eyes, different BOS amplitudes and surfaces (e.g., firm and foam); standard and modified balance error scoring system | RMS acceleration; sway amplitude, velocity, variability and frequency; ellipse and total sway area; 95% ellipsoid sway volume | Higher RMS, total power, mean distance, acceleration range, path length, ellipse and total sway area, 95% ellipsoid sway volume and area in TBI than HS | Self-reported symptoms (e.g., dizziness, headache) correlated with sway path length and postural sway area |
| Neuropathies N = 3 [199,200,201] | N = 3 [199,200,201] | 1 to 2 IMUs on waist and shin | Force plate (COP measures) | Upright stance with open or closed eyes, different BOS amplitudes and surfaces (e.g., firm and foam) | RMS acceleration; range of acceleration; peak velocity; body sway area | Correlation between inertial and COP measures; higher RMS acceleration, acceleration range, and peak velocity; larger body sway area in NP than HS | Vibration perception threshold negatively correlated with postural control |
| Vestibular syndromes N = 4 [196,197,198,199] | N = 4 [196,197,198,199] | 1 to 4 IMUs on head, trunk, waist and legs | Not performed | Upright stance with open or closed eyes, different BOS amplitudes and surfaces (e.g., firm and foam); walking tasks; shortened functional mobility test | Range of acceleration; peak velocity; RMS acceleration; mean power frequency; quotient of Romberg for inertial measures | Higher range of acceleration, peak velocity, RMS acceleration and quotient of Romberg for some inertial measures; smaller mean power frequency in VS than HS | Not significant or not performed |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zampogna, A.; Mileti, I.; Palermo, E.; Celletti, C.; Paoloni, M.; Manoni, A.; Mazzetta, I.; Dalla Costa, G.; Pérez-López, C.; Camerota, F.; et al. Fifteen Years of Wireless Sensors for Balance Assessment in Neurological Disorders. Sensors 2020, 20, 3247. https://doi.org/10.3390/s20113247
Zampogna A, Mileti I, Palermo E, Celletti C, Paoloni M, Manoni A, Mazzetta I, Dalla Costa G, Pérez-López C, Camerota F, et al. Fifteen Years of Wireless Sensors for Balance Assessment in Neurological Disorders. Sensors. 2020; 20(11):3247. https://doi.org/10.3390/s20113247
Chicago/Turabian StyleZampogna, Alessandro, Ilaria Mileti, Eduardo Palermo, Claudia Celletti, Marco Paoloni, Alessandro Manoni, Ivan Mazzetta, Gloria Dalla Costa, Carlos Pérez-López, Filippo Camerota, and et al. 2020. "Fifteen Years of Wireless Sensors for Balance Assessment in Neurological Disorders" Sensors 20, no. 11: 3247. https://doi.org/10.3390/s20113247
APA StyleZampogna, A., Mileti, I., Palermo, E., Celletti, C., Paoloni, M., Manoni, A., Mazzetta, I., Dalla Costa, G., Pérez-López, C., Camerota, F., Leocani, L., Cabestany, J., Irrera, F., & Suppa, A. (2020). Fifteen Years of Wireless Sensors for Balance Assessment in Neurological Disorders. Sensors, 20(11), 3247. https://doi.org/10.3390/s20113247













